Abstract
Over the past few decades, Lake Mead (Nevada-Arizona, USA) has experienced multiple ecosystem stressors including drought, increased water demand, and establishment of invasive species (quagga mussels, gizzard shad). Despite these potential stressors, zooplankton and phytoplankton community dynamics in the pelagic regions of Lake Mead have generally followed consistent seasonal patterns. Long-term monitoring results (2007–2015) show that zooplankton and phytoplankton communities remain relatively stable in Lake Mead on an inter-annual basis, but are susceptible to shifts caused by extreme climate fluctuations and alterations in mixing regimes. A warm winter characterized by low snowpack in 2014/2015 preceded a large bloom of toxic cyanobacteria (Microcystis aeruginosa Kützing) in Las Vegas Bay the following summer. Large bloom events are rare in Lake Mead; however, under future climate scenarios, these types of events may become more frequent. Because of the consistency of plankton community dynamics over an extended period of time, Lake Mead offers an ideal system for the study of future climate change impacts. This study aims to characterize the response of plankton communities in Lake Mead to both linear and dynamic environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lake Mead, located along the lower Colorado River (Nevada-Arizona, U.S.A.), is the largest reservoir by volume within the U.S. (Holdren & Turner, 2010). Over the last few decades, Lake Mead has been experiencing ongoing threats to water quality from multiple compounding factors. Since 2000, drought conditions have persisted in reservoirs throughout the southwestern U.S. Average water stage has fallen over 40 m in Lake Mead and inflows have been reduced by over 3,000 Mm3/year (Moreo & Swancar, 2013). Invasive quagga mussels (Dreissena bugensis Andrusov, 1897) were first discovered in Lake Mead in 2007 (LaBounty & Roefer, 2007), but likely became established in the lower Colorado River ca. 2003 (McMahon, 2007). Since introduction, quagga mussels have spread throughout Lake Mead and downstream reservoirs (Wong et al., 2010). Other factors that may be impacting the ecosystem and water quality in Lake Mead include establishment of gizzard shad [Dorosoma cepedianum (Lesueur, 1818)] and rapid population growth in the city of Las Vegas and surrounding area, contributing to significantly increased treated wastewater inflow from the Las Vegas Wash (Holdren & Turner, 2010). It is unclear to what extent climate change might impact Lake Mead and other Colorado River reservoirs, although the majority of models predict declines in average annual river discharge over the next 50 years (Rajagopalan et al., 2009).
Decreases in reservoir water levels pose unknown threats to the ecosystem, and the long-term ecological impacts of invasive dreissenid mussels on southwestern reservoirs are still unclear. Drought conditions have the potential to negatively impact overall water quality in lakes and reservoirs via reduced inflows and outflows, warmer surface temperatures and prolonged stratification, increased water residence time, increased nutrient concentrations, and increased frequency of harmful algal blooms (Mosley, 2015). Murdoch et al. (2000) predict that the combined effects of prolonged drought and warming air temperatures will increase the residence time of point-source nutrients and pollutants in lakes and reservoirs as a result of decreased inflow and outflow. In the Great Lakes region of the U.S., dreissenid mussels have caused significant shifts in water quality and ecosystem function by increasing water clarity and reducing phytoplankton populations (Caraco et al., 1997; Fahnenstiel et al., 2010), in turn reducing populations at higher trophic levels (zooplankton and fish) (Bridgeman et al., 1995; MacIsaac, 1996; Pace et al., 1998).
Despite the potential impacts of quagga mussels on plankton ecology in invaded lakes and reservoirs, little evidence exists demonstrating significant changes in either zooplankton or phytoplankton populations in response to the invasion in Lake Mead (Beaver et al., 2010; Wong et al., 2010; Turkett, 2016). Drought impacts on zooplankton appear to be minimal, as long-term trends in zooplankton community composition follow regular seasonal patterns closely tied to temperature and mixing cues (Beaver et al., 2010). The results of these studies show that the established food web in this system has remained essentially unaltered a decade post-quagga invasion and drought initiation. However, previous studies in many different types of ecosystems have indicated that response of individual species to gradually increasing ecological stressors may not be linear (Scheffer et al., 2001). Many studies have shown that loss of resilience in lake ecosystems can go undetected for several years until a critical threshold of change occurs, which may be induced via abnormal climatic, biological, or hydrological events (Scheffer et al., 2001; Zambrano et al., 2001; Ji et al., 2018). This type of catastrophic change often requires extensive restoration efforts to re-establish biological communities (Jeppesen et al., 2007; Ji et al., 2018). In 2015, warm temperatures reduced winter snowpack and increased thermal stratification during a period of traditional mixing and were followed by a large, toxic bloom of Microcystis aeruginosa Kützing in Lake Mead. The compounding effects of low water elevation due to reduced inflows (which in turn reduces wind-driven mixing, as surface waters are more protected by heightened canyon walls), earlier onset of seasonal stratification and increased surface water temperatures provided environmental conditions that have elsewhere led to blooms of M. aeruginosa (Jacoby et al., 2000; Cao et al., 2006). Cyanobacterial blooms tend to thrive under warm, highly stratified conditions (Paerl & Huisman, 2008), and can lead to production of a suite of potent toxins that threaten humans and wildlife. Although this type of toxic bloom event has been rare amongst Colorado River reservoirs thus far, it may be a foreshadowing of an increasing frequency of such events to come under warming climate conditions, suggesting that Lake Mead may be vulnerable to major ecological disruptions in the future.
This study examines seasonal and inter-annual dynamics of zooplankton and phytoplankton in open water regions of Lake Mead over a nine-year period following the introduction of quagga mussels (2007–2015), including an abnormally warm year that resulted in a large bloom of toxic cyanobacteria. The relationship between zooplankton biomass and water temperature, as well as phytoplankton biovolume and water temperature, was explored using quadratic regression and niche centroid analysis in order to determine optimal temperature conditions for dominant species. Canonical correlation analysis (CCA) was then used to determine how fluctuations in water quality variables may affect relative biomass or biovolume of individual taxa. Recently, Beaver et al. (2010) documented a high degree of regularity in seasonal patterns of zooplankton abundance and community composition between 2000 and 2009. Given the long-term duration of unchanging plankton community dynamics under increasing ecological stress, along with the 2015 bloom event that coincided with an extreme climate fluctuation, we suggest that plankton in Lake Mead may be experiencing a threshold, rather than linear response to environmental change. Since inter-annual variability has been relatively low thus far, Lake Mead represents an excellent system for the study of climate change impacts in both the near-term and long-term future. Results underscore the importance of the linkages amongst hydrology, nutrients, phytoplankton, and higher trophic levels in Colorado River reservoirs, and highlight the potential impacts of climate change on these important ecosystems.
Materials and methods
Study sites
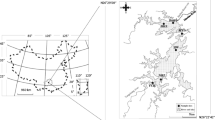
Lake Mead, a subtropical monomictic system, is impounded by Hoover Dam, which supplies power generation to a large portion of the southwestern United States. It is an economically important reservoir as it provides municipal water for the City of Las Vegas and the surrounding area, supplying about 90% of municipal water needs in the Las Vegas Valley. Releases from Hoover Dam are critical for water supply to downstream reservoirs such as Lake Mojave (Nevada-Arizona) and Lake Havasu (Arizona-California). The Lake Mead National Recreation Area is one of the largest in the country, with a public launch capacity for over 3,000 boats (Holdren & Turner, 2010). Lake Mead also serves as habitat for several varieties of sportfish, as well as the endangered razorback sucker [Xyrauchen texanus (C.C. Abbott, 1860)], native to the Colorado River. Major tributaries of Lake Mead are the Colorado River (97% of inflow), Virgin River, and the Las Vegas Wash. Varied water inflows from each tributary result in three major, ecologically unique regions of Lake Mead: Las Vegas Bay, Overton Arm, and Colorado River. Las Vegas Bay, the most downstream basin, collects the combined flows from the Overton Arm, the Las Vegas Wash and the main stem of the Colorado River and consists of four sampling stations (LVB sites, Fig. 1). Overton Arm collects the combined drainages of the Virgin and Muddy Rivers and consists of six sampling stations (VR sites). The Colorado River enters Lake Mead at the northern end, nearly 97 km upstream of Hoover Dam, and consists of seven sampling stations (CR sites). Samples from each station in Las Vegas Bay, Overton Arm, and the Colorado River were collected quarterly (winter, spring, summer, fall) from January 2007 through November 2015 by the Bureau of Reclamation Resources Management Office in Boulder City, Nevada.
Sample collection/laboratory analyses
Water quality
Water quality profiles were conducted along the thalweg of the Colorado River (site CR394.0 to CR342.5) from the bottom to the surface of each sampling station using a multi-parameter submersible sonde unit (Manta Multiprobe, Eureka, Austin) capable of measuring depth, dissolved oxygen concentration, pH, electrical conductivity, and temperature. The bottom depth for each station was recorded as the maximum station depth, and temperature data were recorded in 5-m intervals from the bottom depth to 50 m, followed by 2-m intervals from 50 to 20 m, and finally in 1-m intervals from 20 m to the surface. Thermal profiles of Lake Mead were produced using Surfer®12 (Golden Software, LLC). For the purpose of this study, temperature data from the top five meters of each station were used for statistical analyses. Secchi depth was determined using a viewscope and a 20-cm disc. Water samples for total phosphorus determination were collected as a composite of the 0–5 m strata using a pool hose, and at the 20, 100 m (when applicable), and at the maximum depth using a Kemmerer sample bottle. All samples were kept on ice and shipped overnight to Sierra Foothill Laboratory, a certified and accredited laboratory in the state of California, for analysis. Samples to be analyzed for total phosphate as phosphorus (P) were immediately acidified in the field and digested and analyzed using EPA method SM4500-P,E, with a reporting limit of 0.002 mg/l as P.
Zooplankton
Water samples collected for zooplankton analysis from 2007 to 2015 were obtained from the top 0–30 m (or identified maximum depth) using a 64-µm mesh Wisconsin-style plankton net. The net was washed into 250-ml Nalgene bottles and preserved with 2% Lugol’s solution, and promptly shipped on ice to BSA Environmental Services, Inc. Upon arrival at the laboratory, sample volumes were measured and homogenized aliquots examined on Wilovert inverted microscopes at ×100 magnification (up to ×1000 if necessary). All samples were analyzed using the Utermöhl sedimentation technique. A minimum tally of 200 organisms per sample was reached (or the entire sample analyzed), and specimens were identified to the lowest taxonomic level possible. For each identified taxa, up to 10 individual specimen length and width measurements were recorded (or less for more rare taxa) for the calculation of biomass estimates based on established length/width relationships (Dumont et al., 1975; McCauley, 1984; Lawrence et al., 1987). Zooplankton carbon content was assumed to be 50% of dry mass (Latja & Salonen, 1978).
Phytoplankton
Phytoplankton samples were collected as 0-5 m composites using a pool hose and stored in 250-ml Nalgene bottles. Each sample was immediately preserved with 2% Lugol’s solution and iced in the field, then shipped to BSA Environmental Services, Inc. for laboratory analysis. Phytoplankton slides were prepared using standard membrane filtration technique in order to preserve cell structure for examination at high magnification (McNabb, 1960).
Filtered phytoplankton samples were analyzed on Leica DMLB compound microscopes at ×630 magnification for random field and full strip counts, as well as ×400 magnification for half-filter counts to further ensure complete species reporting. As per Lund et al. (1958), a minimum of 400 natural units (colonies, filaments, unicells) were enumerated to the lowest possible taxonomic level from each sample to ensure accuracy within 90% confidence limits. Cell biovolumes, based on measurements of 10 organisms per taxon when possible, were estimated for all identified phytoplankton taxa using formulae for solid geometric shapes that most closely match cell shape (Hillebrand et al., 1999). PCR was performed following microscopic classification to confirm the identification of Microcystis aeruginosa (Nübel et al., 1997), a known toxin-producing species (Chorus & Bartram, 1999). Phytoplankton carbon content was estimated as described in Rocha & Duncan (1985). C-phytoplankton was divided by 106 in order to achieve comparable units with C-zooplankton (µg/l) and carbon ratios (C-zooplankton:C-phytoplankton) were calculated using whole-lake means for each sampling event. Mean carbon ratios were plotted against whole-lake mean surface temperatures, and a regression equation that best fit the data, along with the associated coefficient of determination, was calculated using Microsoft Excel.
Algal toxins
Whole water grab samples for toxin analysis were collected in August, 2015 between 0 and 5 m depth from 19 open water sites throughout the reservoir, as well as two near-shore sites and one beach site. Samples were collected with buffer (TRIZMA, 2-chloroacetamide), held on ice during transport to Texas A&M University Corpus Christi, and processed within 6 h of receipt. 60-ml subsamples were concentrated on SPE C18 cartridges, then eluted with 1 ml 75% MeOH and the elutes were analyzed for the detection of Microcystin-LR using HPLC/MS following EPA method 544.
Temperature intervals and niche centroids
The relationship between water temperature and daphnid biomass was examined using both a quadratic regression model and niche centroid analysis. Spring and summer (May and August 2007–2015) samples were used for the quadratic regression model. Mean biomass values were calculated for both Daphnia pulex complex and Daphnia galeata mendotae Birge, 1918 for each respective water temperature interval: 16–18, 18–20, 20–22, 22–24, 24–26, 26–28, 28–30, and 30–32°C. All sampling points with temperatures that fell within an interval range were used to calculate biomass means. An equation that best fits the data and the associated coefficient of determination were calculated using Microsoft Excel.
Niche centroid analysis provides a weighted average of an environmental variable (in this case, surface water temperature) in sites at which an individual taxon occurs. Niche centroid temperatures calculated for different taxa can be compared and interpreted as the “optimum” temperature at which that taxon is likely to experience highest biomass or biovolume (infer maximal growth). The relationship between water temperature and biomass/biovolume was quantified using the equation described in ter Braak and Verdonschot (1995):
where yik is the density of species k in the ith sample; y+k is the summed biomass/biovolume of species k in all samples, and xi is the water temperature in the ith sample.
For the niche centroid analysis, Daphnia pulex complex and Daphnia galeata mendotae biomass were individually analyzed, as well as Cyclotella spp., Rhodomonas spp., and Microcystis spp. biovolume.
Statistical analyses
Zooplankton
34 zooplankton taxa identified from samples collected between January 2007 and November 2015 were used in Canonical Correlation Analyses (CCA) using PRIMER 6 (Clarke & Gorley, 2006). Samples with missing or unavailable water quality data were excluded from the analysis, resulting in 587 individual samples. The zooplankton data set was standardized and a Principal Component Analysis (PCA) was performed, which identified a few statistically significant species that explained a large percentage of the total variance within the data set (D. pulex complex and D. galeata mendotae). Subsequently, a Bray–Curtis resemblance matrix of the standardized zooplankton biomass data was used in a CCA (using the Canonical Analysis of Principal Coordinates (CAP) function of the PERMANOVA+ add-on in PRIMER 6) with a sample-matched, normalized matrix of 4 water quality variables: water temperature, Secchi depth, station depth, and total phosphorus. Following the CCA, samples were plotted on ordination diagrams using the first two CAP axes, and the biomass values of D. pulex complex and D. galeata mendotae were superimposed over the CCA plot.
Phytoplankton
Prior to analyses, phytoplankton biovolume data were reduced to include only the taxa that constituted greater than or equal to 5% of the total sample biovolume for at least one sample, resulting in 79 taxa. A PCA on the standardized phytoplankton biovolume data set identified Rhodomonas spp. and Cyclotella spp. as significant genera contributing to explained variability. Both genera, as well as Microcystis spp., were then superimposed over the zooplankton CCA ordination diagrams.
Results
Water quality
The Colorado River was the deepest, clearest region of Lake Mead with a mean station depth of 89.4 m and mean Secchi depth of 9.5 m (Table 1). The lowest mean depth and clarity was observed in Las Vegas Bay (41.9 and 6.5 m, respectively). In contrast, Las Vegas Bay was the warmest region with a mean temperature of 20.7°C, and also exhibited the highest mean phosphorus concentration (14.2 μg/l). Temperature and total phosphorus concentrations in Overton Arm and Colorado River displayed narrow differences (20.0, 20.1°C and 7.1, 5.6 μg/l, respectively). Annual mean winter water temperatures from the top 5 m of the water column support the shifting temperature–density pattern observed in the winter for Las Vegas Bay, Overton Arm, and Colorado River regions of Lake Mead (Table 2, Fig. 2). Differences in winter water temperatures from all three basins were determined to be statistically significant (P < 0.05, two sample t test assuming equal variance).
Thermal profiles
Thermal profiles of the Colorado River thalweg from February 2012–2015 (Fig. 3) display the shifting temperature–density relationship in Lake Mead, which has a water column that has historically been either completely or partially mixed in winter. In February 2012, relative uniformity in temperature at the downstream end of the lake (near station CR342.5) was observed, ranging from about 12.5°C at the surface of the lake to 9.5°C at the lake bed (Fig. 3A). Similar uniformity was observed in February 2013 and February 2014 (Fig. 3B, C). The thermal profile from February 2015 (Fig. 3D) portrayed a strongly stratified water column that has maintained a distinct epilimnion (surface water temperatures approximately 13–16°C above 30 m depth) and hypolimnion (water temperatures approximately 12–13°C below 30 m depth). Thermal profiles from station CR346.4 during winter sampling from 2007 to 2015 also show consistency in winter mixing regimes from 2007 to 2014 (Fig. 4). In winter 2015, water temperatures from about 40 m depth down to the lake bed were approximately 12°C, consistent with previous years; however water temperatures in the top 40 m of the water column at station CR346.4 ranged from about 13–14.5°C, while in the top 3 m of the water column temperatures ranged from 14.9 to 16.3°C. In all previous winters, surface water temperatures did not go above 13.4°C.
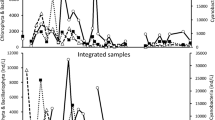
Zooplankton and phytoplankton seasonality
Zooplankton biomass was primarily dominated by D. pulex complex in all regions of Lake Mead, secondarily by D. galeata mendotae. Generally, peak zooplankton populations were in winter and spring (February, May) when water temperatures were 12–14 and 18–25°C, respectively (Fig. 5). Throughout the study, zooplankton populations were considerably higher in Las Vegas Bay than Overton Arm and Colorado River. Maximum D. pulex complex and D. galeata mendotae populations occurred in Las Vegas Bay; D. pulex complex in winter 2011 (mean biomass 537.5 µg d.w./l) and D. galeata mendotae in spring 2013 (mean biomass 73.8 µg d.w./l). No D. pulex complex was observed in Las Vegas Bay in summer 2010, summer 2013, spring, summer, and fall 2014, or summer 2015. D. galeata mendotae was not observed in Las Vegas Bay in winter 2008, winter 2009, winter 2014, or spring 2014.
Phytoplankton biovolume in all regions of Lake Mead was divided into four categories: Cyclotella spp., Microcystis spp., other cyanobacteria, and other phytoplankton. Peak phytoplankton biomass was primarily observed in spring and summer (May, August) when water temperatures were between 18 and 30°C (Fig. 6). Comparable to zooplankton, phytoplankton populations were generally higher in Las Vegas Bay than Overton Arm and Colorado River. Both Cyclotella spp. and Microcystis spp. mean biovolume peaked within Las Vegas Bay in 2015. Cyclotella spp. was highest in summer 2015 (2.4 × 109 µm3/l), whereas Microcystis spp. was highest in winter 2015 (6.1 × 108 µm3/l).
Mean monthly C-zooplankton:C-phytoplankton ratios showed a moderately strong correlation (R2 = 0.57, Fig. 7) to water surface temperatures, which were indicative of lake mixing regimes. Higher Z:P ratios were generally observed at lower surface temperatures, which tend to occur during mixis while lower Z:P ratios were observed at higher temperatures, which typically occur during periods of thermal stratification.
Toxicity
In the spring and summer of 2015, large floating colonies of Microcystis spp. were observed in the top 2 m of the water column throughout the reservoir. Microcystin concentrations ranged from non-detect (3 sites in the upper reaches of the Virgin River Arm) to 4,696 (Hemenway Harbor) μg/l (Fig. 8). Concentrations were highest in the three near-shore sites. In open water sites, mean microcystin concentration was 1.48 μg/l.
Temperature intervals and niche centroids
A relationship between spring/summer daphnid biomass and water temperature was revealed by both the temperature interval regression and the niche centroid analysis (Fig. 9, Table 3). The temperature interval regression models for D. pulex complex and D. galeata mendotae showed differing temperature preferences for the two species. The regression for D. pulex complex (R2 = 0.48) showed peak biomass between 16 and 18°C, with declines in biomass past about 24°C. The regression for D. galeata mendotae (R2 = 0.32) showed a trend of rising biomass until about 24–26°C, and then declines in biomass at higher temperatures. Very high biomass (137.75 µg d.w./l) of D. galeata mendotae was observed at station LVB3.5 in spring of 2012, producing a very high average biomass for that species at the 24–26°C interval (mean = 10.29 µg d.w./l at 24–26°C, R2 = 0.58 when outlier is excluded). Despite the influence of the outlier value for D. galeata mendotae, the results seen from temperature interval regressions imply a seasonal succession pattern in which D. pulex complex is dominant early on in the season when water temperatures are cooler, followed by a gradual rise in D. galeata mendotae biomass as temperatures warm and D. pulex complex biomass declines. The niche centroid analysis displayed similar results, suggesting that highest D. pulex complex biomass would be found at 16.7°C and highest D. galeata mendotae biomass at 22.3°C.
Mean annual spring and summer (May, Aug) Daphnia pulex complex biomass versus surface (0–5 m) water temperature interval (black, y = − 0.145x2 + 4.836x − 12.04, R2 = 0.48) and Daphnia galeata mendotae biomass versus surface (0–5 m) water temperature interval (gray, y = −0.329x2 + 15.88x − 176.5, R2 = 0.32) for all three regions of Lake Mead (n = 276)
Canonical correlation analysis
Canonical correlation analysis (CCA) compared 34 zooplankton taxa and 4 water quality variables, producing two canonical axes with strong correlations (Table 4). Axes one and two explained 61 and 12% of the total variance, respectively. Water temperature exhibited a strong positive correlation with the first axis (0.600) and a strong negative correlation with the second axis (− 0.640). Secchi depth displayed a strong negative correlation with the first axis (− 0.637), and however was weakly correlated with the second axis. A moderate negative correlation with the first axis and a strong negative correlation with the second axis was observed in regard to station depth (− 0.449 and − 0.547, respectively). Total phosphorus was weakly correlated with the first axis and displayed a strong positive correlation with the second axis (0.535).
For each of the 587 samples analyzed in the CCA, D. pulex complex and D. galeata mendotae biomass were superimposed onto the ordination diagrams to illustrate correlations with the water quality variables (Fig. 10A, B). D. pulex complex was negatively correlated with water temperature indicating a preference for cooler water, and was positively correlated with water clarity (Secchi depth), which was highest in the Colorado River. D. galeata mendotae was negatively correlated with water clarity and positively correlated with temperature, indicating a preference for more turbid, warmer water.
As with the zooplankton biomass, Cyclotella spp., Rhodomonas spp. and Microcystis spp. biovolumes were overlaid onto the zooplankton CCA ordination diagrams to illustrate correlations with the water quality variables (Fig. 11A–C). All three genera displayed similar patterns: Cyclotella spp., Rhodomonas spp., and Microcystis spp. biovolumes were correlated with warm water temperatures and greater water clarity (Secchi depth), which were reflected by the niche centroid temperature optima (25.0, 21.5, and 22.4°C, respectively).
Discussion
Consistency in plankton communities
Previous studies of the zooplankton communities (e.g., Burke 1977; Paulson & Baker 1983; Wilde 1984; Axler et al., 1988; Wilde & Paulson, 1988; Sollberger & Paulson, 1992; Loomis, 2009) and phytoplankton communities (Everett, 1972; Koening et al., 1972; Staker et al., 1974; Baker & Paulson, 1980; Paulson et al., 1980; Evans & Paulson, 1983; Paulson & Baker, 1983; Prentki & Paulson, 1983; Janik, 1984) of Lake Mead have been limited in scope and duration and were largely conducted before the current drought conditions, invasion of quagga mussels and establishment of gizzard shad. Consequently, their value for assessing water quality under current environmental conditions is limited. However, studies examining zooplankton communities prior to current environmental disturbances (drought, invasive species) show that both D. pulex complex and D. galeata mendotae dominated the crustacean zooplankton community (Burke, 1977), and exhibited similar seasonal succession patterns to what was observed in this study (dominance of D. pulex complex in winter followed by D. galeata mendotae in spring, Wilde, 1984). The most recent long-term analysis of zooplankton community dynamics in Lake Mead (Beaver et al., 2010) found that biomass of the dominant zooplankton taxa (Daphnia spp.) generally peaked in the winter/early-spring and was lowest during summer. Results from this study show that seasonal patterns in Daphnia dominance have continued through the current study period, as similar patterns were observed across all three basins. The results of these two studies cover, together, a 15-year period (2000–2015) showing little change in Daphnia dynamics and minimal shifts in community composition.
Phytoplankton composition and seasonality in this study also showed similar trends to what has been observed in the past (Staker et al., 1974; LaBounty & Burns, 2005; Turkett, 2016), with highest biovolume occurring in Boulder Basin (Las Vegas Bay sites and downstream CR sites) and annual biovolume peaks during the summer. The seasonal pattern observed for phytoplankton, as well as niche centroid analysis performed for three major taxa, indicate that phytoplankton biovolume is strongly tied to temperature cues and water clarity. Although not explored in this report, previous studies have determined that Lake Mead is severely phosphorus-limited (Paulson & Baker, 1983; LaBounty & Burns, 2005) and that past phytoplankton blooms have occurred in response to sudden, increased nutrient inputs (LaBounty & Burns, 2005). Alignment of zooplankton and phytoplankton data display an inverse correlation in terms of seasonal shifts in total biomass/biovolume, suggesting that grazing may help limit phytoplankton growth in the winter, when Daphnia are at their most abundant. Alternatively, zooplankton growth might be limited by phytoplankton, as phytoplankton biovolume in Lake Mead is generally much lower than in other U.S. reservoirs (Turkett, 2016).
A recent study of seasonal food web dynamics in Lake Taupō, New Zealand (Stewart et al., 2017) indicated that in large, deep oligotrophic monomictic lakes, the control of the food web may alternate between two states; during the stratified period, primary production may be limited by nutrient availability (bottom-up control), while during mixing, primary production may be more strongly controlled by grazing (top-down control). In Lake Mead, zooplankton:phytoplankton carbon ratios also vary seasonally, with highest ratios observed during mixing periods (winter) most years. This suggests that mixis is critical in terms of structuring plankton communities and setting boundaries on primary production. Long-term trends in chlorophyll from Beaver et al. (2010) showed a decline in Las Vegas Bay, but no change between 2000 and 2007 in other reservoir basins. Over the course of this study, no significant increases or decreases in total phytoplankton biovolume were observed. Species composition also remained relatively consistent on a seasonal and inter-annual basis, save for a few isolated instances of high Microcystis spp. biovolume, the highest of which occurred in Las Vegas Bay in 2015 following abnormally strong thermal stratification in the winter of that year.
Detailed taxonomic analysis revealed a seasonal succession within the daphnid community, showing that the larger D. pulex complex is dominant during winter, while the smaller D. galeata mendotae makes more significant contributions to total biomass during spring and summer. Co-occurrence of D. pulex complex and D. galeata mendotae was common in a recent survey of reservoir systems in the western U.S. (Beaver et al., 2014), implying that zooplankton community dynamics observed in Lake Mead are likely to be comparable elsewhere in the region. CCA performed in this study revealed that surface temperature was a strong driver of species variation within the zooplankton community. Both niche centroid analysis and temperature interval regressions showed a lower temperature optimum for D. pulex complex than for D. galeata mendotae. These results indicate that seasonal shifts between the two daphnid species are closely tied to changes in surface water temperatures. In Lake Mead surface temperatures range from about 11 to 31°C over the course of the year, with annual thermal stratification during summer months (Holdren & Turner, 2010). Habitat partitioning via diel vertical migration in deep lakes with hypolimnetic refugia is thought to be a response to increased zooplanktivory (Jeppesen et al., 2003), which generally is higher during the summer. In both Lake Mead and Lake Powell (the second largest U.S. reservoir, located upstream from Lake Mead on the Colorado River), large-bodied D. pulex complex are nearly absent from the epilimnion during the summer, with largest populations located in cooler strata below 10 m at open water sites, while small-bodied D. galeata mendotae remain in the upper 10 m and are usually found in warm, productive bays influenced by nutrient inflows (Beaver, unpublished data). Similar habitat partitioning between daphnid species with differing thermal tolerances and vertical migration tendencies has been observed in other studies (Stich & Lampert, 1981; Leibold, 1991; Seda et al., 2007).
Zooplankton form a critical link in the Lake Mead food web for both forage fish (threadfin shad, Loomis et al., 2011) and sportfish such as largemouth bass (Wilde & Paulson, 1988) and striped bass (Wilde & Paulson, 1989a), as well as the native, endangered razorback sucker (Marsh & Langhorst, 1988). Because of their contribution to total zooplankton biomass, Daphnia are the pivotal intermediaries in energy transfer to higher trophic levels in Lake Mead and other southwestern U.S. reservoirs. Threadfin shad spawning coincides with daphnid peaks and continues through June in lower Colorado River reservoirs (Wilde & Paulson, 1988, 1989b; Mueller & Horn, 2004; Beaver et al., 2010; Loomis et al., 2011). Annual productivity of sportfish is strongly related to interactions among nutrients, zooplankton and phytoplankton in reservoirs throughout North America (Stockner et al., 2000). The lack of significant changes in both the zooplankton and phytoplankton communities in Lake Mead implies that, despite climatic and ecological disturbances, major forage and sportfish communities (and thus recreational fishing activities) are not currently threatened by disruptions in the food web at lower trophic levels.
Effects of invasive species and drought
Empirical data from the Great Lakes region indicate that dreissenid mussels have both direct and indirect effects on zooplankton populations via consumption of small zooplankters (rotifers and small cladocerans) and competition for food (phytoplankton) (MacIsaac, 1996). Competition between the two groups has the potential to decouple established trophic linkages among nutrients, phytoplankton, zooplankton, and planktivorous fish leading to increased water clarity, reduced turbidity, and altered phytoplankton and zooplankton composition (e.g., Fahnenstiel et al., 2010; Kerfoot et al., 2010; Kissman et al., 2010). Results from the CCA show that D. pulex complex exhibits higher biomass under increased water clarity in Lake Mead. As such, it is possible that filtration by quagga mussels might benefit D. pulex complex by improving water clarity and expanding the extent of preferable habitat for the large cladoceran. On the other hand, increased water clarity has improved the reaction distance for size-selective visual predators which most negatively impacts large-bodied daphnids, such as D. pulex complex (Vinyard & O’Brien, 1976; Miner & Stein, 1993).
A minor decrease in chlorophyll and an increase in water clarity in Boulder Basin (including Las Vegas Bay sites and station CR346.4) post-quagga invasion compared to pre-quagga invasion has been documented (Wong et al., 2010, 2014); however, changes in these parameters were found to be closely associated with declines in phosphorus (human-mediated efforts) and cannot be directly attributed to quagga filtering. A similar decrease in chlorophyll was observed by Beaver et al. (2010); however, this decline was also concurrent with large reductions in phosphorus inputs. Thus, it is difficult to say whether quagga mussels have contributed to declines in phytoplankton, as would be expected following invasion in smaller lakes or reservoirs. The apparently low impact that dreissenid mussels have had on plankton communities in Lake Mead is likely due to specificities in basin morphology, as Lake Mead is much deeper and has a longer water residence time (1–3 years, LaBounty & Burns, 2005) than most eastern U.S. systems that have been impacted by quaggas (Bridgeman et al., 1995; Fanslow et al., 1995; Caraco et al., 1997; Turkett, 2016). Dreissenid filtration rate is dependent on particle concentration, and is accelerated when particle concentration is low (Sprung & Rose, 1988). Filtration speed is also impacted by particle size, temperature, population size, and quality of food available (i.e., ingestion vs. production of pseudofeces) (Turkett, 2016 and references therein). Because of the comparatively large volume of water in Lake Mead, expected ecosystem effects of quagga filtration (i.e., reduction in zooplankton and phytoplankton) may be delayed.
Juvenile (0-year) gizzard shad feed primarily on zooplankton, but may feed on detritus or phytoplankton as they grow (Dettmers & Stein, 1992). Previous studies have shown that gizzard shad have the capacity to nearly eliminate crustacean zooplankton from lakes, if densities are high enough (Dettmers & Stein, 1992, 1996). The expansion and proliferation of gizzard shad throughout the lower Colorado River has been well documented; however, few studies have specifically examined the impacts of invasion on Colorado River reservoir ecosystems (Webber & Jones, 2013). Introductions of gizzard shad and quagga mussels into Lake Mead likely occurred within 4–5 years of each other. Both species are known to consume phytoplankton as a food source; however, the presence of quagga mussels appears to have had minimal impact on slowing the increase in gizzard shad populations in Lake Mead (Wong & Gerstenberger, 2011). While quagga mussels are known for improvement of water clarity in lakes after becoming established (Limburg et al., 2010), gizzard shad often have the opposite effect. Benthic foraging by gizzard shad can disturb sediment in lakes, which may cause increases in total nutrients, reduced water clarity and increases in phytoplankton abundance (Schaus & Vanni, 2000). Thus far, however, neither species has had significant impacts on water clarity or overall phytoplankton abundance in Lake Mead. Both quagga mussels and gizzard shad have expanded in Lake Mead since the time of their introduction; however, declines in zooplankton have not been observed, despite the potential for both species to decimate populations. As with quagga mussels, most previous studies of the impacts of gizzard shad on plankton communities were conducted in smaller, shallower lakes (e.g., Pierce et al., 1981; Dettmers & Stein, 1996; Schaus et al., 2010); it is likely that the depth of Lake Mead restricts interactions between the benthos and the epilimnion to the shallow inflow areas.
Limited studies exploring the effects of drought on plankton communities have shown either an increase in total zooplankton abundance (Olds et al., 2014), a shift from dominance of cladocerans to copepods (Havens et al., 2007), a greater contribution from Daphnia spp. (Beaver et al., 2013) or no significant shift in phytoplankton or zooplankton populations (McGowan et al., 2005). Long-term trends in lake water surface temperature worldwide show that the majority of lakes, regardless of geographical location, have warmed over the last few decades and that surface water warming to closely tied to rising air temperatures; however, the degree and character of surface water warming are highly variable and dependent on specific lake morphology (Butcher et al., 2015; Kraemer et al., 2015; O’Reilly et al., 2015). Accordingly, deeper lakes and reservoirs such as Lake Mead may exhibit slower ecosystem response to the effects of drought and increasing air temperatures.
Occurrence of toxic cyanobacteria
In the past, Lake Mead has experienced periods of high productivity (due to elevated phosphorus before 2002) and blooms of non-toxic species (LaBounty & Burns, 2005). Since about 2000, Microcystis spp. has been observed in Lake Mead, often appearing in high abundances in net tow samples (Beaver, unpublished data). However, the 2015 bloom of M. aeruginosa was the first event, to our knowledge, in which harmful toxins were detected in Lake Mead. Although toxin levels were lower in pelagic zones of the reservoir, all basins experienced some degree of toxicity. Traditionally, cyanobacterial blooms have been associated with high phosphorus inputs, contradicting what was observed in Lake Mead; however, there is growing evidence that non-N2-fixing cyanobacteria (such as Microcystis spp.) can outcompete other cyanobacterial taxa under low-P conditions, as long as nitrogen concentrations are sufficiently high (Paerl & Otten, 2013). Since total nitrogen was not analyzed in this study, it is difficult to say if this was the case. Our analyses show that Microcystis spp. had a thermal optimum during the study period of ~ 22°C. Previous studies have found that Microcystis spp. can thrive at temperatures between 15 and 30°C (Chorus & Bartram, 1999) and does best under highly stratified conditions in which it can regulate buoyancy to occupy the surface layer of the water column. During the 2015 bloom event, large colonies of M. aeruginosa were observed several meters below the water’s surface. Previous long-term studies on the German lake Müggelsee show that toxic cyanobacteria can dominate phytoplankton communities once thermal stratification thresholds are reached, regardless of ambient air temperatures (Wagner & Adrian, 2009; Huber et al., 2012), and enclosure experiments show that cyanobacterial blooms can actually help to prolong stratified conditions (Kumagai et al., 2000). Overall toxicity of Microcystis spp. has also been linked to increased temperatures (Davis et al., 2009).
In Lake Mead, the likelihood of toxic bloom reoccurrence may be exacerbated by populations of quagga mussels. In dreissenid-invaded systems in the Great Lakes region of the United States, invasion has often been followed by establishment of annual Microcystis spp. blooms (Lavrentyev et al., 1995; Vanderploeg et al., 2002). Selective filtration by quaggas favors Microcystis spp., as mussels preferentially discard that species in favor of diatoms and other phytoplankton. As a result, microcystin concentrations have been shown to be higher in lakes with established populations of dreissenid mussels (Knoll et al., 2008). Likewise, invasion of gizzard shad may increase likelihood of phytoplankton blooms if zooplankton predators are significantly decreased. The potential thermal overlap between dominant Daphnia spp. and Microcystis spp. indicates that these taxa could co-occur. Daphnia are susceptible to inhibitory impacts to food ingestion (Rohrlack et al., 1999) and death from microcystin poisoning (Rohrlack et al., 2005). Recurrences of toxic blooms could be detrimental to the dominant zooplankton of Lake Mead and could cause cascading reductions in higher trophic levels.
The 2015 M. aeruginosa bloom followed high water temperatures and earlier than normal stratification in Lake Mead resulting from a combination of low winter precipitation and warm spring and summer air temperatures. Similar conditions were observed during that time period across most western states in the U.S. (Mote et al., 2016) and were likely related to higher than average off-shore sea surface temperatures that persisted from the winter of 2013/2014 through the spring/summer of 2015 (Bond et al., 2015). Varying ecological consequences from across the western U.S. and eastern Pacific coastline were reported following the 2014/2015 warm weather anomaly (Cavole et al., 2016), including multiple instances of organisms appearing outside of their expected range. There are many examples of sea surface temperature anomalies causing droughts and disrupting plankton composition in lakes and reservoirs particularly in shallow productive systems. Prolonged droughts create favorable conditions for cyanobacteria by increasing water residence time, thermal stability, and surface temperature, (e.g., Bouvy et al., 2000; Reichwaldt & Ghadouani, 2012; Brasil et al., 2015) while creating conditions less favorable for cladocerans (Havens et al., 2017; Ji et al., 2017). The fact that the summer of 2015 was ubiquitously warm in western U.S. states leads to the conclusion that biological consequences during that time period (including the 2015 M. aeruginosa bloom in Lake Mead) stem primarily from anomalous ocean conditions that have since dissipated. However, it remains unclear whether this type of scenario will be more common in the future as drought in the southwestern U.S. persists.
Non-linear responses to change
Ecological theory suggests that biological responses to environmental stress may not exhibit linear reactions to gradual change; instead, gradual degradation in environmental quality may cause a system to slowly lose resiliency in non-quantifiable ways until a threshold is reached, inducing drastic changes in population dynamics (Scheffer et al., 2001). Lake Mead has certainly experienced gradual environmental change over the last 15 years, as drought has steadily decreased reservoir water levels and invasive species have spread and multiplied (Fig. 12). The 2015 M. aeruginosa bloom in Lake Mead may be an example of a non-linear response to environmental change. Up until 2015, surface water temperatures largely remained consistent on an inter-annual basis. Likewise, phytoplankton seasonal dynamics and community composition have been relatively stable, indicating that the effects of drought on zooplankton and phytoplankton have been minimal thus far. The unusually warm weather in 2015 (and associated effects on snowpack and thermal stratification) demonstrated that phytoplankton composition can change dramatically when water temperatures rise a few degrees.
Monthly mean surface water elevation for Lake Mead (open circles) from 2000 to 2015. Black squares represent mean surface water elevation in February of each year. Black diamonds represent mean winter surface water temperature (0–5 m) in Las Vegas Bay (2007–2015). Major disturbances affecting Lake Mead are denoted with black arrows
The morphology of Lake Mead (specifically in terms of size and depth relative to many North American reservoirs) has likely been a factor contributing to the resilience of this ecosystem; however, given predictions about climate warming and continued proliferation of quagga mussels and gizzard shad, it is probable that environmental stress will continue to grow in scope and that impacts to the food web will become more severe. Lake Havasu, located approximately 250 km downstream from Lake Mead, also experienced a large bloom of M. aeruginosa in the summer of 2015. Although the 2015 bloom appears to be an isolated incident thus far in Lake Mead, Lake Havasu has been experiencing annual recurrences of high M. aeruginosa densities in the past several years (H. McMaster, unpublished data). This may be due in part to the differences in morphology and hydrology between the two reservoirs, as Lake Havasu is shallower and warmer on average than Lake Mead. Both Lake Mead and Lake Havasu contain a reservoir of M. aeruginosa cells that are present in the phytoplankton community nearly every year. The fact that Lake Havasu experiences higher densities of M. aeruginosa annually than Lake Mead suggests that the smaller, shallower reservoir may have already crossed an environmental threshold to where high abundances of cyanobacteria are seen during the stratified period every summer. This indicates that Lake Mead may be closer to an irreversible ecological tipping point, where similar toxic bloom events could occur more frequently in a climatically warmer future.
Conclusions
Lake Mead has not demonstrated significant impacts to established trophic level relationships since the beginning of the drought and subsequent invasion by invasive species in the southwestern U.S. Despite multiple ongoing environmental stressors such as drought, invasion of dreissenid mussels, and the establishment of gizzard shad, temporal and spatial patterns of zooplankton and phytoplankton in Lake Mead remained comparable to historic trends during the study period.
The 2015 bloom of M. aeruginosa in Lake Mead was likely a result of anomalous weather conditions (i.e., decreased snowpack and above average air temperatures); however, these conditions may become more commonplace as the climate warms. The compounding effects of climate warming, persistent drought, increasing water demand, and expansion of invasive species have unknown consequences for the future health of the Lake Mead ecosystem. Because seasonal patterns have been relatively consistent on an inter-annual basis, shifts in zooplankton and phytoplankton species composition and phenology could be early signs of changing environmental conditions, indicative that Lake Mead might be experiencing a threshold response to environmental disturbances. Continuation of current monitoring efforts will shed light on the 2015 M. aeruginosa bloom in Lake Mead and will reveal whether the combination of reduced flow and warmer surface temperatures are indeed predictable catalysts for the occurrence of toxic blooms in southwestern U.S. reservoirs.
References
Axler, R., L. Paulson, P. Vaux, P. Sollberger & D. H. Baepler, 1988. Fish aid – the Lake Mead fertilization project. Lake and Reservoir Management 4: 125–135.
Baker, J. R. & L. J. Paulson, 1980. Influence of Las Vegas Wash density current on nutrient availability and phytoplankton growth in Lake Mead. Proceedings of the Symposium of Surface Water Impoundments ASCE. June 2–5, 1980, Minneapolis, MN.
Beaver, J. R., T. E. Tietjen, B. J. Blasius-Wert, J. E. Kirsch, T. C. Rosati, G. C. Holdren, E. M. Kennedy, R. M. Hollis, C. E. Teacher, K. M. Buccier & S. K. Evans, 2010. Persistence of Daphnia in the epilimnion of Lake Mead, Arizona-Nevada, during extreme drought and expansion of invasive quagga mussels (2000–2009). Lake and Reservoir Management 26: 273–282.
Beaver, J. R., D. E. Jensen, D. A. Casamatta, C. E. Tausz, K. C. Scotese, K. M. Buccier, C. E. Teacher, T. C. Rosati, A. D. Minerovic & T. R. Renicker, 2013. Response of phytoplankton and zooplankton communities in six reservoirs of the middle Missouri River (USA) to drought conditions and a major flood event. Hydrobiologia 705: 173–189.
Beaver, J. R., C. E. Tausz, T. R. Renicker, G. C. Holdren, D. M. Hosler, E. E. Manis, K. C. Scotese, C. E. Teacher, B. T. Vitanye & R. M. Davidson, 2014. The late summer crustacean zooplankton in western USA reservoirs reflects ecoregion, temperature and latitude. Freshwater Biology 59: 1173–1186.
Brasil, J., J. L. Attayde, F. R. Vasconcelos, D. D. F. Dantas & V. L. M. Huszar, 2015. Drought-induced water-level reduction favors cyanobacteria blooms in tropical shallow lakes. Hydrobiologia 770: 145–164.
Bridgeman, T. B., G. L. Fahnenstiel, G. A. Lang & T. F. Nalepa, 1995. Zooplankton grazing during the zebra mussel (Dreissena polymorpha) colonization of Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 567–573.
Bond, N. A., M. F. Cronin, H. Freeland & N. Mantua, 2015. Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophysical Research Letters 42: 3414–3420.
Bouvy, M., D. Falcao, M. Marinho, M. Pagano & A. Moura, 2000. Occurrence of Cylindrospermopsis (Cyanobacteria) in 39 Brazilian tropical reservoirs during the 1998 drought. Aquatic Microbiology 23: 13–27.
Burke, T. A., 1977. The limnetic zooplankton community of Boulder Basin, Lake Mead in relation to the metalimnetic oxygen minimum. MS Thesis, University of Nevada, Las Vegas.
Butcher, J. B., D. Nover, T. E. Johnson & C. M. Clark, 2015. Sensitivity of lake thermal and mixing dynamics to climate change. Climatic Change 129: 295–305.
Cao, H.-S., F.-X. Kong, L.-C. Luo, X.-L. Shi, Z. Yang, X.-F. Zhang & Y. Tao, 2006. Effects of wind and wind-induced waves on vertical phytoplankton distribution and surface blooms of Microcystis aeruginosa in Lake Taihu. Journal of Freshwater Ecology 21: 231–238.
Caraco, N. F., J. J. Cole, P. A. Raymond, D. L. Strayer, M. L. Pace, S. E. Findlay & D. T. Fischer, 1997. Zebra mussel invasion in a large, turbid river: phytoplankton response to increased grazing. Ecology 78: 588–602.
Cavole, L. M., A. M. Demko, R. E. Diner, A. Giddings, I. Koester, C. M. Pagniello, M. L. Paulsen, A. Ramirez-Valdez, S. M. Schwenck, N. K. Yen & M. E. Zill, 2016. Biological impacts of the 2013–2015 warm-water anomaly in the Northeast Pacific: winners, losers, and the future. Oceanography 29: 273–285.
Chorus, I. & J. Bartram, 1999. Toxic Cyanobacteria in Water: A Guide to Public Health Significance. World Health Organization, E&FN Spon, London: 400.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth.
Davis, T. W., D. L. Berry, G. L. Boyer & C. J. Gobler, 2009. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8: 715–725.
Dettmers, J. M. & R. A. Stein, 1992. Food consumption by larval gizzard shad: zooplankton effects and its implications for reservoir communities. Transactions of the American Fisheries Society 121: 494–507.
Dettmers, J. M. & R. A. Stein, 1996. Quantifying linkages among gizzard shad, zooplankton and phytoplankton in reservoirs. Transactions of the American Fisheries Society 125: 27–41.
Dumont, H. J., I. Van De Velde & S. Dumont, 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19: 75–97.
Evans, T. D. & L. J. Paulson, 1983. The influence of Lake Powell on the suspended sediment-phosphorus dynamics of the Colorado River inflow to Lake Mead. Aquatic Resource Management of the Colorado River Ecosystem. In Adams, V. D. & V. A. Lamarra (eds), Aquatic Resource Management of the Colorado River Ecosystem. Ann Arbor Scientific Publishers, Ann Arbor: 57–68.
Everett, L. G., 1972. A mathematical model of primary productivity and limnological patterns in Lake Mead. PhD Dissertation, University of Arizona.
Fahnenstiel, G., T. Nalepa, S. Pothoven, H. Carrick & D. Scavia, 2010. Lake Michigan lower food web: long-term observations and Dreissena impact. Journal of Great Lakes Research 36: 1–4.
Fanslow, D. L., T. F. Nalepa & G. A. Lang, 1995. Filtration rates of the zebra mussel (Dreissena polymorpha) on natural seston from Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 489–500.
Havens, K. E., T. E. East & J. R. Beaver, 2007. Zooplankton response to extreme drought in a large subtropical lake. Hydrobiologia 589: 187–198.
Havens, K. E., G. Ji, J. R. Beaver, R. S. Fulton & C. E. Teacher, 2017. Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change. Hydrobiologia. https://doi.org/10.1007/s10750-017-3425-7.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Holdren, G. C. & K. Turner, 2010. Characteristics of Lake Mead, Arizona-Nevada. Lake and Reservoir Management 26: 230–239.
Huber, V., C. Wagner, D. Gerten & R. Adrian, 2012. To bloom or not to bloom: contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 169: 245–256.
Jacoby, J. M., D. C. Collier, E. B. Welch, F. J. Hardy & M. Crayton, 2000. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Canadian Journal of Fisheries and Aquatic Sciences 57: 231–240.
Janik, J. J., 1984. The role of nannoplankton in the phytoplankton dynamics of four Colorado River reservoirs (Lakes Powell, Mead, Mohave, and Havasu). MS Thesis, University of Nevada, Las Vegas.
Jeppesen, E., J. P. Jensen, C. Jensen, B. Faafeng, D. O. Hessen, M. Søndergaard, T. Lauridsen, P. Brettum & K. Christoffersen, 2003. The impact of nutrient state and lake depth on top-down control in the pelagic zone of lakes: a study of 466 lakes from the temperate zone to the arctic. Ecosystems 6: 313–325.
Jeppesen, E., M. Meerhoff, B. A. Jacobsen, R. S. Hansen, S. Sondergaard, J. P. Jensen, T. L. Lauridsen, N. Mazzeo & C. W. C. Branco, 2007. Restoration of shallow lakes by nutrient control and biomanipulation – the successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285.
Ji, G., K. E. Havens, J. R. Beaver & R. S. Fulton, 2017. Response of zooplankton to climate variability: drought creates a perfect storm for cladocerans in shallow eutrophic lakes. Water 9: 764.
Ji, G., K. E. Havens, J. R. Beaver & T. L. East, 2018. Recovery of plankton from hurricane impacts in a large shallow lake. Freshwater Biology 63: 366–379.
Kerfoot, W. C., F. Yousef, S. A. Green, J. W. Budd, D. J. Schwab & H. A. Vanderploeg, 2010. Approaching storm: disappearing winter bloom in Lake Michigan. Journal of Great Lakes Research 36: 30–41.
Kissman, C. E., L. B. Knoll & O. Sarnelle, 2010. Dreissenid mussels (Dreissena polymorpha and Dreissena bugensis) reduce microzooplankton and macrozooplankton biomass in thermally stratified lakes. Limnology and Oceanography 55: 1851–1859.
Knoll, L. B., O. Sarnelle, S. K. Hamilton, C. E. Kissman, A. E. Wilson, J. B. Rose & M. R. Morgan, 2008. Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Canadian Journal of Fisheries and Aquatic Sciences 65: 448–455.
Koenig, E. R., R. W. Tew & J. E. Deacon, 1972. Phytoplankton successions and lake dynamics in Las Vegas Bay, Lake Mead, Nevada. Journal of the Arizona Academy of Science 7: 109–112.
Kraemer, B. M., O. Anneville, S. Chandra, M. Dix, E. Kuusisto, D. M. Livingstone, A. Rimmer, G. Schadlow, E. Silow, L. M. Sitoki, R. Tamatamah, Y. Vadeboncoeur & P. B. McIntyre, 2015. Geophysical Research Letters 42: 4981–4988.
Kumagai, M., S. Nakano, C. Jiao, K. Hayakawa, S. Tsujimura, T. Nakajima, J.-J. Frenette & A. Quesada, 2000. Effect of cyanobacterial blooms on thermal stratification. Limnology 1: 191–195.
LaBounty, J. F. & N. M. Burns, 2005. Characterization of Boulder Basin, Lake Mead, Nevada-Arizona, USA – based on analysis of 34 limnological parameters. Lake and Reservoir Management 21: 277–307.
LaBounty, J. F. & P. Roefer, 2007. Quagga mussels invade Lake Mead. LakeLine 27: 17–22.
Latja, R. & K. Salonen, 1978. Carbon analysis for the determination of individual biomass of planktonic animals. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 20: 2556–2560.
Lavrentyev, J. P., W. S. Gardner, J. F. Cavaletto & J. R. Beaver, 1995. Effects of the zebra mussel (Dreissena polymorpha Pallas) on protozoa and phytoplankton from Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 545–557.
Lawrence, S. G., D. F. Malley, W. J. Findlay, M. A. MacIver & I. L. Delbaere, 1987. Method for estimating dry weight of freshwater planktonic crustaceans from measures of length and shape. Canadian Journal of Fisheries and Aquatic Science 44: 264–274.
Liebold, M. A., 1991. Trophic interactions and habitat segregation between competing Daphnia species. Oecologia 86: 510–520.
Limburg, K. E., V. A. Luzadis, M. Ramsey, K. L. Schulz & C. M. Mayer, 2010. The good, the bad, and the algae: perceiving ecosystem services and disservices generated by zebra and quagga mussels. Journal of Great Lakes Research 36: 86–92.
Loomis, E. M., 2009. Trophic interactions associated with introduction of the invasive quagga mussel in Lake Mead, Nevada. MS Thesis. University of Nevada, Las Vegas.
Loomis, E. M., J. C. Sjoberg, W. H. Wong & S. Gerstenberger, 2011. Abundance and stomach content analysis of threadfin shad in Lake Mead, Nevada: do invasive quagga mussels affect this prey species? Aquatic Invasions 6: 157–168.
Lund, J. W., G. Kipling & E. D. LeCren, 1958. The inverted microscope method of estimating algal numbers and the statistical basis of estimates by counting. Hydrobiologia 11: 143–170.
MacIsaac, H. J., 1996. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. American Zoologist 36: 287–299.
Marsh, P. C. & D. R. Langhorst, 1988. Feeding and fate of wild larval razorback sucker. Environmental Biology of Fishes 21: 59–67.
McCauley, E., 1984. The estimation of the abundance and biomass of zooplankton in samples. In Downing, J. A. & F. H. Rigler (eds), A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters. Blackwell Scientific Publications, Oxford: 228–265.
McGowan, S., P. R. Leavitt & R. I. Hall, 2005. A whole-lake experiment to determine the effects of winter droughts on shallow lakes. Ecosystems 8: 694–708.
McMahon, R. F., 2007. Analysis of the shell length distributions of quagga mussel (Dreissena rostriformis bugensis) taken from Lake Mead and Mohave on the Colorado River drainage during Jan–Mar 2007. U.S. Bureau of Reclamation
McNabb, C. D., 1960. Enumeration of freshwater phytoplankton concentrated on the membrane filter. Limnology and Oceanography 5: 57–61.
Miner, J. G. & R. A. Stein, 1993. Interactive influence of turbidity and light on larval bluegill (Lepomis macrochirus) foraging. Canadian Journal of Fisheries and Aquatic Sciences 50: 781–788.
Moreo, M. T. & A. Swancar, 2013. Evaporation from Lake Mead, Nevada and Arizona, March 2010 through February 2012. USGS Scientific Investigations Report 2013–5229.
Mosley, L. M., 2015. Drought impacts on the water quality of freshwater systems; review and integration. Earth-Science Reviews 140: 203–214.
Mote, P. W., D. E. Rupp, S. Li, D. J. Sharp, F. Otto, P. F. Uhe, M. Xiao, D. P. Lettenmaier, H. Cullen & M. R. Allen, 2016. Perspectives on the causes of exceptionally low 2015 snowpack in the western United States. Geophysical Research Letters 43: 10980–10988.
Mueller, G. & M. J. Horn, 2004. Distribution and abundance of the pelagic fish in Lake Powell, Utah and Lake Mead, Arizona-Nevada. Western North American Naturalist 64: 306–311.
Murdoch, P. S., J. S. Baron & T. L. Miller, 2000. Potential effects of climate change on surface water quality in North America. Journal of the American Water Resources Association 36: 347–366.
Nübel, U., F. Garcia-Pichel & G. Muyzer, 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Applied and Environmental Microbiology 63: 3327–3332.
Olds, B. P., B. C. Peterson, K. D. Koupal, C. W. Schoenebeck, K. M. Farnsworth-Hoback & W. W. Hoback, 2014. Zooplankton density increases in an irrigation reservoir during drought conditions. Transactions of the Nebraska Academy of Sciences 34: 27–32.
O’Reilly, C. M., S. Sapna, D. K. Gray, S. E. Hampton, J. S. Read, R. J. Rowley, P. Schneider, J. D. Lenters, P. B. McIntyre, B. M. Kraemer, G. A. Weyhenmeyer, D. Straile, B. Dong, R. Adrian, M. G. Allan, O. Anneville, L. Arvola, J. Austin, J. L. Bailey, J. S. Baron, J. D. Brookes, E. de Eyto, M. T. Dokulil, D. P. Hamilton, K. Havens, A. L. Hetherington, S. N. Higgins, S. Hook, L. R. Izmest’eva, K. D. Joehnk, K. Kangur, P. Kasprzak, M. Kumagai, E. Kuusisto, G. Leshkevich, D. M. Livingstone, S. MacIntyre, L. May, J. M. Melack, D. C. Mueller-Navarra, M. Naumenko, P. Noges, T. Noges, R. P. North, P.-D. Plisnier, A. Rigosi, A. Rimmer, M. Rogora, L. G. Rudstam, J. A. Rusak, N. Salmaso, N. R. Samal, D. E. Schindler, S. G. Schladow, M. Schmid, S. R. Schmidt, E. Silow, M. E. Soylu, K. Teubner, P. Verburg, A. Voutilainen, A. Watkinson, C. E. Williamson & G. Zhang, 2015. Rapid and highly variable warming of lake surface waters around the globe. Geophysical Research Letters 42: 10773–10781.
Pace, M. L., S. E. Findlay & D. Fischer, 1998. Effects of an invasive bivalve on the zooplankton community of the Hudson River. Freshwater Biology 39: 103–116.
Paerl, H. W. & J. Huisman, 2008. Blooms like it hot. Science 320: 57–58.
Paerl, H. W. & T. G. Otten, 2013. Harmful cyanobacterial blooms: causes, consequences and controls. Microbial Ecology 65: 995–1010.
Paulson, L. J. & J. R. Baker, 1983. Interrelationships among nutrients, plankton and striped bass in Lake Mead. University of Nevada, Las Vegas. Lake Mead Limnological Research Center Technical Report 10.
Paulson, L. J., J. R. Baker & J. E. Deacon, 1980. The limnological status of Lake Mead and Lake Mohave under present and future powerplant operations of Hoover Dam. Lake Mead Limnological Research Center Technical Report 1.
Pierce, R. J., T. E. Wissing & B. A. Megrey, 1981. Aspects of the feeding ecology of gizzard shad in Acton Lake, Ohio. Transactions of the American Fisheries Society 110: 391–395.
Prentki, R. T. & L. J. Paulson, 1983. Historical patterns of phytoplankton productivity in Lake Mead. In Adams, V. D. & V. A. Lamarra (eds), Aquatic Resource Management of the Colorado River Ecosystem. Ann Arbor Scientific Publishers, Ann Arbor: 105–123.
Rajagopalan, B., K. Nowak, J. Prairie, M. Hoerling, B. Harding, J. Barsugli, A. Ray & B. Udall, 2009. Water supply risk on the Colorado River: can management mitigate? Water Resources Research 45: W08201.
Reichwaldt, E. S. & A. Ghadouani, 2012. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: between simplistic scenarios and complex dynamics. Water Research 46: 1372–1393.
Rohrlack, T., M. Henning & J.-G. Kohl, 1999. Does the toxic effect of Microcystis aeruginosa on Daphnia galeata depend on microcystin ingestion rate? Archiv für Hydrobiolgie 146: 385–395.
Rohrlack, T., K. Christoffersen, E. Dittmann, I. Nogueira, V. Vasconcelos & T. Börner, 2005. Ingestion of microcystins by Daphnia: intestinal uptake and toxic effects. Limnology and Oceanography 50: 440–448.
Rocha, O. & A. Duncan, 1985. The relationship between cell carbon and cell volume in freshwater algal species used in zooplanktonic studies. Journal of Plankton Research 7: 279–294.
Schaus, M. H. & M. J. Vanni, 2000. Effects of omnivorous gizzard shad on phytoplankton and nutrient dynamics: role of sediment feeding and fish size. Ecology 81: 1701–1719.
Schaus, M. H., W. Godwin, L. Battoe, M. Coveney, E. Lowe, R. Roth, C. Hawkins, M. Vindigni, C. Weinberg & A. Zimmerman, 2010. Impact of the removal of gizzard shad (Dorosoma cepedianum) on nutrient cycles in Lake Apopka, Florida. Freshwater Biology 55: 2401–2413.
Scheffer, M., S. Carpenter, J. A. Foley, C. Folke & B. Walker, 2001. Catastrophic shifts in ecosystems. Nature 413: 591–596.
Seda, J., K. Kolarova, A. Petrusek & J. Matchacek, 2007. Daphnia galeata in the deep hypolimnion: spatial differentiation of a “typical epilimnetic” species. Hydrobiologia 594: 47–57.
Sollberger, P. J. & L. J. Paulson, 1992. Littoral and limnetic zooplankton communities in Lake Mead, Nevada-Arizona, USA. Hydrobiologia 237: 175–184.
Sprung, M. & U. Rose, 1988. Influence of food size and food quantity on the feeding of the mussel Dreissena polymorpha. Oecologia 77: 526–532.
Staker, R. D., R. W. Hoshaw & L. G. Everett, 1974. Phytoplankton distribution and water quality indices for Lake Mead (Colorado River). Journal of Phycology 10: 323–331.
Stewart, S. D., D. P. Hamilton, W. T. Baisden, M. Dedual, P. Verburg, I. C. Duggan, B. J. Hicks & B. S. Graham, 2017. Variable littoral-pelagic coupling as a food web response to seasonal changes in pelagic primary production. Freshwater Biology 62: 2008–2025.
Stich, H.-B. & W. Lampert, 1981. Predator evasion as an explanation of diurnal vertical migration by zooplankton. Nature 293: 396–398.
Stockner, J. G., E. Rydin & P. Hyenstrand, 2000. Cultural oligotrophication: causes and consequences for fisheries resources. Fisheries 25: 7–14.
ter Braak, C. J. F. & P. F. T. Verdonschot, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Ecology 57: 255–289.
Turkett, W., 2016. Impacts to phytoplankton after the establishment of quagga mussels in Lake Mead, Nevada. PhD Dissertation, University of Nevada, Las Vegas.
Vanderploeg, H. A., T. F. Nalepa, D. J. Jude, E. L. Mills, K. T. Holeck, J. R. Liebig, I. R. Grigorovich & H. Ojaveer, 2002. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 1209–1228.
Vinyard, G. L. & W. J. O’Brien, 1976. Effects of light and turbidity on the reactive distance of bluegill (Lepomis macrochirus). Journal of the Fisheries Board of Canada 33: 2845–2849.
Wagner, C. & R. Adrian, 2009. Cyanobacteria dominance: quantifying the effects of climate change. Limnology and Oceanography 54: 2460–2468.
Webber, P. A. & M. T. Jones, 2013. Continued gizzard shad (Dorosoma cepedianum) range expansion in the Colorado River basin. Western North American Naturalist 73: 110–112.
Wilde, G. R., 1984. Seasonal and spatial heterogeneity in the limnetic zooplankton community of Lake Mead. MS Thesis, University of Nevada, Las Vegas.
Wilde, G. R. & L. J. Paulson, 1988. Food habits of young-of-the-year large-mouth bass in Lake Mead and Lake Mohave, Arizona-Nevada. The Great Basin Naturalist 48: 458–488.
Wilde, G. R. & L. J. Paulson, 1989a. Food habits of subadult striped bass in Lake Mead, Arizona-Nevada. The Southwestern Naturalist 34: 118–123.
Wilde, G. R. & L. J. Paulson, 1989b. Temporal and spatial variation in pelagic fish abundance in Lake Mead determined from echo grams. California Fish and Game 75: 218–223.
Wong, W. H. & S. L. Gerstenberger, 2011. Quagga mussels in the western United States: monitoring and Management. Aquatic Invasions 6: 125–129.
Wong, W. H., T. Tietjen, S. Gerstenberger, G. C. Holdren, S. Mueting, E. Loomis, P. Roefer, B. Moore, K. Turner & I. Hannoun, 2010. Potential ecological consequences of invasion of the quagga mussel (Dreissena bugensis) into Lake Mead, Nevada-Arizona. Lake and Reservoir Management 26: 306–315.
Wong, W. H., G. C. Holdren, T. Tietjen, S. Gerstenberger, B. Moore, K. Turner & D. C. Wilson, 2014. Effects in invasive quagga mussels (Dreissena rostriformis bugensis) on chlorophyll and water clarity in Lakes Mead and Havasu of the Lower Colorado River Basin, 2007–2009. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts and Control, 2nd ed. CRC Press, Boca Raton: 495–508.
Zambrano, L., M. Scheffer & M. Martinez-Ramos, 2001. Catastrophic response of lakes to benthivorous fish introduction. Oikos 94: 344–350.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: David Philip Hamilton
Rights and permissions
About this article
Cite this article
Beaver, J.R., Kirsch, J.E., Tausz, C.E. et al. Long-term trends in seasonal plankton dynamics in Lake Mead (Nevada-Arizona, USA) and implications for climate change. Hydrobiologia 822, 85–109 (2018). https://doi.org/10.1007/s10750-018-3638-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3638-4