Abstract
The use of diatoms as bio-indicators of water quality is common in temperate regions worldwide. However, less attention has been accorded to sub-tropical regions, particularly in Australia. This study assessed the value of diatom communities to infer water quality in a sub-tropical riverine system. Epilithic diatom and water samples were collected monthly from an east Australian river. Principal components analysis showed that the Lower Catchment sites were more strongly influenced by the measured environmental variables differentiating them from the Upper Catchment sites. Canonical Correspondence Analysis showed electrical conductivity and total phosphorus strongly influenced the diatom community distribution. The study revealed diatom species that are robust bio-indicators of water quality in this sub-tropical catchment. Cocconeis placentula, C. placentula var lineata, Gomphonema spec 2 and Tabellaria flocculosa were identified as indicators of moderate water quality. Bacillaria paradoxa, Navicula cryptocephala, Navicula mutica var mutica and Achnanthes fogedii were identified as indicators of poor water quality. This study identified that diatoms are effective indicators of water quality. Further research is required to develop a diatom biological index applicable to sub-tropical east Australian river systems to improve the effectiveness of environmental monitoring and sustainable river management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing human demands on finite water resources has placed a burden on global riverine ecosystems (Wetzel, 2001; Dodson, 2005; Dodds & Whiles, 2010). As world population continues to grow exponentially, demand from multiple anthropogenic uses and ensuing degradation of water resources, is accelerating at an alarming rate (Wetzel, 2001; Jewitt, 2002; Dodson, 2005; Verhoeven & Setter, 2010). Freshwater ecosystems have seen declines in biodiversity far greater than any terrestrial ecosystem globally and are increasingly vulnerable to anthropogenic influence and environmental change (Dudgeon et al., 2006). Broad scale land use change alters hydrological, chemical and biological cycles resulting in catchment modification and degradation (Davies & Nelson, 1994; Hanson et al., 1994; Eyre, 1997; Peters et al., 1997). Unfortunately the result is the declining health of riverine ecosystems with subsequent loss of water quality and biodiversity (Wetzel, 2001; Millennium Ecosystem Assessment, 2005; Verhoeven & Setter, 2010). It is therefore becoming increasingly important to manage the health and integrity of natural water resources effectively to ensure a continued fresh water supply for both human populations and natural systems (Bunn et al., 1999; Preston, 2009; Axelrod, 2011). This has led to an increased focus on catchment scale research and the development of bio-indicators for assessment and monitoring programs to identify the health of riverine ecosystems.
The use of aquatic biota in environmental impact assessments and stream monitoring programs has gained momentum worldwide over the last few decades (Bellinger et al., 2006; Atazadeh et al., 2007). Bio-monitoring is a valuable management tool in assessment and achievement of water quality objectives and environmental values (Herricks & Schaeffer, 1985; ANZECC, 2000). It is integral to assess changes in riverine ecosystems whether they are due to changes in water quality, physical habitat or biological interactions (ANZECC, 2000). The use of bio-indicators gives a more thorough evaluation of environmental conditions. Physical and chemical analyses provide indications of water quality at the time of sampling while biotic parameters show environmental conditions over a temporal range (Battarbee et al., 2001; Philibert et al., 2006; Salomoni et al., 2006; Smol, 2008; Li et al., 2010; Lobo et al., 2010; Bere & Tundisi, 2011).
Benthic macro invertebrates have generally been the most commonly used group of organisms (Resh & Jackson, 1993; Norris & Norris, 1995; Atazadeh et al., 2007; Resh, 2008).
A move towards incorporating benthic diatoms into bio-monitoring programs particularly in Europe, North America and some parts of Australia has been occurring over the past few decades and are increasingly the preferred group of bio-indicators (Atazadeh et al., 2007; Kelly et al., 2009a; Smol & Stoermer, 2010; Almeida & Feio, 2012; Elias et al., 2012). Diatoms are a widely used, tested and proven bio-indicator. Diatoms have been used and researched extensively as bio-indicators of water quality, particularly in the temperate regions of the Northern Hemisphere (Kelly & Whitton, 1998; Wu & Kow, 2002; Bellinger et al., 2006; Salomoni et al., 2006; Bere & Tundisi, 2011). Over the last decade they have become an integral component of policy and legislation in environmental monitoring. In 2000, the Water Framework Directive (EC, 2000) was introduced in the European Union requiring the use of biological indicators (aquatic macrophytes, fishes, invertebrates and phytobenthos) in the water quality evaluation, diatoms being the preferred indicator group by most European nations (Kelly et al., 2009a; Almeida & Feio, 2012; Elias et al., 2012). The United States Environmental Protection Agency systematically uses diatoms in their water quality assessments in many states (Smol & Stoermer, 2010).
Although considerable research effort has been given to temperate regions of the world, less attention has been paid to sub-tropical and tropical regions (Wu & Kow, 2002; Bellinger et al., 2006; Bere & Tundisi, 2011). Recently, a number of studies have been undertaken in temperate sub-tropical Brazil, developing methodology for the implementation of diatom-based monitoring programs in ecological status of riverine ecosystems (Salomoni et al., 2006, 2011; Lobo et al., 2010; Bere & Tundisi, 2011; Bohm et al., 2013). However, research in Australia has predominantly been focused in temperate zones (Blinn et al., 2004; Gell et al., 2005; Newall & Walsh, 2005; Newall et al., 2006; Haynes et al., 2011). Limited studies in sub-tropical regions have concentrated on paleolimnological studies (Parr et al., 2004; Taffs et al., 2008; Logan et al., 2010; Tibby & Taffs, 2011; Logan & Taffs, 2013), or on the estuarine environment (Townsend & Gell, 2005; Logan et al., 2010; Tibby & Taffs, 2011; Logan & Taffs, 2013), with comparatively negligible research effort on freshwater lotic systems (Lake, 1995; Blinn & Bailey, 2001; Mosisch et al., 2001).
Diatom indices have been well developed in the Northern Hemisphere, particularly in Europe and the US. However, the applicability of these indices to Australian rivers has not been widely researched. Diatoms have been under-utilised in Australia. Certainly, there are gaps in Australian diatom research, particularly in sub-tropical and tropical zones. The Diatom Index for Australian Rivers (DIARs) was developed by Chessman et al. (1999) as a tool (using 55 indicator genera) for assessing common anthropogenic stressors in eastern NSW and Victoria. This index assigned each genus with a number from 1 to 10 according to their inferred sensitivity to a general range of stressors. This index was later improved upon by Chessman et al. (2007) using data from four Australian states and the Australian Capital Territory, extending the index to a species level version, the Diatom Species Index for Australian Rivers (DSIARs). This new index could potentially give a more accurate assessment of stream health, as it identifies species sensitivity variations within a genus, and negates previous problems with changes in diatom genera taxonomy (Chessman et al., 2007).
The data used to develop the DSIAR was sourced predominantly from temperate zones within Australia. This could limit its use in sub-tropical regions as it may not include enough datasets from sub-tropical and tropical zones for effective assessment in those areas. Lack of research in these climate zones may have limited the datasets available for inclusion in the DSIAR. These need to be developed to enhance the use of diatoms as bio-indicators, and implemented routinely and systematically as standard world’s best practice in the assessment and monitoring of river health in Australia.
The aim of this study was to assess the diatom assemblages and water quality of a sub-tropical east Australian riverine system to identify a correlation between community composition and water chemistry and to assess the applicability of temperate region-based diatom indices to a sub-tropical river system.
Methods
Study area and sampling design
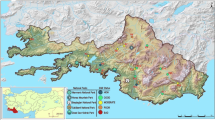
Coopers Creek (Fig. 1) is one of 23 sub-catchments of the Richmond River in northern NSW, with a total stream length of approximately 70 km (Singh et al., 2009). The sub-catchment is divided into two management zones. The Upper Coopers Creek Management Zone is characterised by steep forested slopes interspersed with small cleared rural properties, predominantly macadamia plantations. Much of the Upper Catchment is within NSW National Parks and Wildlife Service estates and has minimal anthropogenic influence. The Lower Coopers Creek Management Zone has extensively cleared valleys with little remaining remnant vegetation and wide floodplains with high productivity (Rous Water, 2009b). Land cleared for agriculture is dominated by beef and dairy production and macadamia orchards, the predominant contributors to diffuse and point source pollution contributing to poor water quality (Morand, 1994; Aplin et al., 1999; Rous Water, 2009a; Singh et al., 2009). The riparian zones along the creeks are minimal and exotic weeds are a significant problem (Morand, 1994; Rous Water, 2009a).
Eight sample sites within the Coopers Creek sub-catchment (Fig. 1) were selected based on ease of access and appropriateness for diatom sampling, such as riffle zones with suitable substrata, depth/photic zone and light regime and were sampled monthly from March to August 2014. Sites 1–4 in the Upper Catchment had extensive riparian zones and were mostly within protected areas Sites 5–8 in the Lower Catchment were highly disturbed with minimal riparian zones, adjacent to macadamia orchards, nurseries and cattle grazing (dairy and beef).
At each site in situ physicochemical parameters were measured and samples were taken for nutrient and diatom analysis. A YSI 556 MPS multi meter was used onsite to determine water temperature, electrical conductivity (EC), pH and dissolved oxygen (DO). Water samples were collected in 1 L plastic bottles which were rinsed three times before sample collection at a depth of 200 mm. Samples were collected, kept on ice and transported back to the Southern Cross University Environmental Analysis Laboratory for further analysis [total dissolved salts, total suspended solids (TSS), phosphate (OP), nitrate (NO3), nitrite (NO2), ammonium (NH3), total nitrogen (TN), total phosphorus (TP)] by a National Association of Testing Authorities accredited laboratory using standard APHA procedures (2012).
Epilithic diatom samples were obtained at each site following the method of Kelly et al. (1998). A sample of 1–3 ml was collected from a minimum of five rocks, randomly selected and sampled at a depth of approximately 200 mm. The upper surfaces of rocks were sampled by removing the algal growth with a toothbrush and collecting the sample in a labelled 70 ml plastic specimen container. All samples were kept on ice and transported back to the laboratory for further processing.
Laboratory analysis
Diatom samples were processed following the method of Battarbee et al. (2001). Soluble salts and carbonaceous material were removed with 10 % hydrochloric acid (HCl). Organic matter was removed by oxidation with 10 % hydrogen peroxide (H2O2). Two slides were prepared for each sample at a high and low density using the mounting medium Naphrax. A minimum of 300 diatom frustules (Battarbee et al., 2001; Chessman et al., 2007) were counted at ×1000 magnification with oil emersion using an Olympus BX51 compound microscope. Diatoms were identified to species level wherever possible using information and photographic plates from a variety of publications including; Foged (1978), Krammer & Lange-Bertalot (1986, 1988, 1991a, b), Vyverman (1995), Hodgson et al. (1997) and Sonneman et al. (2000). All photos of diatom species are archived with the author.
Statistical analysis
Multivariate statistical analyses were used to identify major environmental gradients, explore diatom–environment relationships and identify environmental variables that explained independent portions of the variance in the diatom data. Data were analysed using the statistical package R (R Development Core Team, 2006). Prior to statistical analysis, each environmental variable was checked for skewness, and EC, TP and TSS were log(x + 1) transformed. Principal components analysis (PCA) was performed on the environmental data to determine the major environmental gradients. Parametric t test analyses were performed to determine significant differences in environmental variables and diversity between Upper and Lower Catchment sites (level of significance α = 0.05). Cluster analysis based on Euclidean distance was conducted on the water quality dataset. Detrended Correspondence Analysis with detrending by segments and down weighting of rare species was performed on the species data to establish whether species distribution was unimodal or linear. As gradient lengths were greater than two standard deviation units, unimodal ordination techniques were used (Ter Braak, 1995). Species data were log(x + 1) transformed in an attempt to stabilise the variance in the dataset (Birks et al., 2001).
A series of Canonical Correspondence Analyses (CCA) were performed with scaling focused on inter-species distances, biplot scaling and down weighting of rare species. Variance inflation factors (VIFs) were identified and any environmental variables with VIFs >10 were removed. A series of CCAs of each environmental variable alone was performed, followed by CCAs of individual environmental variables with the remainder as co-variables (i.e., forward selection) to determine which made independent, significant contributions to explaining the variation in the species data (i.e., P < 0.05, based on 999 Monte Carlo permutation tests without Bonferroni or other adjustments). Variance partitioning was used to determine the amount of variation explained by each variable and the interactions between them.
The counts of each diatom taxon were expressed as a percentage of the total valves counted (relative abundance). Diatom community diversity was calculated for each sample using Shannon’s diversity index (Cooper, 1995). Dominant diatom taxa >5% was included in the statistical analysis following Taffs et al. (2008), Bere & Tundisi (2009), Antón-Garrido et al. (2013) and O’Driscoll et al., (2014). Dominant taxa and species diversity was portrayed through stratigraphical diagrams using the program C2 (Juggins, 2007). The correlation between the dominant diatom species and the environmental parameters was calculated and those with an r 2 value >0.5 portrayed to explore species–environment relationships.
Results
Physicochemical data
Water quality analyses identified a trend of higher TP (P = 5.55E−05), TN (P = 0.009) and EC (P = 8.87E−09) values in the Lower Catchment, differentiating them from the Upper Catchment (Fig. 2; Table 1). TP values were high across all sites with the highest means in the Lower Catchment almost four times the ANZECC trigger value guideline (0.020 mg/l; ANZECC, 2000). Most sites recorded TN mean values exceeding the ANZECC trigger value (0.250 mg/l; ANZECC, 2000). Upper Catchment sites recorded lower conductivity (mean 61 μS/cm) than Lower Catchment sites (mean 91.9 μS/cm). All sites recorded acidic values slightly lower than optimum pH. DO levels were below the ANZECC trigger value optimum of 90–110% saturation at all sites (highest 70.5 % sat) (see supplementary information for full dataset).
Analysis of environmental data
The PCA showed no clear dominant environmental parameter influencing the sites (Fig. 3). Temperature, EC, TP and pH are negatively correlated with the first PC axis. TN and DO are correlated with the second PC axis. The Upper Catchment sites predominantly fall in the positive PC axis 1 values. Site 2 had a strong correlation with DO. Site 8 had a strong correlation with temperature and TSS. Site 3 was notably different from all other sites, clustered in the upper right quadrant of the PCA. The Lower Catchment sites were more strongly influenced by the variables measured. The axis lengths were >2; therefore, a CCA analysis was conducted to explore species/environment relationships.
Cluster analysis of selected environmental variables (Fig. 4) indicated a distinction between the four sites of the Upper Coopers Creek (Sites 1–4) and the four sites of the Lower Coopers Creek Catchment (Sites 5–8). Sites separated at the first level are predominantly associated with those only of the Upper Catchment and sites predominantly associated with the Lower Catchment. At the next level, there are six groups: Group A—made up of three outliers of the Upper Catchment sites, Group B—mostly the Lower Catchment Sites 6–8, Group C—mostly Site 5, Group D—Sites 3 and 4 of the Upper Catchment, Group E—Sites 1, 2 and 4 and Group F—mostly Sites 1 and 2 with one of each Sites 3 and 4.
An initial CCA indicated that the environmental data explained 35.48 % of the variation in the diatom data (Table 2). As all VIFs were <10, all environmental variables were retained. TP, pH and EC were correlated with axis 1 and DO, TSS, temperature and TN were correlated with axis 2 (Fig. 5). Six variables explained independent portions of the variance in the diatom data (as determined by forward selection) and CCA of these variables indicated they explained 30.5 % of the variation in the diatom data. Variance partitioning indicated that 42 % of the variation in the diatom data was due to these environmental variables alone and the total interaction between them was 11.6 % (Fig. 6). EC explained the most variation (12.62 %), followed by TP (10.54 %), pH (7.65 %) and TN (4.98 %).
Diatoms
A diverse assemblage of diatom species was recorded from Coopers Creek Catchment (full dataset in supplementary information). There were 33 genera identified with a total of 98 species. Species diversity, determined by Shannon’s diversity index, did not differ significantly between the Upper (2.2205) and Lower (2.3735) Catchment sites as demonstrated by mean of their probability of significance (P = 0.27). Site 2 recorded the highest diatom diversity (Table 3; mean 2.7396), while Site 4 recorded the lowest diatom diversity (mean 1.5595). There were 13 species (relative abundance >5 %) recorded at all sites across the catchment: Achnanthes fogedii Håkansson, Achnanthes saxonica Krasske ex Hustedt, Bacillaria paradoxa Gmelin, Eunotia pirla Carter & Flower, Frustulia rhomboides (Ehrenberg) De Toni, Gomphonema angustatum (Kutzing) Rabenhorst, Gomphonema gracile Ehrenberg, Gomphonema parvulum (Kützing) Kützing, Gyrosigma angulatum (Quekett) Griffith & Henfrey, Navicula cincta (Ehrenberg) Ralfs, Nitzschia capitellata Hustedt, Nitzschia palea (Kützing) Smith and Planothidium haynaldii Schaarschmidt. The diatom communities were generally dominated by three–five species (relative abundance >20 %) at each site.
Community composition varied across sites with a distinction between dominant species in the Upper and Lower Catchments (Fig. 7). In the Upper Catchment, Sites 1 and 2 show similar diatom community composition (Figs. 5, 7) with the dominant species P. haynaldii, N. cincta, G. angulatum, N. palea, A. fogedii and Cocconeis placentula var lineata (Ehrenberg) van Heurck. The community composition of Sites 3 and 4 are quite different to Sites 1 and 2 and each other. The dominant species at Site 3 were Gomphonema spec 2 (Vyverman 1995) and Tabellaria flocculosa (Roth) Kützing, neither of which were identified as being significant in the other Upper or Lower Catchment sites. G. angustatum was another dominant species at this site and was recorded at all sites. Site 4 was dominated by three species, C. placentula var lineata, C. placentula Ehrenberg and G. angustatum. G. spec 2 showed a negative correlation (r 2 −0.50) with TP (Fig. 8). The four most dominant species of the Upper Catchment were C. placentula var lineata, C. placentula, G. spec 2 and T. flocculosa.
In the Lower Catchment Site 5 was dominated by A. saxonica, A. fogedii and N. cincta. Sites 6–8 had similar diatom community composition with B. paradoxa, Navicula cryptocephala Kützing, N. palea and P. haynaldii identified as the dominant species at all three sites (Fig. 7). Three species, Navicula mutica var mutica Kützing, Navicula constans Hustedt and N. capitellata, were identified as being >5 % relative abundance at all sites in the Lower Catchment. B. paradoxa (r 2 0.65) and N. constans (r 2 0.51) had a positive correlation with TP, while N. constans (r 2 0.62) and N. capitellata (r 2 0.62) showed positive correlations with EC (Fig. 8). The four most dominant species of the Lower Catchment were B. paradoxa, N. cryptocephala, N. mutica var mutica and A. fogedii. The ecological tolerances and common diatom index values of the dominant species of the Coopers Creek Catchment are summarised in Table 4.
Discussion
Physicochemical data
Natural processes such as rainfall, flood events, functional riparian zones and seasonal variations in temperature contribute to water quality changes and seasonal variation (Singh et al., 2005; Gay & Ferguson, 2012). Anthropogenic activities such as land clearing and intensive agriculture may cause a decline in water quality (Aplin et al., 1999; Smith et al., 1999; Perna & Burrows, 2005; Dudgeon et al., 2006; Dodds & Whiles, 2010). There was little seasonal variability captured in this study because sampling was conducted across a dry year; hence most variability in the dataset could be attributed to anthropogenic impacts and catchment characteristics. PCA analysis showed that the Lower Catchment sites were more strongly influenced by the measured environmental variables. The cluster analysis demonstrated Upper and Lower sites are different. This may be attributed to greater anthropogenic influence and more intensive land use activities contributing to poorer water quality in the Lower Catchment. The environmental parameters measured, particularly nutrient loads, were specifically selected because they are strong indicators of anthropogenic influence, meeting the project aims.
The distinction between Upper and Lower sites was evident and is indicative of the intensive agricultural practices of the Lower Catchment influencing both higher nutrient levels and EC. TP concentrations exceeded the ANZECC (2000) trigger value (0.020 mg/l) for freshwater streams by as much as 4.5 times and TN almost two times at some sites. The data in this study did not indicate the origins of phosphorus; though they can generally be attributed to the Catchment’s geology and soils derived from volcanic activity of the Mount Warning Volcano (Morand, 1994; Rous Water 2009a, b). Fertiliser additions from land use, particularly macadamia plantations, are sources of higher nutrients in the Lower Catchment and likely responsible for the greatest effect on water quality (Rous Water, 2009a). EC results (P = 8.87E−09) clearly demonstrate a differentiation between Upper (mean 61 μS/cm) and Lower (mean 91.9 μS/cm) management areas. Generally lower river reaches have higher conductivity though these results represent a significant increase. They are also indicative of the surrounding land use activities with greater anthropogenic influence in the Lower Catchment. The low pH values recorded may be influenced by the acidity of the Catchment’s geology and soils derived from volcanic activity of the Mount Warning Volcano (Morand, 1994; Rous Water, 2009a, b). The acidifying effect of nitrogen fertilisers used heavily in macadamia production may also be a heavy contributor (Morand, 1994).
CCA and variance partitioning showed that EC, TP and pH were the most important variables in explaining the variation in diatom composition and it is these parameters that are generally selected for evaluation of ecological tolerances in the common diatom indices. There was a significant amount of variation in the species dataset not explained by measured parameters and hence further research needs to be conducted to explain the main determining factors in the diatom community.
Diatom diversity and community composition
The Coopers Creek Catchment showed a high level of species diversity compared to riverine studies of a similar scale in both Australia and other sub-tropical regions (Blinn & Bailey, 2001; Bellinger et al., 2006; Newall et al., 2006). Diversity was slightly higher in the Lower Catchment, however, the difference was not significant (P = 0.27). This diversity pattern is consistent with the intermediate disturbance hypothesis (Connell, 2002) which suggests that higher diversity is maintained under intermediate scales of disturbances. Our result is similarly consistent with other studies (Chessman, 1986; Blinn & Bailey, 2001; Sonneman et al., 2001; Bellinger et al., 2006) which found lower or no significant difference in species diversity in less impacted or undisturbed streams. They are also consistent with the findings of Stenger-Kovács et al. (2014) who found a linear relationship between diversity and stream order where diversity increased by 10 % per unit of stream order. Stenger-Kovács et al. (2014) suggest that these results could be explained by geological differences and higher nutrient loading in higher order streams as land use becomes more extensive and intensive as stream order increases.
Community composition was similar throughout Sites 1, 2 and 4 of the Upper Catchment and within all sites of the Lower Catchment (Figs. 5, 7). PCA showed that Site 3 was very different from all the other sites in diatom community composition and had little relationship with the environmental variables measured. This site had the least anthropogenic influence of all sites with all areas upstream in protected areas. The physical and chemical parameters measured may not fully capture the factors influencing community composition (Kelly et al., 2001; Chessman et al., 2007). CCA results showed that 35.48 % of the species variation was explained by the measured environmental factors across the catchment, suggesting other variables may be the key to understanding community composition and relationships to environment, particularly at Site 3. Considering the land use of the catchment, organic pollution, trace metals and toxic chemicals such as pesticides, may be playing a greater role in community composition in the Lower Catchment sites (Kelly et al., 2001).
Key indicator species
Community composition of the Upper Catchment was dominated by four species, C. placentula, C. placentula var lineata, G. spec 2 and T. flocculosa which were not evident in the Lower Catchment (Fig. 7). G. spec 2 and T. flocculosa are known as having low to medium tolerance to common anthropogenic stressors, while C. placentula and C. placentula var lineata have been reported as having varying degrees of tolerance to eutrophication and organic pollution in the common diatom indices and literature (IPS: very sensitive, TDI and PTI: moderate, DSIAR and DIAR: very tolerant; Table 4). However, these two species were predominantly found in the Upper Catchment and so along with G. spec 2 and T. flocculosa, were designated as key indicator species of moderate health in the Coopers Creek Catchment. Four species: B. paradoxa, N. cryptocephala, N. mutica var mutica and A. fogedii, were dominant in the Lower Catchment. These species generally show greater tolerance to high nutrient concentrations and pollution (Table 4) and were considered the key indicator species of poor health in the Coopers Creek Catchment.
Species with significant correlations with individual environmental variables can also be considered as key indicator species (Cooper, 2001; Potapova et al., 2004; Weilhoefer & Pan, 2007). In the Upper Catchment, G. spec 2 had a negative correlation (r 2 −0.50) with TP. Limited information has been recorded on the ecological tolerance of G. spec 2, though the Gomphonema genus is generally tolerant of moderate pollution (Chessman et al., 1999). However, results of this study indicated sensitivity of this species to TP, as evidenced by its high abundance in sites with lower concentrations of TP, particularly Site 3.
Contradictions are generally known to occur in the literature of diatom ecology with regard to ecological preferences of species. Research suggests that ecological tolerances of diatom species may also differ across climatic regions and natural variations in water quality (Kelly et al., 2005; Bellinger et al., 2006; Philibert et al., 2006; Bere & Tundisi, 2011; Besse-Lototskaya et al., 2011; Tan et al., 2013). This questions the applicability of Northern Hemisphere and temperate region indices for use in sub-tropical regions of Australia (Newall et al., 2006; Chessman et al., 2007; Tan et al., 2013) and also highlights the need for more research into the ecological tolerance of diatoms in both Australia and internationally (Hermany et al., 2006; Lobo et al., 2010; Besse-Lototskaya et al., 2011). Taxonomic uncertainty is also an issue that needs further attention as misidentification of diatom species is not unusual and can lead to errors in assemblage–environment relationships and subsequent misrepresentation of the trophic status of a river system (Lobo et al., 2010; Besse-Lototskaya et al., 2011; Rimet & Bouchez, 2012).
In the Lower Catchment, B. paradoxa was dominant at sites with the highest TP concentrations. Research suggests this species is a strong indicator of anthropogenic influence due to its association with high P concentrations (Table 4) (Chessman, 1986; Sonneman et al., 2000; Blinn & Bailey, 2001; Kelly et al., 2005; Dela-Cruz et al., 2006). Little ecological information is available for N. constans which was identified as correlating with TP. N. capitellata and N. constans showed positive correlations with EC. Research indicates that N. capitellata is a strong indicator of heavy pollution (Table 4) which supports the results of this study (Sonneman et al., 2000; Kelly et al., 2005; Dela-Cruz et al., 2006). Bahls et al. (1985) reported that P. haynaldii had low tolerance to TN concentrations over 0.3 mg/l. This observation was reflected in our results suggesting P. haynaldii may be a good indicator of low TN concentrations. These species/environmental variable relationships are consistent with land use activities and level of anthropogenic influence and are considered the key indicator species of individual environmental variables in the catchment.
Diatom indices
Diatoms are increasingly used as bio-indicators of water quality, particularly with regard to anthropogenic stressors impacting biological integrity and ecosystem health (Reid et al., 1995; Kelly et al., 1998, 2009b; Chessman et al., 1999, 2007; Gómez & Licursi, 2001; Li et al., 2010; Bere & Tundisi, 2011; Tan et al., 2013). However, as most of the research and indices developed have been centred on temperate regions, they may not be applicable in a sub-tropical environment in Australia (Newall et al., 2006; Chessman et al., 2007).
The results of this study have revealed the need to develop catchment-based calibration sets or indices, as many species identified in this study did not appear in the DSIAR or commonly used indices of the Northern Hemisphere (DSIAR 50 %, PTI 32 % and TDI 26 %) (Kelly & Whitton, 1995; Muscio, 2002; Chessman et al., 2007; Kelly & Yallop, 2012). Only 14 % of diatom species in this study were included in the ‘reliable diatom taxa list’ developed by Besse-Lototskaya et al. (2011), which evaluated the European diatom trophic indices. This seems to be a common occurrence. Salomoni et al. (2011) found that the Water Quality Biological Index (WQBI; Lobo et al., 2004) developed for rivers in the southern Brazilian Region, was not adequate and subsequently developed a Gravatai WQBI to reflect local environmental characteristics. Research also suggests that the performance of some indices is proportional to the number of species included in the index applied, with lower proportions resulting in lower performance of the index (De la Rey et al., 2004; Newall et al., 2006; Besse-Lototskaya et al., 2011; Tan et al., 2013). Based on the results of the Coopers Creek study, it may be feasible to construct a diatom index suitable for sub-tropical rivers in Australia which would include a greater number of species, increasing the effectiveness of the potential index for use by management agencies.
Conclusion
The use of diatoms as bio-indicators of water quality is well developed in the Northern Hemisphere; however, it is an underdeveloped science in Australia with few diatom river indices available, and all developed in temperate climate zones. Many of the species identified in this study did not appear in the DSIAR or other commonly used indices of the Northern Hemisphere. This study showed that diatoms have potential value as bio-indicators of sub-tropical river health with several species identified as indicators of water quality. It has also revealed the need for expansion of this project, spatially and temporally, to other sub-tropical catchments to build a full dataset for the region.
References
Almeida, S. F. P. & M. J. Feio, 2012. DIATMOD: diatom predictive model for quality assessment of Portuguese running waters. Hydrobiologia 695: 185–197.
Antón-Garrido, B., S. Romo & M. J. Villena, 2013. Diatom species composition and indices for determining the ecological status of coastal Mediterranean Spanish lakes. Paper presented at the Anales del Jardín Botánico de Madrid, pp 122–135.
ANZECC, 2000. Australian and New Zealand guidelines for fresh and marine water quality. The guidelines, Vol. 1. ANZECC, Canberra: 3.1-1–3.5-10.
APHA, 2012. Standard methods for the examination of water and wastewater, 22nd ed. APHA, Washington, DC: 1–180.
Aplin G, P. Beggs, G. Brierley, H. Cleugh, P. Curson, P. Mitchell et al., 1999. Water resource management: an inevitable global crisis? In: Global Environmental Crises: an Australian perspective, 2nd edn. Oxford University Press, Melbourne, pp 117–139.
Atazadeh, I., M. Sharifi & M. G. Kelly, 2007. Evaluation of the Trophic Diatom Index for assessing water quality in River Gharasou, western Iran. Hydrobiologia 589: 165–173.
Axelrod, J., 2011. Water crisis in the Murray–Darling Basin: Australia attempts to balance agricultural need with environmental reality. Sustain Dev Law Policy 12: 12–53.
Bahls, L. L., E. Weber & J. Jarvie, 1985. Ecology and distribution of major diatom ecotypes in the southern Fort Union coal region of Montana. US Geological Survey 1289, 22nd ed. United States Government Printing Office, Washington, DC: 1–151.
Battarbee, R. W., V. J. Jones, R. J. Flower, N. G. Cameron, H. Bennion, L. Carvalho, et al., 2001. 8. Diatoms. In Smol, J., H. Birks & W. Last (eds.), Tracking environmental change using lake sediments. Terrestrial, algal, and siliceous indicators, Vol. 3. Kluwer Academic Publishers, Dordrecht: 155–202.
Bellinger, B. J., C. Cocquyt & C. M. O’Reilly, 2006. Benthic diatoms as indicators of eutrophication in tropical streams. Hydrobiologia 573: 75–87.
Bere, T., & Tundisi, J. (2009). Weighted average regression and calibration of conductivity and pH of benthic diatom assemblages in streams influenced by urban pollution–São Carlos/SP, Brazil. Acta Limnologica Brasiliensia 21: 317–325.
Bere, T. & J. G. Tundisi, 2011. Applicability of borrowed diatom-based water quality assessment indices in streams around São Carlos-SP, Brazil. Hydrobiologia 673: 179–192.
Besse-Lototskaya, A., P. F. Verdonschot, M. Coste & B. Van de Vijver, 2011. Evaluation of European diatom trophic indices. Ecol Indic 11: 456–467.
Birks, H. H., H. Birks, R. Flower, S. Peglar & M. Ramdani, 2001. Recent ecosystem dynamics in nine North African lakes in the Cassarina Project. Aquat Ecol 35: 461–478.
Blinn, D. W. & P. C. E. Bailey, 2001. Land-use influence on stream water quality and diatom communities in Victoria, Australia: a response to secondary salinization. Hydrobiologia 466: 231–244.
Blinn, D., S. Halse, A. Pinder & R. Shiel, 2004. Diatom and micro-invertebrate communities and environmental determinants in the western Australian wheatbelt: a response to salinization. Hydrobiologia 528(1–3): 229–248.
Bohm, J. S., M. Schuch, A. Düpont & E. A. Lobo, 2013. Response of epilithic diatom communities to downstream nutrient increases in Castelhano Stream, Venâncio Aires City, RS, Brazil. J Environ Prot 2013(4): 20–26.
Bunn, S. E., P. M. Davies & T. D. Mosisch, 1999. Ecosystem measures of river health and their response to riparian and catchment degradation. Freshw Biol 41: 333–345.
Chessman, B. C., 1986. Diatom flora of an Australian river system: spatial patterns and environmental relationships. Freshw Biol 16: 805–819.
Chessman, B., I. Growns, J. Currey & N. Plunkett-Cole, 1999. Predicting diatom communities at the genus level for the rapid biological assessment of rivers. Freshw Biol 41: 317–331.
Chessman, B. C., N. Bate, P. A. Gell & P. Newall, 2007. A diatom species index for bioassessment of Australian rivers. Mar Freshw Res 58: 542–557.
Connell, J. H., 2002. Diversity in tropical rain forests and coral reefs. Found Trop For Biol Class Pap Comment 199: 259.
Cooper, S. R., 1995. Chesapeake Bay watershed historical land use: impact on water quality and diatom communities. Ecol Appl 5: 703–723.
Cooper S (2001) Estuarine paleoenvironmental reconstructions using diatoms. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and Earth Sciences. Cambridge University Press, Cambridge, pp 352–366.
Davies, P. E. & M. Nelson, 1994. Relationships between riparian buffer widths and the effects of logging on stream habitat, invertebrate community composition and fish abundance. Mar Freshw Res 45: 1289–1305.
De la Rey, P., J. Taylor, A. Laas, L. Van Rensburg & A. Vosloo, 2004. Determining the possible application value of diatoms as indicators of general water quality: a comparison with SASS 5. Water SA 30: 325–332.
Dela-Cruz, J., T. Pritchard, G. Gordon & P. Ajani, 2006. The use of periphytic diatoms as a means of assessing impacts of point source inorganic nutrient pollution in south-eastern Australia. Freshw Biol 51: 951–972.
Dodds W, Whiles M (2010) Freshwater ecology, concepts and environmental applications of limnology. Elsevier, Inc., San Diego, California, pp 1–553.
Dodson, S., 2005. Introduction to limnology. McGraw Hill, New York.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. Kawabata, D. J. Knowler, C. Leveque, et al., 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81: 163–182.
EC, 2000. Directive 2000/60/EC of the European Parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Off J Eur Communities L327: 1–72.
Elias, C. L., N. Vieira, M. J. Feio & S. F. P. Almeida, 2012. Can season interfere with diatom ecological quality assessment? Hydrobiologia 695: 223–232.
Eyre, B., 1997. Water quality changes in an episodically flushed sub-tropical Australian estuary: a 50 year perspective. Mar Chem 59: 177–187.
Foged, N., 1978. Diatoms in eastern Australia. Bibl Phycol Band 41: 1–243.
Gay J, Ferguson A (2012) Review of water quality in Rocky Mouth Creek, Final Report. In: Aquatic biogeochemical and ecological research. Richmond River County Council, p 46.
Gell, P. A., Sonneman, J. A., Reid, M., Illman, M. A., & Sincock, A. J. (1999). An illustrated key to common diatom genera from Southern Australia CRCFE and MDBC Identification Guide No. 26. (pp. 1–63). Cooperative Research Centre for Freshwater Ecology.
Gell, P., J. Tibby, J. Fluin, P. Leahy, M. Reid, K. Adamson, S. Bulpin, A. MacGregor, P. Wallbrink, G. Hancock & B. Walsh, 2005. Accessing limnological change and variability using fossil diatom assemblages, south-east Australia. River Res Appl 21(2–3): 257–269.
Gómez, N. & M. Licursi, 2001. The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquat Ecol 35: 173–181.
Grudzinska, I., Saarse, L., Vassiljev, J., & Heinsalu, A. (2014). Biostratigraphy, shoreline changes and origin of the Limnea Sea lagoons in northern Estonia: the case study of Lake Harku. Baltica 27: 15–24.
Hanson, G. C., P. M. Groffman & A. J. Gold, 1994. Denitrification in riparian wetlands receiving high and low groundwater nitrate inputs. J Environ Qual 23: 917–922.
Haynes, D., R. Skinner, J. Tibby, J. Cann & J. Fluin, 2011. Diatom and foraminifera relationships to water quality in The Coorong, South Australia, and the development of a diatom-based salinity transfer function. J Paleolimnol 46(4): 543–560.
Hermany, G., A. Schwarzbold, E. Lobo, M. Oliveira & R. Santa Cruz do Sul, 2006. Ecology of the epilithic diatom community in a low-order stream system of the Guaíba hydrographical region: subsidies to the environmental monitoring of southern Brazilian aquatic systems. Acta Limnol Bras 18(1): 9–27.
Herricks, E. E. & D. J. Schaeffer, 1985. Can we optimize biomonitoring? Environ Manag 9: 487–492.
Hodgson D, Vyverman W, Tyler P (1997) Diatoms of meromictic lakes adjacent to the Gordon River, and of the Gordon River estuary in south-west Tasmania. J. Cramer Publishing, Stuttgart, pp 1–173.
Jewitt, G., 2002. Can Integrated Water Resources Management sustain the provision of ecosystem goods and services? Phys Chem Earth A/B/C 27(11–22): 887–895.
Juggins, S., 2007. C2: software for ecological and palaeoecological data analysis and visualisation 709 (version 1.5). Newcastle University, Newcastle.
Kelly, M. & B. Whitton, 1995. The trophic diatom index: a new index for monitoring eutrophication in rivers. J Appl Phycol 7: 433–444.
Kelly, M. G. & B. A. Whitton, 1998. Biological monitoring of eutrophication in rivers. Hydrobiologia 384: 55–67.
Kelly, M. K. & M. Yallop, 2012. A streamlined taxonomy for the Trophic Diatom Index. Environment Agency, Bristol: 24.
Kelly, M. G., A. Cazaubon, E. Coring, A. Dell’Uomo, L. Ector, B. Goldsmith, et al., 1998. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10: 215–224.
Kelly, M. G., C. Adams, A. C. Graves, J. Jamieson, J. Krokowski, E. B. Lycett, et al., 2001. The trophic diatom index: a user’s manual. Revised edition. R&D Technical Report E2/TR2. Environment Agency, Bristol, p 135.
Kelly, M. G., H. Bennion, E. J. Cox, B. Goldsmith, J. Jamieson, S. Juggins, et al., 2005. Common freshwater diatoms of Britain and Ireland: an interactive key. Environment Agency. http://craticula.ncl.ac.uk/EADiatomKey/html/taxon13160100.html. Accessed 11 Sept 2014.
Kelly, M., L. King, B. Ní Chatháin, 2009a. The conceptual basis of ecological-status assessments using diatoms. In: Paper presented at the biology and environment: proceedings of the Royal Irish Academy, pp 175–189.
Kelly, M., H. Bennion, A. Burgess, J. Ellis, S. Juggins, R. Guthrie, et al., 2009b. Uncertainty in ecological status assessments of lakes and rivers using diatoms. Hydrobiologia 633: 5–15.
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae 1. Teil: Naviculaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds.), Süßwasserflora von Mitteleuropa. Gustav Fischer, Stuttgart: 876.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae 2. Teil.: Bacillariaceae, Epithemiaceae, Surirellaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds.), Süßwasserflora von Mitteleuropa. Gustav Fischer, Stuttgart: 596.
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds.), Die Süßwasserflora von Mitteleuropa. Gustav Fischer, Stuttgart: 576.
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gesamtliteraturverzeichnis Teil 1-4. In Ettl, H., G. Gärtner, J. Gerloff, H. Heynig & D. Mollenhauer (eds.), Die Süßwasserflora von Mitteleuropa. Gustav Fischer, Stuttgart: 437.
Lake, P., 1995. Of floods and droughts: river and stream ecosystems of Australia. In Cushing, C. E., K. W. Cummins & G. W. Minshall (eds.), River and stream ecosystems, ecosystems of the world. Elsevier, Amsterdam: 659–694.
Li, L., B. Zheng & L. Liu, 2010. Biomonitoring and bioindicators used for river ecosystems: definitions, approaches and trends. Procedia Environ Sci 2: 1510–1524.
Lobo, E., V. Callegaro, G. Hermany, D. Bes, C. Wetzel & M. Oliveira, 2004. Use of epilithic diatoms as bioindicators from lotic systems in southern Brazil, with special emphasis on eutrophication. Acta Limnol Bras 16(1): 25–40.
Lobo, E. A., C. E. Wetzel, L. Ector, K. Katoh, S. Blanco & S. Mayama, 2010. Response of epilithic diatom communities to environmental gradients in subtropical temperate Brazilian rivers. Limnetica 29(2): 323–340.
Logan, B. & K. H. Taffs, 2013. Relationship between diatoms and water quality (TN, TP) in sub-tropical east Australian estuaries. J Paleolimnol 50(1): 123–137.
Logan, B., K. H. Taffs, B. D. Eyre & A. Zawadski, 2010. Assessing changes in nutrient status in the Richmond River estuary, Australia, using paleolimnological methods. J Paleolimnol 46(4): 597–611.
Millennium Ecosystem Assessment, 2005. Ecosystems and human well-being: wetlands and water synthesis. World Resources Institute, Washington, DC: 1–68.
Morand DT (1994) Soil landscapes of the Lismore–Ballina 1: 100 000 sheet: Mullumbimby, Byron Bay, Casino, Kyogle. Dept. of Conservation and Land Management, Soil Conservation Service, pp 1–234.
Mosisch, T. D., S. E. Bunn & P. M. Davies, 2001. The relative importance of shading and nutrients on algal production in subtropical streams. Freshw Biol 46(9): 1269–1278.
Muscio, C. 2002. The diatom pollution tolerance index: assigning tolerance values. City of Austin-Watershed Protection and Development Review Department, pp 1–17.
Newall, P. & C. J. Walsh, 2005. Response of epilithic diatom assemblages to urbanization influences. Hydrobiologia 532(1–3): 53–67.
Newall, P., N. Bate & L. Metzeling, 2006. A comparison of diatom and macroinvertebrate classification of sites in the Kiewa River system, Australia. Hydrobiologia 572: 131–149.
Norris, R. H. & K. R. Norris, 1995. The need for biological assessment of water quality: Australian perspective. Aust J Ecol 20: 1–6.
O’Driscoll, C., de Eyto, E., Rodgers, M., O’Connor, M., Asam, Z.-u.-Z., Kelly, M., & Xiao, L. (2014). Spatial and seasonal variation of peatland-fed riverine macroinvertebrate and benthic diatom assemblages and implications for assessment: a case study from Ireland. Hydrobiologia 728: 67–87.
Parr, J. F., K. H. Taffs & C. M. Lane, 2004. A microwave digestion technique for the extraction of fossil diatoms from coastal lake and swamp sediments. J Paleolimnol 31(3): 383–390.
Perna, C. & D. Burrows, 2005. Improved dissolved oxygen status following removal of exotic weed mats in important fish habitat lagoons of the tropical Burdekin River floodplain, Australia. Mar Pollut Bull 51: 138–148.
Peters, N. E, G. R. Buell, E. A. Frick, 1997. Spatial and temporal variability in nutrient concentrations in surface waters of the Chattahoochee River Basin near Atlanta, Georgia. In: Hatcher KJ (ed) Proceedings of the 1997 Georgia water resources conference, Athens, Georgia, 20–22 March 1997, pp 103–112.
Philibert, A., P. Gell, P. Newall, B. Chessman & N. Bate, 2006. Development of diatom-based tools for assessing stream water quality in south-eastern Australia: assessment of environmental transfer functions. Hydrobiologia 572: 103–114.
Potapova, M. G., D. F. Charles, K. C. Ponader & D. M. Winter, 2004. Quantifying species indicator values for trophic diatom indices: a comparison of approaches. Hydrobiologia 517: 25–41.
Preston, B. J., 2009. Water and ecologically sustainable development in the courts. Macquarie J Int Comp Environ Law 6: 129.
R Development Core Team, 2006. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
Reid, M. A., J. C. Tibby, D. Penny & P. A. Gell, 1995. The use of diatoms to assess past and present water quality. Aust J Ecol 20: 57–64.
Resh, V. H., 2008. Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ Monit Assess 138: 131–138.
Resh, V. H. & J. K. Jackson, 1993. Rapid assessment approaches to biomonitoring using benthic macroinvertebrates. In Rosenberg, D. M. & V. H. Resh (eds.), Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall, New York: 195–233.
Rimet, F. & A. Bouchez, 2012. Biomonitoring river diatoms: implications of taxonomic resolution. Ecol Indic 15: 92–99.
Rous Water (2009a) Wilsons River CMP state of the catchment draft. Rous Water, Lismore, p 115.
Rous Water (2009b) Wilsons River catchment management plan. Rous Water, Lismore, p 22.
Salomoni, S. E., O. Rocha, V. L. Callegaro & E. A. Lobo, 2006. Epilithic diatoms as indicators of water quality in the Gravataí River, Rio Grande do Sul, Brazil. Hydrobiologia 559(1): 233–246.
Salomoni, S. E., O. Rocha, G. Hermany & E. A. Lobo, 2011. Application of water quality biological indices using diatoms as bioindicators in the Gravataí River, RS, Brazil/Aplicação de índices biológicos da qualidade água utilizando diatomáceas como bioindicadoras no rio Gravataí, RS, Brazil. Braz J Biol 71(4): 949–959.
Singh, K. P., A. Malik & S. Sinha, 2005. Water quality assessment and apportionment of pollution sources of Gomti River (India) using multivariate statistical techniques—a case study. Anal Chim Acta 538: 355–374.
Singh, I., N. Flavel & M. Bari, 2009. Coopers Creek Water Sharing Plan. Socio-economic impact assessment of changes to the flow rules. NSW Department of Water and Energy, Sydney: 1–36.
Smith, V. H., G. D. Tilman & J. C. Nekola, 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut 100: 179–196.
Smol, J. P., 2008. Pollution of lakes and rivers: a paleoenvironmental perspective, 2nd ed. Blackwell Publishing, Malden.
Smol, J. P. & E. F. Stoermer, 2010. The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge.
Sonneman, J. A., A. J. Sincock, J. Fluin, M. A. Reid, P. Newall, J. C. Tibby, et al., 2000. An illustrated guide to common stream diatom species from temperate Australia. The Cooperative Research Centre for Freshwater Ecology Identification guide No. 33, pp 1–166.
Sonneman, J. A., C. J. Walsh, P. F. Breen & A. K. Sharpe, 2001. Effects of urbanization on streams of the Melbourne region, Victoria, Australia. II. Benthic diatom communities. Freshw Biol 46: 553–565.
Stenger-Kovács, C., L. Tóth, F. Tóth, É. Hajnal & J. Padisák, 2014. Stream order-dependent diversity metrics of epilithic diatom assemblages. Hydrobiologia 721(1): 67–75.
Taffs, K. H., L. J. Farago, H. Heijnis & G. Jacobsen, 2008. A diatom-based Holocene record of human impact from a coastal environment: Tuckean Swamp, eastern Australia. J Paleolimnol 39(1): 71–82.
Tan, X., F. Sheldon, S. E. Bunn & Q. Zhang, 2013. Using diatom indices for water quality assessment in a subtropical river, China. Environ Sci Pollut Res 20: 4164–4175.
Ter Braak, C. J. F., 1995. Ordination. In Jongman, R. H., C. J. Ter Braak & O. F. Van Tongeren (eds.), Data analysis in community and landscape ecology. Cambridge University Press, Cambridge: 91–173.
Thompson, R. M. & P. S. Lake, 2010. Reconciling theory and practise: the role of stream ecology. River Res Appl 26: 5–14.
Tibby, J. & K. H. Taffs, 2011. Palaeolimnology in eastern and southern Australian estuaries. J Paleolimnol 46(4): 503–510.
Townsend, S. A. & P. A. Gell, 2005. The role of substrate type on benthic diatom assemblages in the Daly and Roper Rivers of the Australian wet/dry tropics. Hydrobiologia 548(1): 101–115.
Verhoeven, J. T. A. & T. L. Setter, 2010. Agricultural use of wetlands: opportunities and limitations. Ann Bot 105: 155–163.
Vyverman, W., 1995. Diatoms from Tasmanian mountain lakes: a reference data set (TASDIAT) for environmental reconstruction and a systematic and autecological study. Bibl Diatomol Band 33: 1–192.
Weilhoefer, C. L. & Y. Pan, 2007. Relationships between diatoms and environmental variables in wetlands in the Willamette Valley, Oregon, USA. Wetlands 27: 668–682.
Wetzel, R. G., 2001. Limnology, lake and river ecosystems, 3rd ed. Academic Press, San Diego: 850.
Wu, J.-T. & L.-T. Kow, 2002. Applicability of a generic index for diatom assemblages to monitor pollution in the tropical River Tsanwun, Taiwan. J Appl Phycol 14: 63–69.
Yu, S.-Y., Berglund, B. E., Andrén, E., & Sandgren, P. (2004). Mid-Holocene Baltic Sea transgression along the coast of Blekinge, SE Sweden–ancient lagoons correlated with beach ridges. GFF 126: 257–272.
Acknowledgments
This Project was supported by an Australian Research Council Linkage Grant (LP130100498). We would like to thank Southern Cross University for their support with field and laboratory expenses. We thank Joanne Austin, Murphy Birnberg, Sarah Hembrow, Louise O’Neil, Daniela Stott and Matt Veness for their assistance with field work and Nev Minch for the preparation of figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisák
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Oeding, S., Taffs, K.H. Are diatoms a reliable and valuable bio-indicator to assess sub-tropical river ecosystem health?. Hydrobiologia 758, 151–169 (2015). https://doi.org/10.1007/s10750-015-2287-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2287-0