Abstract
Biomonitoring of least disturbed areas is a crucial issue to accurately assess the ecological status of surface waters and ensure their sustainability. Diatom metrics are important tools for the assessment of environmental conditions of lotic ecosystems to achieve environmental sustainability. The present study was aimed to evaluate the ecological status of least disturbed areas in the Antalya River basin (Turkey) using diatom indices developed from different ecoregion. Diatom species such as Cymbella excisa, Achnanthidium minutissimum, Cocconeis euglypta, and Cocconeis placentula were the most contributing species to the dissimilarity of sampling stations between rainy and dry seasons. The first two axes of canonical correspondence analysis (CCA) elucidated a strong (86%) correlation between diatom taxa and stressors. Results of the Monte Carlo permutation test indicated that total phosphorus (TP), water temperature, Tin (Sn), total organic carbon, zinc, and pH are significantly influential ecological factors on the distribution of diatom taxa among sampling stations. The Spearman correlation rank test indicated that diatom indices had significant positive and negative correlations with TP gradient. Results revealed that diatom indices developed from different ecoregions have different scores for the eco-assessment of similar sampling stations. European diatom indices mostly showed similar behavior in the bioassessment of the ecological status of rivers in the Antalya region compared to the different ecoregion. Among European diatom indices, TIT was more competitive and could give better results in the bio-evaluation of rivers in the Antalya River basin. Using diatom indices developed from different ecoregions may lead to an erroneous assessment of water quality. Accordingly, ecoregional specific diatom metrics are needed to accurately determine the surface water quality.
Graphical abstract

Eco-assessment of least disturbed areas of the Antalya River basin: application of diatom indices from different ecoregions: Abuzer Çelekli, Ömer Lekesiz, Tolga Çetin
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities threaten the health of surface waters worldwide, which is one of the major environmental problems. Bio-assessing water quality of lotic ecosystems is getting more important to attain environmental sustainability since the application of the European WFD-Water Framework Directive (Hering et al. 2010; Birk et al. 2012; Kelly et al. 2014; Çelekli and Lekesiz 2020).
The information on the occurring taxa of various ecosystems is used in biological indices to assess the water quality of surface waters (e.g., Rott et al. 1999; Kelly et al. 2008; Çelekli et al. 2019). Diatom assemblages as robust bio-indicators of spatial and temporal changes in environmental conditions are used to decipher integrated environmental information due to their presence or absence in different ecosystems (e.g., Potapova et al. 2004; Della Bella et al. 2012; Delgado and Pardo 2015).
Biological assessment has been applied since the 1900s (Kolkwitz and Marson 1908). Reliable information about water quality is required to achieve realistic objectives based on biological quality tools to ensure the biotic integrity of ecosystems. Concerning these reasons, several diatom indices have been developed in different ecoregions for the evaluation of the ecological status of streams and rivers. Some of them are pollution sensitive index-IPS (Cemagref 1982), eutrophication and/or pollution index-diatom-EPI-D (Dell’Uomo 2004), trophic index-TI (Rott et al. 1999), diatom trophic index-TDI (Kelly et al. 2008), and trophic index Turkey-TIT (Çelekli et al. 2019).
Rivers can be grouped according to typological categories like altitude, catchment area, geology, hydro-morphological dynamic, etc., for which the reference conditions are defined as the least disturbed regions (i.e., human impacts are absent or minimal). These conditions are important for the bioassessment approach to establish classification systems and water class boundaries. Biological metrics should reflect both the least disturbed areas and different types of environmental (physical, chemical, and hydro-morphological) disturbances under pressures of human activities (Borja et al. 2011; Feio et al. 2014). Stressor gradient variation at each typology can change the biological composition and their abundance. Diatom metrics based on the entire diatom assemblages have different responses to different stressors under the monitoring and reference conditions at different stream typologies (Çelekli et al. 2019; Charles et al. 2021; Salinas-Camarillo et al. 2021).
Several rivers have various hydro-morphological and catchment properties, which are under pressure from natural and anthropogenic factors in the Antalya River system. Studies dealing with the bioassessment of least disturbed conditions in the Antalya River basin (Turkey) using diatom indices have not been found in literature. Hence, the eco-assessment of rivers in the Antalya River basin using different diatom indices and multivariate approaches is the first attempt taking into account the WFD. Considering that the purposes of this study were to test differences (i) in the diatom-stressors interactions in the least disturbed areas with different typologies using multivariate approaches, (ii) in ecological optima of diatom assemblages in different water body types using weighted average regression model, and (iii) in the assessment of ecological status of least disturbed water bodies by the use of various diatom indices developed from different ecoregions. The suitability of diatom indices to assess the ecological integrity of these river systems was used to identify the monitored environmental changes in the Antalya River basin.
Materials and methods
The Antalya River basin
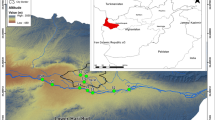
The Antalya River basin is located between the Taurus Mountains and the Mediterranean Sea in southwest Turkey (Fig. 1). Antalya River basin is represented by the Aksu, Manavgat, and Köprüçay sub–basins from the Late Cenozoic, is located within the Isparta Angle, situated between the Mid Miocene Aegean and Lycian arcs and the Late Eocene Taurus arc within the Alpine chain (Çiner et al. 2008). It is one of the most important catchments of Anatolia due to water potential and socio-economic activities. This basin consists of different rivers (Dim, Kargı, İnasar, Boyalı, Göynük, Sapadere, Köprüçay, and Yazılı) that discharge their waters to the Mediterranean Sea.
Location of sampling stations in the Antalya River basin. Sampling stations are symbolized by R1-R36 and their full names are given in Table 1
Two different climatic types are found in the Antalya River basin; a Mediterranean climate with dry summers and mild, moist winters is observed in the south part of the basin and a Central Anatolia continental climate with hot, dry summers and cold, snowy winters occurs in the north part of the basin. The highest region (2835 m asl.) of the basin is located in the northern mountains that have low temperatures, intense precipitation, and snow, whereas the south plain areas are generally warmer with intense rainfall and evaporation. This basin is characterized by high spatiotemporal variability of precipitation and the dominant vegetation as maquis and pines.
Sampling
Geographical variables (altitude, latitude, and longitude) of sampling stations were determined with a geographical positioning system (Garmin Vista HCx model GPS). Samples of water and diatoms were collected from 36 sampling stations (named from R1 to R36) of 31 streams (Fig. 1) in the spring, summer, and fall seasons of 2017. The typological criteria like altitude, geology, slope, and precipitation according to the implementation of the EU Water Framework Directive (Directive 2000) were used to group the lotic ecosystems. Hydro-geographical features of rivers are given in Table 1. The flow regime (F) in the rivers is evaluated according to the intermittent (F1) and permanent (F2). A-altitude (A1 < 800 m, A2 800–1600 m, and A3 > 1600 m), S-slope (S1 < 2% and S2 > 2%), G-geology (G1 high and G2 low mineralization), P-precipitation (P1 < 400 mm and P2 ≥ 400 mm), and D-drainage area (D1-wet and D2-dry regions) as typological criteria were also used for the classification of streams. The least disturbed areas were determined according to land use maps. The information was obtained from the Ministry of Agriculture and Forestry, General Directorate of Water Management.
Water temperature (oC), pH, electrical conductivity (EC μS/cm), salinity (ppt), and dissolved oxygen concentration (DO, mg/L) in situ were measured by a YSI professional plus oxygen–temperature meter. Water samples were maintained in coolers with ice packs for chemical analysis during the transfer to the laboratory. For sampling, epilithic diatoms, at least five sun-merged stones in the riffle parts were randomly handled, and the upper surface of stones was scraped with a toothbrush in 100 mL of distilled water as a composite sample per site (European Committee for Standardization 2014). Diatoms were fixed by adding a Lugol solution with glycerol.
Laboratory analyses
A few nutrients (total phosphorus (TP), total nitrogen (TN), nitrite-nitrogen (N-NO2), and nitrate-nitrogen (N-NO3)) from water samples were analyzed and biochemical oxygen demand (BOD5) was measured following standard methods (APHA 2012). Heavy metals (e.g., copper, iron, nickel, aluminum, cobalt, lead, arsenic) in the water samples were determined by using an inductively coupled plasma-optical emission spectrometry (Perkin Elmer, Optima 2100 DV).
Epilithic diatom samples were treated with hot acids (H2SO4:HNO3 as 2:1) and mounted with Naphrax to prepare permanent slides according to the standard method of the European Committee for Standardization (2014). More than 500 valves were counted for each diatom slide under a light microscope (Olympus BX53 model attached a DP73) at 1000× magnification. Several taxonomic books (Krammer 2000, 2002; Lange-Bertalot 2001; Bey and Ector 2013; Lange-Bertalot et al. 2017) were used to identify diatom species.
Bioassessment of sampling stations
Diatom indices developed from different regions such as Eutrophication and/or Pollution Index-Diatom (EPI-D) in Italy (Dell’Uomo 2004), Trophic Index (TI) in Austria (Rott et al. 1999), Pollution Sensitivity Index (IPS) in France (Cemagref 1982), Trophic Diatom Index (TDI) in England (Kelly et al. 2008), Duero Diatom Index (DDI) in Spain (Álvarez-Blanco et al. 2013), Trophic Diatom Index for Lakes (TDIL) in Hungary (Stenger-Kovács et al. 2007), Trophic Water Quality Index (TWOI) in Brazil (Lobo et al. 2015), Diatom Ecological Quality Index (DEQI) in Mexico (Salinas-Camarillo et al. 2021), Diatom Species Index Australian Rivers (DSIAR) in Australia (Chessman et al. 2007), Richmond River Diatom Index (RRDI) in Australia (Oeding and Taffs 2017), and Trophic index Turkey (TIT) in Turkey (Çelekli et al. 2019) were used to determine the environmental conditions of rivers in the Antalya River basin (Turkey). A few diatom indices like IPS, DSIAR, and DDI have inverse correlations to environmental pollution whereas others have direct relationships.
Statistical analyses
Means and standard deviations of environmental variables of sampling stations for three seasons were determined, using the descriptive analysis (SPSS version 15.0, USA). Duncan’s multiple range test was performed to determine whether there were differences in physico-chemical data among sampling stations. The percentile analysis was performed to calculate the 25th, 50th, and 75th percentiles of data. Diatom metrics-environment and environment-environment interactions were tested with the Spearman correlation test. Sampling stations were grouped as spring, summer, and fall, and then nonparametric multivariate methods (ANOSIM-one-way similarity analysis and SIMPER-the Analysis of Similarity Percentage) were used to compare sampling stations based on their diatom composition. The ANOSIM test was used to determine whether there was a significant difference in the diatom compositions of sampling stations among sampling seasons. Bray Curtis (similarity coefficient) was used to test the null hypothesis (H0) which states that there is no difference in the diatom composition of sampling stations among the sampling seasons. SIMPER analysis was used to determine which diatom species contribute to the dis/similarity among stations within each season. In the SIMPER analysis, species with at least 1% contribution to dis/similarity between groups were considered. Community Analysis Package version 4.1.3 software (Seaby and Henderson 2007) was used for the analyses of ANOSIM and SIMPER. Results of detrended correspondence analysis performed on the diatom species list indicated that the gradient lengths of the first two axes are greater than 3.0. Thus, a CCA-canonical correspondence analysis (ter Braak and Šmilauer 2002) using CANOCO 4.5 software was suggested to elucidate relationships between 8 environmental variables and 122 diatom species in the Antalya River basin. To reduce skewness and to obtain a normal distribution except pH (ter Braak and Šmilauer 2002), environmental data were transformed (ln(x+1)). The Monte Carlo permutation test was performed to determine which explanatory factors significantly affect the distribution of diatom assemblages. The weighted averaging regression model (Juggins and ter Braak 1992) was carried out to determine the optima of diatom species for environmental stressors.
Results
Physical and chemical variables
Physicochemical variables changed among the sampling stations in the Antalya River basin and the descriptive results are summarized in Table 2. The lowest mean water temperature value (10.0 °C) was recorded in Kuz River (R32) located at 1047 m asl., while Karpuz River (R10) had the highest value (26.6 °C) located at 29 m asl. The Antalya River system had slightly alkaline waters. The highest mean pH value (8.84) was recorded in station R12 (Akçay River), and Köprüçay River (R20) showed the lowest pH level (7.57). Relatively low values of water electrical conductivity (EC) were measured in Kuru River (R6), Köprüçay River (R30), and Utuce river (R16) as the following 265, 267, and 276 μS/cm, respectively. The highest mean EC value (760 μS/cm) was recorded in Cevizler River (R11). Salinity showed similar spatio-temporal patterns to EC gradient in surveyed these rivers (for more see Table 2).
Sampling stations in the Antalya River system had mostly low nutrient gradient especially total phosphorous (TP). The highest mean TP was found in Sapadere River (R2) with 15.0 μg/L, whereas the lowest value (4.3 μg/L) was determined in Utuce river (R16). Station R16 had also the lowest nitrate (43 μg/L N-NO3) and low CaCO3 (106 mg/L) values. Karagöz River (R15) consisted of the highest mean CaCO3 with 291 mg/L. Mainly low heavy metal concentrations were found in the sampling stations of the Antalya River system during the study time. Besides, results of other metals such as aluminum, cobalt, lead, arsenic, titanium, and vanadium are not given because their values are very low or sometimes not detected.
Diatom assemblages-environment relationships
A total of 201 diatom taxa were identified in the Antalya River basin. Of the diatom species, 122 (Supplementary 1) occurred more than once and had a relative abundance above 1%, and were used in the multivariate statistical analyses. During the study, Cymbella excisa, Achnanthidium minutissimum, Cocconeis placentula var. euglypta, Cymbella excisiformis, Diatoma moniliformis, Encyonopsis minuta, Navicula cryptotenella, Navicula tripunctata, and Nitzschia dissipata were frequently found.
ANOSIM indicated that the difference between the spring and fall groups is significant as well as between spring and summer groups. On the other hand, there was no significant difference in the diatom composition of sampling stations between the fall and the summer seasons.
According to SIMPER, a 14.94% within-group similarity was found for the spring season. Contributions of Cymbella excisa, Fragilaria capucina, and Cocconeis placentula to this similarity are 43.6%, 11.46%, and 9.74%, respectively. The in-group similarity of the fall season is 14.35%. Cocconeis euglypta, Achnanthidium minutissimum, and C. placentula were the most contributing species to within-group similarity of fall season showing 41.25%, 15.64%, and 7.57%, respectively. The within-summer similarity rate was calculated as 10.98%. C. euglypta, C. placentula, and Cocconeis lineata were the most contributing species to the within-group similarity of summer season with a percentage rate of 38.96%, 15.69%, and 6%, respectively.
In terms of SIMPER dissimilarity rate between the spring and fall was 94%. The most contributing species to this difference are Cymbella excisa (7.98%), Cocconeis euglypta (7.95%), Achnanthidium minutissimum (5.52%), and Cocconeis placentula (5.44%). The dissimilarity rate between spring and summer was 92%. Cocconeis euglypta (9.76%), C. excisa (8.11%), C. placentula (5.47%), and Cocconeis lineata (4.14%) are the most contributing species to this dissimilarity. The dissimilarity rate between the fall and summer was calculated as 87.34%.
A strong correlation (0.860) between diatom assemblages and environmental variables was indicated by the first two axes of CCA. Total phosphorus (TP), Tin (Sn), pH, total organic carbon (TOC), water temperature, and zinc (Zn) played significant roles on the distribution of diatom taxa among sampling stations in the basin (Fig. 2). In the CCA ordination, relatively pollution-tolerant species (e.g., C. lineata, Cocconeis euglyptoides, Diatoma vulgaris, Nitzschia palea, Nitzschia paleacea, Navicula associate, Navicula cryptotenella, Navicula cryptocephala, and Reimeria sinuata) showed close relationships with TP on the positive side associating with R2, R17, R9, and R26. Pollution-sensitive diatom species (e.g., C. excisa, A. minutissimum, Diatoma ehrenbergii, Diatoma tenuis, Encyonema silesiacum, Encyonopsis minuta, and Hannaea arcus) were located on the opposite region of the pollution-tolerant species. Pollution-sensitive diatom species were associated with R4, R11, R15, R19, R23, and R33 sampling stations. Results of the weighted average (WA) regression approved the preferences of diatom assemblages in the CCA ordination (Fig. 2). Above-mentioned pollution-tolerant species had TP optima above 75% of TP gradient (7.5 μg/L), whereas, pollution-sensitive species preferred TP optima in the range of 4.5–5.5 μg/L in the Antalya River basin. Some diatoms, Navicula lanceolata, Navicula cari, Navicula trivialis, Amphora ovalis, and Fragilaria crotonensis, displayed association with TOC. This association is supported by the results of WA regression. These diatom species preferred TOC values above 75% (11.26 mg/L) of this variable in the Antalya river system.
Ecological status of rivers
The bioassessment of sampling stations using various diatom indices is given in Table 3. Diatom indices developed from different ecoregions showed different scores and ecological status from a high to a bad condition in the Antalya river systems. The EPI-D had the highest percentage of high ecological status (61%), followed by TIT (47%), TI (39%), and IPS (36%), whereas it was not found by the assessment of TDIL, TWQI, DDI, TDI, RRDI, and DSIAR (Fig. 3). DDI developed in Spain cannot differentiate the ecological status of the stations but it presented a good ecological status for all stations. Similarly, TDIL, TWQI, RRDI, and DSIAR made a small distinction in the environmental conditions of the sampling stations.
Results of the Spearman correlation analysis (Table 4) indicated that TP gradient displays significantly positive correlations with TIT (p < 0.01, r = 0.802), TI (p < 0.01, r = 0.601), EPI-D (p < 0.01, r = 0.526), TDI (p < 0.01, r = 0.537), DEQI (p < 0.01, r = 0.589), RRDI (p < 0.01 r = 0.663), TDIL (p < 0.01, r = 0.482), and TWQI (p < 0.05, r = 0.284), whereas negatively significant correlations with IPS (p < 0.01, r = − 0.614), DSIAR (p < 0.01, r = − 0.376), and DDI (p < 0.01, r = − 0.366).
Deterioration of R2 station was represented by several diatom indices, which indicated a poor ecological status based on TIT, TI, and DSIAR; TWQI, TDI, and RRDI indicated a bad ecological status. However, results of EPI-D, TDIL, DEQI, and DDI indicated that R2 had a good environmental condition. European diatom indices, TIT, TI, EPI-D, and IPS mainly showed similar behavior to differentiate the environmental conditions of the sampling stations in the Antalya River basin (Fig. 3 and Table 3). Results of these indices mostly indicated that the least disturbed areas are R1, R4, R15, R16, R21-24, R27, R32, R33, and R36 with high ecological status.
Discussion
Human population demand water resources for various purposes such as drinking water, irrigation, fishing, power production, and recreation activities according to the water quality of the rivers, which lead to the alternation of these ecosystems. TP and TN gradients in the some of sampling stations (e.g., R1, R4, R15, R16, R21-24, R27, R32, R33, R36) in the present are lower than reference values of Portuguese territory (Feio et al. 2012) and are similar to the least disturbed condition in European Mediterranean rivers (Feio et al. 2014). Results of the EC indicated that R3-6, R16-17, R19, R22, R24-25, R28-31, and R35 have the first-class water quality, while others are the second-class water quality. Conductivity of freshwaters may vary according to the geological background as well. The EC is related to the total content of the ions in aquatic ecosystems and may be used to determine the extent and nature of modification to water geochemistry due to anthropogenic activities and so it is accepted as a good monitoring variable (Das et al. 2006; Çelekli et al. 2019). The enhancement of sulfates and other ions concentrations can cause an increase in EC level to indicate the deterioration of water bodies. Deterioration of watercourses is not only based on these factors because there are hundreds of chemical variables like nutrients, pesticides, salinity, turbidity, and smell (Merga et al. 2020). Measuring all of them is expensive and impractical. Besides, the water chemistry can give restricting knowledge about water conditions because its sampling time indicates instant information of the water quality and ignores temporal changes in aquatic ecosystems.
The findings of the present study revealed that the physicochemical environment in rivers (Fig. 2) regulates the biological condition of diatom assemblages, in agreement with previous studies (Feio et al. 2020; Freitas et al. 2021). Diatom compositions in sampling stations significantly changed among sampling seasons according to findings of ANOSIM. The difference between spring and fall is higher than between other seasons, but no significant difference between summer and fall was found. This could be due to the effects of rainy and dry seasons. Diatom species such as C. excisa, A. minutissimum, C. euglypta, and C. placentula are the most contributing species to the dissimilarity between rainy and dry seasons. Cymbella excisa and F. capucina had high contribution in the rainy season, while the contributions of A. minutissimum, C. euglypta, and Cocconeis lineata were high in the dry season. Besides, the SIMPER findings indicated that the mentioned species were also the most contributing species to sampling stations within-season similarity. It is understood that a few diatom species play a significant role in the differentiation between and within groups.
Diatom composition and abundance in sampling stations had great contribution to determine the water quality. Sampling stations R2, R9, R17, and R26 were characterized by moderately pollution-tolerant species associated with TP gradient (Fig. 2) such as N. palea, N. paleacea, C. lineata, C. euglyptoides, N. associate, N. cryptocephala, and R. sinuata. This association is also confirmed by the WA findings. Mentioned species had TP optima higher than 75th percentile of TP gradient in the Antalya basin. Nitzschia palea as a pollution-tolerant species has a wide tolerance to a broad range of nutrient values from low in the least disturbed areas to high in the wastewater (Çelekli and Şahin 2021), but high nutrient values favor it (Winter and Duthie 2000). Nitzschia palea is categorized as a high TP indicator by databases of diatom indices (e.g., Rott et al. 1999; Dell’Uomo 2004; Lobo et al. 2015; Çelekli et al. 2019), but its exact minimum and optimum values were not achieved by diatom datasets. This species as a pollution-tolerant taxon has been found in pristine and more degraded conditions due to its tolerance to a wide range of nutrient gradients (Szczepocka and Szulc 2009; Çelekli et al. 2019). Besides, Holmes and Taylor (2015) reported that N. paleacea has high tolerance to heavy metal levels and occurs in nutrient-enriched waters with a moderate to high EC. This diatom preferred a slightly alkaline condition (pH = 8.24) in the Antalya basin and it was also found in similar conditions in the Great Fish River, South Africa (Holmes and Taylor 2015).
Pollution-sensitive species such as C. excisa, A. minutissimum, D. ehrenbergii, D. tenuis, E. silesiacum, E. minuta, and H. arcus were associated to R4, R11, R15, R16, R19, R23, and R33. Results of the WA regression approved the preferences of these diatom assemblages in the ordination (Fig. 2). Among pollution-sensitive species, C. excisa and A. minutissimum had great contribution not only to the dissimilarity between rainy and dry seasons but also to sampling stations within-season similarity. Mentioned pollution-sensitive species preferred low TP optima (4.5–5.5 μg/L) in the Antalya River basin. Cymbella excisa was dominantly found in less polluted ecosystems (Gómez and Licursi 2001), and also it is known as pollution-sensitive species (Potapova et al. 2004; Delgado et al. 2012) and considered as a low trophic indicator species (Wang et al. 2014; Çelekli et al. 2019). TP optima value of A. minutissimum was smaller than the 25th percentile in the Antalya basin, in agreement with the findings in the west Mediterranean river basin (Çelekli and Lekesiz 2020). Achnanthidium minutissimum was also reported as abundant species in Mediterranean karst springs (Lai et al. 2020), Alpine springs (e.g., Falasco and Bona 2011; Cantonati et al. 2012), and in reference sites in southern Brazilian rivers (Freitas et al. 2021). This diatom species had negative correlations with nutrients and EC (p < 0.05) in the Hantangang River, Korea (Cho et al. 2020). Achnanthidium minutissimum is an indicator of low nutrient concentrations and had high relative abundance in the reference areas (Potapova and Charles 2007; Çelekli et al. 2019; Chen et al. 2020). Achnanthidium minutissimum shows common distribution in freshwater bodies worldwide, which makes it a global indicator organism for assessing water quality (Çelekli and Lekesiz 2020; Freitas et al. 2021). This diatom species was commonly found in the least disturbed sampling stations in the Antalya river system and it is also considered as a reference taxon (Delgado et al. 2010, 2012; Wang et al. 2014; Çelekli et al. 2019).
Pollution-sensitive taxa were not only abundantly found in the least disturbed areas in the Antalya River basin but also were dominantly found in less impacted ecosystems in different ecoregions (Delgado et al. 2012; Wang et al. 2014; Çelekli and Kapı 2019). Besides, low TP preferences of the aforementioned species in the present study reinforce the findings of previous limno-ecological studies (e.g., (Rimet et al. 2004; Gomà et al. 2005; Delgado et al. 2012; Wang et al. 2014; Lobo et al. 2016; Çelekli et al. 2019). Furthermore, most diatom indices indicated that these pollution-sensitive species have low trophic weight values (e.g., Çelekli et al. 2019; Kelly et al. 2008; Rott et al. 1999).
Assessing the ecological status of lotic ecosystems requires the development of integrated approaches that consider the complex relationships between bio-indicator assemblages and ecological factors. Since the application of the European WFD (EC 2009), the biological assessment of surface water quality has been a more important issue to accomplish environmental sustainability. Nowadays, the bioassessment of the ecological condition of surface waters is adopted worldwide (Charles et al. 2021). The results of bioassessment of the sampling stations indicated that diatom indices developed from different ecoregions have different scores resulting in different ecological statuses from a high to a bad condition in the Antalya river systems. The findings of the present indicated that significant differences are observed among ecological status percentages of diatom indices. In the ecological evaluation of the sampling areas, EPI-D has the highest percentage of high ecological status, while it was not seen in the assessment of TDIL, DDI, TDI, DSIAR, and RRDI. The TP gradient (4–15 μg/L) was the most influential environmental factor on the diatom species and their abundance. TP is a relevant chemical and is used to quantify the trophic weight of species in various diatom indices such as TI (Rott et al. 1999), TIT (Çelekli et al. 2019), and EPI-D (Dell’Uomo 2004). The relationship between diatom assemblages and TP gradient in the sampling stations directly affected scores of diatom indices. The present study also revealed significant positive correlations of TP gradient with TIT, TI, EPI-D, TDI, DEQI, RRDI, TDIL, and TWQI, and significantly negative correlations with IPS, DSIAR, and DDI.
European diatom indices like TI (Rott et al. 1999), TIT (Çelekli et al. 2019), EPI-D (Dell’Uomo 2004), and IPS (Cemagref 1982) mostly showed similar behavior in the bioassessment of ecological conditions of sampling stations in the Antalya River basin. The IPS (Cemagref 1982), TI (Rott et al. 1999), and EPI-D (Dell’Uomo 2004) are widely used diatom indices in European water quality assessment incorporating nutrients and hydromorphology (Lobo et al. 2016). Results of these indices mostly indicated that the least disturbed sites are R1, R4, R15, R16, R21-24, R27, R32, R33, and R36 with high ecological status. Distinct tolerant/sensitivity values of diatom species are different in the tested diatom indices database, which could affect the findings in the bioassessment water quality of rivers in the Antalya River basin. Ecological conditions of these sampling stations were not only supported by their low nutrients and low heavy metals values but also assisted by the least disturbed areas in terms of streambed consists of rocks, stones, and sands, which is surrounded by plane and pine trees. Ecological conditions of these sampling stations are similar to the least disturbed condition in European Mediterranean rivers (Feio et al. 2014) and reference values of Portuguese territory (Feio et al. 2012). Besides, human activities have minimal impact on the mentioned sampling stations. Although, DDI developed in Spain (Álvarez-Blanco et al. 2013) has a significant negative correlation with TP variation, it cannot differentiate the ecological status of sampling stations and presented a good ecological status for all stations in the present study. The trophic weight of diatom species in the Antalya river system varied from 8.1 to 9.7 for the DDI, which resulted in the index scores from 8.88 in R2 to 9.46 in R32 which attributes the classification of good ecological status. This could be a consequence of the high and good ecological quality class boundaries of the DDI being 10.00 and 8.50-9.99, respectively (Álvarez-Blanco et al. 2013), which strongly affect the bioassessment results. The TDI developed in the UK (Kelly et al. 2008) significantly correlated with TP gradient, classifying ecological status of sampling stations as 66.6% good, 30.6% moderate, and 2.8% bad environmental conditions. Another European diatom index is TDIL (Stenger-Kovács et al. 2007) developed for the lentic ecosystems have different dynamics from the lotic ecosystems, which could affect the bioassessment of the ecological status of rivers in the Antalya River basin.
Some indices (DDI, DSIAR, and TDIL) are not sensitive to the variations between sites, since they hardly change even though the environmental conditions change. Conversely, European indices such as TIT, TI, EPI-D, and IPS had significant correlations with environmental variables and they have mainly the ability to different environmental conditions of sampling station. Among them, TIT was more competitive based on the Spearman correlation coefficient and could give better results in the bio-evaluation of rivers in the Antalya River basin. The European indices are not adapted efficiently to the particular hydrological and climatic conditions of this Mediterranean region (e.g., Çelekli et al. 2018; Lazaridou et al. 2018; Çelekli and Bilgi 2019) due to the effects of ecoregion environmental factors on the trophic weight of diatom taxa. Besides, not all of the taxa found in the Antalya river system are included in the taxa list of TIT. Thus, it is necessary to revise it according to preferences of diatom taxa in the present and other studies.
DEQI from Mexico (Salinas-Camarillo et al. 2021), TWQI from Brazil (Lobo et al. 2015), and RRDI and DSIAR developed in Australia had significant correlations with TP gradient in the Antalya river system. Nevertheless, these indices except DEQI were not able to differentiate the ecological status of rivers in the Antalya River basin. Some important variations were seen in the biological evaluation of DEQI. For instance, Naras river sidearm (R13) had a bad ecological status based on the DEQI result, whereas a high environmental condition was found according to TI, TIT, and IPS. Among the species seen in station R13, DEQI only including Fragilaria crotonensis was used in the index calculation. This is limiting the ability to assess the ecological status due to a loss of information. The R13 ecosystem had relatively low nutrients and low heavy metals. Streambed of R13 consists of rocks, stones, and sands, which is surrounded by plane and pine trees. Besides, there is no urbanization, agricultural activity, and land use. Using diatom indices developed from different ecoregions may lead to erroneous evaluation of water quality. Consequently, ecoregional specific diatom indices are developed to accurately determine the ecological status of surface waters. Different ecoregion including geology, climate, topography, vegetation, wildlife, hydrology, and human activities (Omernik 1987; Çelekli and Kapı 2019; Espinosa et al. 2020) strongly affect environmental factors on the trophic weight and indicator values of diatom assemblages (Lobo et al. 2015; Çelekli et al. 2019; Salinas-Camarillo et al. 2021). Another factor is the class boundary of ecological status which varied from a diatom index to another. From that point, there is a crucial need to determine class boundaries of diatom indices for facilitating the evaluation of ecological quality of freshwater bodies. The performed diatom indices did not include all diatom taxa found in the Antalya river system. This seems a limiting factor to assess the water quality. Some indicator taxa may not be considered in the index calculation like the assessment of R13 by DEQI, which could lead to an erroneous assessment of water quality due to a loss of information. Besides, results of the bioassessment were changed in the Antalya River basin due to diatom indices consist of different trophic weight of species list, in agreement with previous studies in which comparing European and American diatom indices (Lavoie et al. 2009) and Anatolia (Çelekli and Kapı 2019; Çelekli and Lekesiz 2020). Concerning the number of taxa in diatom indices, IPS, TI, and DSIAR include more than those of other tested indices in the present study, which is important in bioassessment studies. Diatom indices have a define ecological quality class boundary; however, sampling stations have different river typologies like altitude, flow regime, geology, slope, and precipitation impact ecosystems. Ecological quality class boundaries should be determined especially according to altitude typological criteria (A1 < 800 m, A2 800–1600 m, and A3 > 1600 m). Altitude affects environmental conditions which regulate ecological preferences of diatom species. Considering those points, there is a need to develop class boundaries of diatom indices in the future according to further research with the understanding of biological responses to other environmental predictors from both monitoring and reference conditions.
Conclusion
Results of the present study underline that diatom taxa are ubiquitous indicators and present in a variety of environments, which may cause some variability in the comparison between their sensitivity values. Diatom species such as C. excisa, A. minutissimum, C. euglypta, and C. placentula are the most contributing species to the dissimilarity of sampling stations between rainy and dry seasons in the Antalya region. Results of the present study revealed that the physicochemical variation in rivers with different typologies regulates the biological conditions of diatom species. Diatom distribution was most influenced by TP, water temperature, TOC, Zn, Sn, and pH. Tested diatom indices had significant correlations with TP gradient. The results of bioassessment of the sampling stations exhibited that diatom indices have different scores resulting in ecological status from a high to a bad condition in the Antalya river systems. Results of diatom indices mostly indicated that the least disturbed areas are R1, R4, R15, R16, R21-24, R27, R32, R33, and R36 with high ecological status. The results of bioassessment are complementary to physico-chemical and hydromorphological evaluations of rivers. The results of the present study suggest that although the indices differ markedly in their approach and purpose, their use for assessing stream integrity in the Antalya River basin gave similar results based on European diatom indices except for TDI, DDI, and TDIL. It seems that TIT, TI, EPI-D, and IPS could be suitable diatom metrics for assessing the ecological status of sampling stations in this Mediterranean region. Among them, TIT was more competitive based on the Spearman correlation coefficient and could give better results in the bio-evaluation of rivers in the Antalya River basin. Using diatom indices developed from different ecoregion can lead to erroneous interpretation of water quality. Consequently, ecoregional specific diatom indices should be developed to accurately determine the ecological status of surface waters.
Data availability
Not applicable.
References
Álvarez-Blanco I, Blanco S, Cejudo-Figueiras C, Bécares E (2013) The Duero Diatom Index (DDI) for river water quality assessment in NW Spain: Design and validation. Environ Monit Assess 185(1):969–981
APHA (2012) American Public Health Association. American Water Works Association, Water Environment Federation, Standard Methods for the Examination of Water and Wastewater. 22nd Edition, p1496
Bey M, Ector L (2013) Atlas des diatomées des cours d’eau de la région Rhône-Alpes. Tome 1 Centriques, Monoraphidées. Tome 2 Araphidées, Brachyraphidées. Tome 3 Naviculacées: Naviculoidées. Tome 4 Naviculacées: Naviculoidées. Tome 5 Naviculacées: Cymbelloidées, Gomphonématoidées. Tome 6 Bacillariacées, Rhopalodiacées, Surirellacées, pp1–1182
Birk S, Bonne W, Borja A, Brucet S, Courrat A, Poikane S, Solimini A, van de Bund W, Zampoukas N, Hering D (2012) Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecol Indic 18:31–41
Borja A, Barbone E, Basset A, Borgersen G, Brkljacic M, Elliott M, Garmendia JM, Marques JC, Mazik K, Muxika I (2011) Response of single benthic metrics and multi-metric methods to anthropogenic pressure gradients, in five distinct European coastal and transitional ecosystems. Mar Pollut Bull 62(3):499–513
Cantonati M, Angeli N, Bertuzzi E, Spitale D, Lange-Bertalot H (2012) Diatoms in springs of the Alps: spring types, environmental determinants, and substratum. Freshw Sci 31(2):499–524
Çelekli A, Bilgi F (2019) Bioassessing ecological status of surface waters in the Araban-Yavuzeli catchment (Turkey): application of diatom indices. Turk J Bot 43:597–607
Çelekli A, Kapı E (2019) Ecoregion approach in the assessment of aquatic ecosystems in the west of Gaziantep (Turkey): application of diatom metrics. Ecol Indic 103:373–382
Çelekli A, Lekesiz Ö (2020) Eco-assessment of West Mediterranean basin’s rivers (Turkey) using diatom metrics and multivariate approaches. Environ Sci Pollut Res 27(22):27796–27806
Çelekli A, Şahin G (2021) Bio-assessment of wastewater effluent conditions with algal pollution index and multivariate approach. J Clean Prod 310
Çelekli A, Toudjani AA, Lekesiz HÖ, Çetin T (2018) Ecological quality assessment of running waters in the North Aegean catchment with diatom metrics and multivariate approach. Limnologica 73:20–27
Çelekli A, Toudjani AA, Gümüş EY, Kayhan S, Lekesiz HÖ, Çetin T (2019) Determination of trophic weight and indicator values of diatoms in Turkish running waters for water quality assessment. Turk J Bot 43(1):90–101
Cemagref (1982) Etude des méthodes biologiques d’ appréciation quantitative de la qualité des eaux. - Rapport Q. Ee. Lyon. - A. F. Bassin RhôneMéditerranée-Corse. pp 218
Charles DF, Kelly MG, Stevenson RJ, Poikane S, Theroux S, Zgrundo A, Cantonati M (2021) Benthic algae assessments in the EU and the US: Striving for consistency in the face of great ecological diversity. Ecol Indic 121:107082
Chen X, Zhou W, Luo G, Luo P, Chen Z (2020) Spatial and temporal variations of the diatom communities in megacity streams and its implications for biological monitoring. Environ Sci Pollut Res 27(30):37581–37591
Chessman BC, Bate N, Gell PA, Newall P (2007) A diatom species index for bioassessment of Australian rivers. Mar Freshw Res 58(6):542
Cho I-H, Kim H-K, Lee M-H, Kim Y-J, Lee H, Kim B-H (2020) The effect of monsoon rainfall patterns on epilithic diatom communities in the Hantangang River, Korea. Water 12(5):1471
Çiner A, Karabiyikoğlu M, Monod O (2008) Late Cenozoic sedimentary evolution of the Antalya Basin, southern Turkey. Turk J Earth Sci 17(1):1–41
Das T, Pal AK, Chakraborty SK, Manush SM, Dalvi RS, Sarma K, Mukherjee SC (2006) Thermal dependence of embryonic development and hatching rate in Labeo rohita (Hamilton, 1822). Aquaculture. 255:536–541. https://doi.org/10.1016/j.aquaculture.2006.01.013
Delgado C, Pardo I (2015) Comparison of benthic diatoms from Mediterranean and Atlantic Spanish streams: community changes in relation to environmental factors. Aquat Bot 120(PB):304–314
Delgado C, Pardo I, García L (2010) A multimetric diatom index to assess the ecological status of coastal Galician rivers (NW Spain). Hydrobiologia 644(1):371–384
Delgado C, Pardo I, García L (2012) Diatom communities as indicators of ecological status in Mediterranean temporary streams (Balearic Islands, Spain). Ecol Indic 15(1):131–139
Dell’Uomo A (2004) L’indice diatomico di eutrofizzazione/polluzione (EPI-D) nel monitoraggio delle azque correnti, line guida. Dipartimento di Botanica ed Ecologia, Universita di Camerino
Della Bella V, Pace G, Barile M, Zedde A, Puccinelli C, Ciadamidaro S, Danieli PP, Andreani P, Aulicino FA, Belfiore C (2012) Benthic diatom assemblages and their response to human stress in small-sized volcanic-siliceous streams of central Italy (Mediterranean eco-region). Hydrobiologia 695(1):207–222
Directive (2000) Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for Community action in the field of water policy. OJ L327, 22.12.2000. https://doi.org/10.3000/17252555.L_2009.140.eng
EC (2009) European Committee for Standardization, Water Framework Directive intercalibration technical report. Part 2. In: Poikane S (ed) Lakes. European Commission, Joint Research Centre, Ispra (Italy)
Espinosa MA, Vélez-Agudelo C, Isla FI (2020) Diatom responses to natural and anthropogenic environmental changes in a Patagonian river, Argentina. J S Am Earth Sci 102:102677
European Committee for Standardization (2014) European Committee for Standardization. In: Water quality-guidancefor the routine sampling and preparation of benthic diatoms fromrivers and lakes, vol 13946. European Standard EN, Brussels, p 17
Falasco E, Bona F (2011) Diatom community biodiversity in an Alpine protected area: a study in the Maritime Alps Natural Park. J Limnol 70(2):157
Feio MJ, Aguiar FC, Almeida SFP, Ferreira MT (2012) AQUAFLORA: a predictive model based on diatoms and macrophytes for streams water quality assessment. Ecol Indic 18:586–598
Feio MJ, Aguiar FC, Almeida SFP, Ferreira J, Ferreira MT, Elias C, Serra SRQ, Buffagni A, Cambra J, Chauvin C, Delmas F, Dörflinger G, Erba S, Flor N, Ferréol M, Germ M, Mancini L, Manolaki P, Marcheggiani S, Minciardi MR, Munné A, Papastergiadou E, Prat N, Puccinelli C, Rosebery J, Sabater S, Ciadamidaro S, Tornés E, Tziortzis I, Urbanič G, Vieira C (2014) Least disturbed condition for European Mediterranean rivers. Sci Total Environ 476–477:745–756
Feio MJ, Filipe AF, Garcia-Raventos A, Ardura Gutiérrez A, Calapez AR, Pujante AM, Mortagua A, Múrria C, Díaz de Quijano D, Martins F (2020) Advances in the use of molecular tools in ecological and biodiversity assessment of aquatic ecosystems. Asoc Ibérica Limnol 39(1):419–440
Freitas NCW, Heinrich CG, Etges T, de Souza CG, Lobo EA (2021) Assessment of potential reference sites for evaluating the ecological status of subtropical and temperate Brazilian lotic systems using the epilithic diatom community. Environ Sci Pollut Res 28(7):8698–8708
Gomà J, Rimet F, Cambra J, Hoffmann L, Ector L (2005) Diatom communities and water quality assessment in mountain rivers of the Upper Segre Basin (La Cerdanya, Oriental Pyrenees). Hydrobiologia 551(1):209–225
Gómez N, Licursi M (2001) The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquat Ecol 35(2):173–181
Hering D, Borja A, Carstensen J, Carvalho L, Elliott M, Feld CK, Heiskanen A-S, Johnson RK, Moe J, Pont D (2010) The European Water Framework Directive at the age of 10: a critical review of the achievements with recommendations for the future. Sci Total Environ 408(19):4007–4019
Holmes M, Taylor J (2015) Diatoms as water quality indicators in the upper reaches of the Great Fish River, Eastern Cape, South Africa. Afr J Aquat Sci 40(4):321–337
Juggins S, ter Braak C (1992) Calibrate-Program for species-environment calibration by (weighted averaging) partial least squares regression. Environmental Change Res Cent University College London
Kelly M, Juggins S, Guthrie R, Pritchard S, Jamieson J, Rippey B, Hirst H, Yallop M (2008) Assessment of ecological status in UK rivers using diatoms. Freshw Biol 53(2):403–422
Kelly M, Urbanic G, Acs E, Bennion H, Bertrin V, Burgess A, Denys L, Gottschalk S, Kahlert M, Karjalainen SM, Kennedy B, Kosi G, Marchetto A, Morin S, Picinska-Fałtynowicz J, Poikane S, Rosebery J, Schoenfelder I, Schoenfelder J, Varbiro G (2014) Comparing aspirations: intercalibration of ecological status concepts across European lakes for littoral diatoms. Hydrobiologia 734(1):125–141
Kolkwitz R, Marson M (1908) Ökologie der pflanzlichen Saprobien. Berichte der Deutschen Botanischen Gesellschaft. Ber Dtsch Bot Ges 26:505–519
Krammer K (2000) Diatoms of Europe. In: Lange-Bertalot H (ed) The Genus Pinnularia, Vol. 1–703, vol 1. A. R. G. Gantner Verlag K. G, Ruggel, pp 1–703
Krammer K (2002) Diatoms of the European Inland Waters and Comparable Habitats, Cymbella, vol 3. Gantner Verlag, Ruggell
Lai GG, Padedda BM, Ector L, Wetzel CE, Lugliè A, Cantonati M (2020) Mediterranean karst springs: diatom biodiversity hotspots under the pressure of hydrological fluctuation and nutrient enrichment. Plant Biosyst - Int J Deal Asp Plant Biol 154(5):673–684
Lange-Bertalot H (2001) Navicula sensu stricto and 10 genera separated from Navicula sensu lato, Frus-tulia. – Diatoms of Europe 2. Gantner Verlag, Ruggell, pp 1–526
Lange-Bertalot H, Hofmann G, Werum M, Cantonati M, Kelly MG (2017) In: Kelly MG (ed) Freshwater benthic diatoms of Central Europe: over 800 common species used in ecological assessment, vol 942. Koeltz Botanical Books, Schmitten-Oberreifenberg
Lavoie I, Hamilton PB, Wang YK, Dillon PJ, Campeau S (2009) A comparison of stream bioassessment in Québec (Canada) using six European and North American diatom-based indices. Nova Hedwig Beih 135:37–56
Lazaridou M, Ntislidou C, Karaouzas I, Skoulikidis N (2018) Harmonisation of a new assessment method for estimating the ecological quality status of Greek running waters. Ecol Indic 84:683–694
Lobo EA, Schuch M, Heinrich CG, da Costa AB, Düpont A, Wetzel CE, Ector L (2015) Development of the Trophic Water Quality Index (TWQI) for subtropical temperate Brazilian lotic systems. Environ Monit Assess 187(6):354
Lobo EA, Heinrich CG, Schuch M, Wetzel CE, Ector L (2016) Diatoms as bioindicators in rivers. In: River Algae. Springer International Publishing, Cham, pp 245–271
Merga L, Mengistie A, Faber J, Van den Brink P (2020) Trends in chemical pollution and ecological status of Lake Ziway, Ethiopia: a review focussing on nutrients, metals and pesticides. Afr J Aquat Sci 45(4):386–400
Oeding S, Taffs KH (2017) Developing a regional diatom index for assessment and monitoring of freshwater streams in sub-tropical Australia. Ecol Indic 80:135–146
Omernik JM (1987) Ecoregions of the conterminous United States. Ann Assoc Am Geogr 77(1):118–125
Potapova M, Charles DF (2007) Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol Indic 7(1):48–70
Potapova MG, Charles DF, Ponader KC, Winter DM (2004) Quantifying species indicator values for trophic diatom indices: a comparison of approaches. Hydrobiologia 517(1–3):25–41
Rimet F, Ector L, Cauchie HM, Hoffmann L (2004) Regional distribution of diatom assemblages in the headwater streams of Luxembourg. Hydrobiologia 520(1–3):105–117
Rott E, Pipp E, Pfister P, van Dam H, Ortler K, Binder N, Pall K (1999) Indikationslisten für Aufwuchsalgen in österreichischen Fließgewässer Trophieindikation (sowie geochemische Präferenzen, taxonomische und toxikologische Anmerkungen). Wasserwirtschaftskataster. Bundesministerium für Land-und Forstwirtschaft, Wasserwirtschaftskataster, Wien. p 248
Salinas-Camarillo VH, Carmona-Jiménez J, Lobo EA (2021) Development of the Diatom Ecological Quality Index (DEQI) for peri-urban mountain streams in the Basin of Mexico. Environ Sci Pollut Res 28(12):14555–14575
Seaby RM, Henderson PA (2007) Community Analyses Package (CAP 4.1. 3.). Engl Pisces Conserv Ltd
Stenger-Kovács C, Buczkó K, Hajnal É, Padisák J (2007) Epiphytic, littoral diatoms as bioindicators of shallow lake trophic status: Trophic Diatom Index for Lakes (TDIL) developed in Hungary. Hydrobiologia 589(1):141–154
Szczepocka E, Szulc B (2009) The use of benthic diatoms in estimating water quality of variously polluted rivers. Oceanol Hydrobiol Stud 38(1):17–26
ter Braak CJF, Šmilauer P (2002) CANOCO Reference Manual and CanoDraw for Windows User's Guide: Software for Canonical Community Ordination (version 4.5). http://www.canoco.com
Wang X, Zheng B, Liu L, Li L (2014) Use of Diatoms in river health assessment. Annu Res Rev Biol 4(24):4054–4074
Winter JG, Duthie HC (2000) Epilithic diatoms as indicators of stream total N and total P concentration. J North Am Benthol Soc 19(1):32–49
Acknowledgements
We thank Dr. Mehmet Yavuzatmaca for his help.
Funding
This study was supported by T.R. Ministry of Agriculture and Forestry, General Directorate of Water Management, Çınar Engineering Co., and the Scientific Research Projects Executive Council of Gaziantep University, which supported this research.
Author information
Authors and Affiliations
Contributions
AÇ applied the sampling methodology, collected samples, analyzed physico-chemical variables, identified diatom species, applied statistical analyses, written, reviewed, and edited the article. ÖL collected samples, analyzed physico-chemical variables, identified diatom species, and drawn the map of the studied region. TÇ applied the sampling methodology. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Çelekli, ., Lekesiz, Ö. & Çetin, T. Eco-assessment of least disturbed areas of the Antalya River basin: application of diatom indices from different ecoregions. Environ Sci Pollut Res 29, 790–804 (2022). https://doi.org/10.1007/s11356-021-15394-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15394-0