Abstract
Eutrophication from nutrient over-enrichment is a serious problem for the coastal zone and the estuarine environment globally. Australian estuaries are generally oligotrophic, but assessing whether they are becoming more eutrophic over time is inhibited by a lack of long-term monitoring data. The Richmond River, Australia, provided an ideal example of an estuary under anthropogenic pressure where the use of paleoecological techniques may potentially be used to reconstruct changes in nutrient status over time. Five cores were extracted from depositional areas in the Richmond River, and subjected to paleo-magnetic profiling and loss-on-ignition testing to ensure the core of greatest integrity was selected for further analytical work. Results from diatom and stable isotope analyses combined with 210Pb and AMS 14C dating show a strong indication that the total phosphorus levels in the Richmond River have fluctuated since 1940. Evidence also suggests that these fluctuations are most likely a function of climate. This study has successfully applied paleolimnological techniques to the estuarine environment to produce information that can be used by environmental managers to aid decision making in relation to nutrient control for the Richmond River. Further work using paleolimnological techniques should be aimed at the extraction of a core containing diatoms that pre-date European settlement and include analyses of specific lipids and fatty acid biomarkers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal eutrophication resulting from nutrient over-enrichment is a serious environmental problem (Cloern 2001) that results in detrimental changes in water quality and biological populations (Gikas et al. 2006). Eutrophication not only reduces ecosystem functions, but also has negative effects on the social and economic conditions of the coastal environment (Smith et al. 2008). Estuarine eutrophication requires considerable attention as estuaries are heavily affected by nutrient loading from many anthropogenic land uses. These include non-point sources like fertilizer use in agricultural and residential areas, and runoff from coastal urban and industrial development, as well as diffuse nutrient contributions from the atmosphere and groundwater (Hall and Smol 1999; Glibert et al. 2006; Bigler et al. 2007). Point sources of nutrients, such as ocean outfalls from sewage treatment plants, also aid the eutrophication process (Gabric and Bell 1993; Kronvang et al. 1995).
When management targets are set for estuaries with regard to eutrophication, it is paramount to have information describing how nutrient concentrations have varied over time (Weckström 2006; Saunders and Taffs 2009). A significant predicament when interpreting such changes in estuarine water quality is a lack of long-term monitoring data, with few datasets containing water quality measurements that extend back further than 50 years (e.g. Eyre 1997). In cases where there is an absence of long-term data related to estuarine water quality, historical information stored in the stratigraphic record can be utilized, by employing a paleoecological approach (Cooper et al. 2004).
Paleolimnological techniques based on diatoms can provide information about trends in trophic status over long periods of time. This is achieved by utilising robust statistical methods along with information about the changing abundances of diatom species assemblages in sediment cores to perform quantitative reconstructions of water quality (Tibby and Reid 2004). Although such methods were developed and applied to lakes (Anderson 1993; Reavie et al. 1995; Rosén et al. 2000) rather than the estuarine environment, paleolimnological techniques have the ability to be adapted to the coastal environments, including estuaries (Miller and Risberg 1990; Cooper and Brush 1993; Andrén 1999; Andrén et al. 1999; Colman et al. 2002; Kauppilla et al. 2003; Vaalgamaa 2004; Hilgartner and Brush 2006; Weckström 2006; Saunders and Taffs 2009). Similar work is sparse and considerably novel in Australia, with few studies developing diatom-based transfer functions for retrospective assessment of estuarine water quality conditions (Saunders, submitted; Logan and Taffs, submitted). Other researchers have used diatom species and assemblage data to qualitatively reconstruct past environmental conditions in coastal south eastern Australia (Fluin et al. 2007; Haynes et al. 2007). Two other studies have highlighted the use of paleoecological techniques as an effective management tool for setting rehabilitation targets in the absence of long term monitoring data (Saunders et al. 2008; Taffs et al. 2008).
Characteristically, Australian estuaries have an inclination towards oligotrophic conditions (Scanes et al. 2007). This contrasts to many well studied Northern Hemisphere estuaries which are vertically stratified due to poor mixing. This leads to hypoxic and anoxic bottom waters (Davis and Koop 2006), which are key symptoms of eutrophic conditions (Bricker et al. 2003). However, with upwards of 75% of the population located within 200 km of the coastline (Glibert et al. 2006; SoE 2006), Australian systems are at risk to increases in nutrient loading and hence eutrophication. Water quality within many mainland Australian estuaries is also characterized by irregular flooding events that have a sizeable influence on nutrient cycling and estuarine hydrology (Roy et al. 2001). Flushing of sub-tropical estuaries in Australia is highly episodic, and while the visible symptoms of nutrient enrichment and associated eutrophication may not be evident, this does not imply that they are not becoming more eutrophic over time (Eyre 1997). While no record of these flushing events exists for many sub-tropical estuaries in Australia, it is likely that such episodes, and the nutrient enrichment associated with them, have left signatures in estuarine sedimentary sequences. Thus, paleolimnological methods may identify periods of change to estuarine nutrient levels.
This paper investigates whether past nutrient levels in the Richmond River, an east Australian sub-tropical estuary on the far north coast of New South Wales (NSW), can be assessed using paleolimnological techniques. The Richmond River is a typical example of an estuary modified by European land use activities. The estuary drains a large catchment area and has undergone significant change due to changed land use activities since European settlement, which has eroded its economic, social and environmental values. Thus, it provides an ideal case example to test the application of adapted paleoecological techniques that have been utilized effectively in Northern Hemisphere estuarine systems to a Southern Hemisphere estuary. Specifically, this paper aims to reconstruct historical changes in total phosphorus concentrations in the Richmond River based on diatoms preserved in a dated sediment core. These inferences will be combined with geochemical data to determine if nutrient levels and associated eutrophication effects are becoming more evident over time.
Materials and methods
Study site
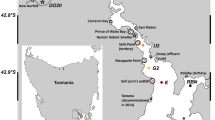
The Richmond River (153.592°E, 28.877°S) is one of the largest coastal drainage systems in NSW, with a catchment area of 6,878 km2 (Fig. 1) and a mean annual discharge of 1 920 000 ML. Over 50% of the catchment area is used for agricultural purposes (Hossain et al. 2004). Two major tributaries, the Wilsons River from the north, and Bungawalbin Creek from the south, merge with the main channel up stream from Woodburn, which is located ca. 40 km from the mouth. Downstream of Woodburn, the Richmond River increasingly becomes estuarine in nature, where it is joined by North Creek at its mouth prior to discharging into the sea.
The climate of coastal northern NSW is humid sub-tropical, with a distinctive pattern of summer to autumn maximum in rainfall. Mean annual rainfall is 1,100 mm in the western part of the catchment, increasing to 1,400 mm along the coast. Daily mean temperatures in the lower part of the Richmond River catchment at Ballina vary from 27 to 31°C in January, to a daily minimum of 6–12°C in July (Hashimoto et al. 2006). This climate regime is controlled by two influences: a sub-tropical high pressure belt during the winter and spring months (June through to October) creating clear, predominately dry weather patterns; and a domination of easterly monsoonal trade winds during the summer and autumn months (November through to May). Tropical cyclones can occur between January and April, creating heavy rainfall and flood events. Thus, the Richmond River estuary is dominated by short lived but large episodic freshwater inputs during the summer months. These inputs can flush the estuary, producing freshwaters to the river mouth. However, little to no discharge of this magnitude occurs during the winter months, which has a significant impact on the hydrology, salinity and biology of the estuary (Eyre and Twigg 1997). The majority of the annual discharge (90%) and flood events for the Richmond River occur during the summer-autumn wet season (Hossain et al. 2004). Hence, flushing times of the Richmond River can vary from 0 to 196 days in length depending of the amount of freshwater discharge (Eyre 1997; Eyre and Twigg 1997; Eyre 1998). The Richmond River catchment also experiences significant intra- and inter-annual variability in nutrient loads and exports (McKee et al. 2000).
The Richmond River catchment was settled by Europeans in 1840, who began introducing land uses such as grazing, agriculture and timber cutting. The development of these industries was rapid, with population increasing to 4,000 by 1870. There was a further rise to a population of above 22,000 by 1891 due to the increasing demand for the developing dairy industry. Between 1920 and 1950 the reliance on sea and rail by this industry diminished in the Richmond River around Ballina, as local infrastructure started to support other modes of transport (Daley 2001). In the most recent half a century, agricultural and grazing activities have increased significantly, with upwards of 75% of the upper Richmond River catchment used for agriculture. Fertilizer use in the catchment has also increased 20 fold over this time. Demographic changes have also been experienced, particularly in Ballina, with a population of over 35,000 listed in the mid 1990s. This has instigated the sub-division of agricultural land for urban development and expansion (Eyre 1997). The Richmond River itself is located in an area that is forecast to be a focal point for increases in population growth over the next 25 years. This is expected to place increasing pressure on the estuary and associated coastal habitats (Smith et al. 2008).
The Richmond River estuary lies within a highly developed catchment area and is currently listed as ‘extensively modified’ by OzCoasts (2008). Increases in vegetation clearing by the agriculture industry and stormwater runoff from urban and industrial areas, have resulted in loss of habitat within wetland, riparian and floodplain areas. Decreasing water quality is a significant issue, with research showing dissolved and fine sediment nitrogen and phosphorus levels have increased by several orders of magnitude since European settlement (Hossain 1998). In particular, total phosphorus loading has shown to have a association with land uses (Eyre and Pont 2003). Hence, the Richmond River is an ideal example of an Australian estuary that has suffered from the effects of anthropogenic eutrophication.
Core collection and selection
The methodology used for this study was based on established methods used for similar research (Siver 1999; Bloom et al. 2003; Reid 2005; Woodward and Shulmeister 2006), but was adapted to take into account the dynamic nature of an estuary. Sites of constant sedimentation for coring can be found in estuaries (Cooper et al. 2004). To ensure that a core of good integrity was selected for analysis, five push cores were extracted from a series of depositional sites in the Richmond River (Fig. 1), using a 1.5 m × 74 mm PVC pipe. Each core was extracted from a current area of deposition within the estuary, away from the main channel of flow. The shortest two cores were RR2 (42 cm) and RR5 (38 cm), and were not used for further analysis. The three longest cores (RR1—69 cm; RR3—74 cm; RR4—92 cm) were selected for organic and carbonate content analyses through the loss-on-ignition (LOI) method described in Heiri et al. (2001) and Bengtsson and Enell (1986) (Figs. 2, 3).
Natural Remanent Magnetisations
Natural Remanent Magnetisations (NRMs) were measured on the three longest sediment cores (RR1, RR3, and RR4) using a 2G Enterprises long-core, 3-axis cryogenic magnetometer at the ANU Black Mountain Paleomagnetic Laboratory, Canberra. To test the stability of NRMs, representative specimens were subject to alternating field demagnetisation. NRMs were stable, with little or no evidence of secondary (overprint) magnetisations. Inferences were made using changes in the magnetic inclination at 2 cm intervals in each core. The results of these analyses determined which core was submitted for 210Pb, Cs137 and AMS 14C dating, along with subsequent diatom and stable isotope analysis.
210Pb and AMS 14C dating
Ten sediment samples were dated using 210Pb dating techniques at the Australian Nuclear Science and Technology Organization (ANSTO), Sydney. The total 210Pb activity was determined by measuring its granddaughter 210Po, which was assumed to be in secular equilibrium with 210Pb. Supported 210Pb was approximated by measuring 226Ra activity (Harrison et al. 2002). The CIC (Constant Initial Concentration) and CRS (Constant Rate of Supply) models indicated a monotonic profile. The results from three 137Cs analyses supported the CIC model, thus sediment ages used in this report will be based on CIC ages. In addition, one AMS 14C date was attained on the sediment phytolith fraction and also processed and measured at ANSTO. Dates were calibrated using the program CALIB (version 5.0). The age range was obtained from the intercepts method (Stuiver and Reimer 1993).
Diatoms
Twenty-seven samples from the selected core were processed for diatom analysis at differing resolutions depending upon the depth of the sample. These generally corresponded with depths at which loss-on-ignition and dating methods were also applied. Diatoms were processed according to the methods outlined in Parr et al. (2004). Slides were examined using an Olympus CX40 compound light microscope fitted with an Olympus DP10 digital camera. Between 300 and 400 diatoms frustules were identified and counted from each sample to determine the diatom community assemblage. Diatoms were counted across several transverses across each slide to ensure that counting was representative. Diatoms were generally identified using the photographs of Witkowski et al. (2000) and Taffs (2005), with reference to Foged (1978) and Gell et al. (1999). Species occurring with >2% relative abundance in at least one sample were included in statistical analysis. The centric to pennate ratio, which is considered an indicator of eutrophication, was calculated following the procedure of Cooper and Brush (1993).
Stable isotopes
Stable isotopes were processed according to in-house methodologies at the Centre for Coastal Biogeochemistry at Southern Cross University. Samples were lyophilised, homogenized and weighed into tin capsules. Capsulated samples were analysed by Dumas combustion using a Thermo FlashEA 1112 coupled online to a Thermo Delta V Plus isotope ratio mass spectrometer. All carbon and nitrogen isotope ratios are expressed in conventional δ notation:
where Rsample and Rstd are the 13C/12C or 15N/14N isotope ratios of the sample and standard, respectively. The δ13C isotope ratio of the samples were determined by comparison with a CO2 reference gas standard (99.996%, δ13CVPDB = −6.317) with all values reported relative to Vienna Pee Dee Belemite standard. The δ15N isotope ratio of the samples was determined by comparison with N2 reference gas standard (99.99%, δ15NAIR = −1.706‰) with all values reported relative to air.
Results
Loss on ignition and paleomagnetic profiling
Both paleo-magnetic profiling and LOI testing provided information on which of the cores was most likely to have the highest integrity for analytical purposes. RR3 was selected as both paleo-magnetic and LOI curves showed smooth characteristics relative to the RR1 and RR4 cores. Significant peaks and troughs in the curves for both the RR1 and RR4 cores may have been indicative of dynamic forces within the estuary, thus signaling that sedimentary layers may have been disturbed at these depths in the cores.
210Pb/AMS 14C dating
The unsupported 210Pb activity profile in the sediment core showed a monotonic pattern of exponential decline. This indicates the core’s suitability for establishing a reliable geochronology for the Richmond River estuary based on a CIC model (Appleby and Oldfield 1983) (Fig. 4). Ten 210Pb dates were obtained for the upper 65 cm of sediment, ranging in age from 7.31 ± 0.89 YBP (2000 AD) at the 4–5 cm horizon, to 84.19 ± 4.68 YBP (1923 AD) at 64–65 cm (Table 1). 137Cs activities were tested at depths of 22–24 cm, 25–27 cm and 32–34 cm. Due to the limited amount of sample available for 137Cs analysis by gamma spectrometry, only three samples from the core were analysed. 137Cs activity was detected at 22–24 cm layer, an indication the sediment was deposited post 1955 (Leslie and Hancock 2008). 137Cs activity was not detected below 25 cm, thus it can be assumed sediment below 25 cm was deposited prior to 1955 (Leslie and Hancock 2008). The 210Pb chronology for this core is in general agreement with the 137Cs chronology. Based on the CIC model, there were two distinct changes in sedimentation rate. From 65 cm to 25 cm, the average sedimentation rate was ca. 1.05 cm per year. The upper 25 cm had an average sedimentation rate of c.a. 0.52 cm per year. Mass accumulation rates for the 0–25 cm and 25–65 cm sections of the core were 0.371 ± 0.018 and 0.41 ± 0.035 g/cm2/year, respectively. One AMS 14C date with a conventional radiocarbon age of 4750 ± 60 BP was obtained to get a basal age for the core, which was calculated to be between 5,312 and 5,583 calibrated years BP (Stuiver and Reimer 1993).
Diatoms
Diatoms were well-preserved from the surface to 34 cm in sediment core RR3. From 34 cm to 40 cm preservation was incomplete with only more robust species present. From 40 cm to the base of the core no diatoms were preserved. Decreasing degrees of diatom preservation with depth is common in estuaries due to the effects of salinity and temperature on the biogenic silica cycle (Natori et al. 2006; Roubeix et al. 2008). The mean number of diatom taxa per sample was >40 species. In total, 156 diatom taxa were identified in sediment core RR3. Of these, 39 occurred at a relative abundance >2% in at least one sample. These species were used for environmental reconstructions. There were fifteen common taxa (Fig. 5) occurring with a relative abundance >4% in at least 2 samples, with seven of these being present in every sample. Cyclotella meneghiniana (Kϋtzing), Nitzschia granulata (Grunow) and Planothidium delicatulum (Kϋtzing) encountered at a relative abundance >20% in at least two samples, having minimum/maximum relative abundances of 6.84 and 35.00, 1.98 and 30.59 and 4.73 and 24.12%, respectively.
The fifteen common diatom taxa, and their relative abundances by percentage, plotted against depth, and years AD inferred from 210Pb age. Centric to pennate taxa ratios are represented by the c:p chart. Diatom inferred total phosphorus is represented by the DI-TP chart. No diatom preservation was recorded below 40 cm
The diatom assemblage of RR3 (Fig. 5) illustrates a fairly consistent trend of dominance by C. meneghiniana, N. granulata and P. delicatulum with minor fluctuations in their relative abundance and in the relative abundance of the less dominant species. The centric to pennate diatom ratio (c:p) fluctuated throughout the core (Fig. 5). The highest values below 34 cm occurred at depths of 34–35 cm (c:p = 0.99), 36–37 cm (c:p = 1.13) and 38–40 cm (c:p = 0.79). The peak values above 34 cm, where diatoms were better preserved, were at depths of 3–4 cm (c:p = 0.66), 10–11 cm (c:p = 0.66) and 15–16 cm (c:p = 0.69). There was a mean of 5.1 centric taxa per sample. This was generally representative of dominance by C. meneghiniana and C. striata (Kϋtzing) and the presence of Actinocyclus normanii (Gregory) and Aulacoseira italica (Ehrenberg).
Total phosphorus reconstruction
Diatom-inferred Total Phosphorus (DI-TP) concentrations were calculated based on the total phosphorus (TP) transfer function developed by Logan et al., unpublished data set. This transfer function (\( r_{\text{jack}}^{2} \) = 0.69, RMSEP = 0.027 log10 mg TP l−1) used surface sediment samples from 52 east Australian sub-tropical estuaries between Coffs Harbour, NSW and Gladstone, Queensland. DI-TP (Fig. 5) indicated that nutrient levels have fluctuated in the Richmond River since 1920. Peaks occurred at depths of 8–9 cm (1994 AD), 10–11 cm (1992 AD), 34–35 cm (1950 AD) and 36–37 cm (1945 AD), and generally coincided with higher relative abundances of C. meneghiniana and N. granulata along with increased c:p ratios. Conversely, lower DI-TP inferences coincided with the decreases in C. meneghiniana and greater influence of less common pennate species. DI-TP did not increase significantly over time. The DI-TP peaks at 34–35 cm and 36–37 cm may have a degree of unreliability due to possible poor preservation of specific species that have lower TP optima than C. meneghiniana and N. granulata, the two species that dominate relative abundances at these depths.
Stable isotopes
Results for δ13C were unchanging, with a maximum of −22.76‰ at 44–46 cm, and a minimum of −23.95‰ at 62–65 cm, and a range of 1.19‰ throughout the core. Percent carbon content was at its highest (2.36%) at the very top of the core, with the lowest value (0.44%) recorded at 44–46 cm. δ15N results showed some variability, with the most enriched section (5.2‰) of the core at 2–3 cm, and the most depleted section (2.27‰) at 8–9 cm. A mixing diagram that plots δ13C against δ15N (Fig. 6) reveals five groups of relevance where some grouping of samples from similar depths occurs, due mainly to enrichment in δ15N in a majority of the younger sediments. Group 1 includes 1–2, 3–4, 4–5, and 6–7 cm; Group 2 includes one sample at 8–9 cm depth. Group 3 includes 12–13, 14–15, 15–16, and 16–17 cm; Group 4 includes 32–34, 34–36, and 38–40 cm; Group 5 includes 62–65 and 71–74 cm. These different groups suggest some variation in nitrogen sources over time in the Richmond River estuary.
Discussion
Coring
The retrieval of an intact sediment core of sufficient integrity was given paramount consideration in the field methods used in this study. Careful selection of less dynamic depositional sites for coring was made, generally in areas removed from the higher energy areas of the Richmond River estuary. Despite this approach, the results of the LOI testing and paleo-magnetic profiling suggest that this does not guarantee the extraction of useful sediment cores. Of the three cores selected for further analyses, only one (RR3) showed some indication from initial testing techniques that it would be suitable for paleoenvironmental analysis. The site from which RR3 was extracted differed from the four others in that it received water on the flood tide only, was located at the mouth of Emigrant Creek, a tributary to the Richmond River, and was protected from most water movement by a fringe of Avicennia mangroves (Hashimoto et al. 2006). This site was also away from the deeper, fast flowing sections of the estuary, which were closer to sites from which the RR4 and RR5 cores were extracted. This highlights the importance of using multiple cores from multiple sites in combination with rapid, inexpensive characterisation techniques when studying estuaries.
Collecting multiple cores and using rapid assessment techniques is critical for making the most of limited resources. The results of 210Pb dating analyses indicated that the characterization of the RR3 core using LOI and paleo-magnetic profiling to identify good integrity within the core was successful. The monotonic profile of unsupported 210Pb showed decay with depth, which was evident from the CIC model. This was supported by 137Cs results. This suggests that the sediment sequence was relatively undisturbed despite being located in very close proximity to a tidally influenced section of the estuary. Sites of stable, continuous sedimentation can be found within estuaries (Cooper et al. 2004), as also highlighted by this study when an appropriate approach is employed. The 210Pb activity at 64.5 cm indicated an age of 1923 AD, which was the last 210Pb date inferred before the basal AMS 14C date of between 5312 and 5583 calibrated years BP (Stuiver and Reimer 1993) at 74 cm. Such a large discrepancy between the two dates over a relatively small amount of sediment (~10 cm) may be indicative of a severe erosion event. This may have taken place during a large flood, or indeed any large erosional event after 5312–5583 cal. yr BP. It is probable that this episode was large enough to breach the protective mangrove fringe that would normally provide protection from higher energy events. During such an event, sediments at the RR3 core site may have been scoured to reveal sediments of the age of our basal date, upon which recent sediment has been deposited.
Diatoms
The reconstruction of water quality conditions that pre-date European settlement was not possible due to no diatom preservation below 40 cm. However, the diatom assemblages identified between the surface sediment sample and 40 cm provided significant paleoenvironmental information. This is particularly important, with no long term nutrient monitoring data existing for the Richmond River. No apparent trend for large increases in total phosphorus concentrations since ~1940 AD can be inferred from the diatom assemblages. This is consistent with Eyre (1997), who found that phosphorus concentrations in the Richmond River have remained quite constant, despite large increases in agricultural activities in the Richmond River estuary floodplain over the past five to six decades.
The diatom stratigraphy observed here in combination with the transfer function developed by Logan et al., unpublished data set suggests that nutrient concentrations in the Richmond River since 1940 have been in a state of flux, and are controlled by climate more than anthropogenic activities. The two lowest DI-TP values were recorded at depths of 5–6 cm (0.07 mg/L) and 35–36 cm (0.08 mg/L), however samples from these depths were not used for 210Pb dating, so comparison to annual rainfall for these specific depths was not possible. Other dated samples can be compared to the rainfall record. The third lowest DI-TP (0.09 mg/L) at a depth of 24–25 cm corresponds to around 1959 AD (±2 years). Annual rainfall for the year 1960 was 1,185 mm (BOM 2009). This year lies within error margin for 210Pb dating, and is well below the recorded rainfall average of 1,817 mm (BoM 2009). The highest DI-TP (0.205 mg/L) value was recorded at a depth of 8–9 cm, which corresponds to around 1992 AD (±1 years) based on 210Pb dating results. Average annual rainfall for the years 1991–1993 was 1,411 mm, which is more than 200 mm above the annual average for the period of 1960 (BoM 2009). Unsurprisingly this suggests fluctuating nutrient levels in the Richmond River have a positive relationship with increases in precipitation.
Previous work by Eyre (1997) on the relationship between nutrient levels and rainfall for the Richmond River catchment provides support for inferences based on the diatom data. Eyre (1997) indicated that the estuary may receive up to 90% of its nutrient loading during episodic flood events. Hence, non point source nutrient contributions from terrestrial runoff are a cause for elevated nutrient concentrations in times of higher rainfall. These increases and decreases are reflected in the diatom sedimentary record. This is shown by the increased presence of Cyclotella meneghiniana, shown previously to be a nutrient tolerant species (Cooper and Brush 1993; Whitmore et al. 1996; Koster et al. 2005; Weckström 2006). A majority of the DI-TP peaks in the diatom reconstruction are also accompanied by higher (>0.65) c:p ratios. The average number of taxa per assemblage was >40, while the number of centric taxa present in each assemblage was 5.1 species, with a maximum of six centric species in any one sample. C. meneghiniana was the most dominant of these centric taxa in all cases, providing support for the view that increases in nutrients in the Richmond River are accompanied by increased abundances and dominance of this species. Furthermore, increases in the abundance of Navicula arenaria, shown previously to have preference for unpolluted environments (Admiraal and Peletier 1979), are experienced when the relative abundances of C. meneghiniana are at their lowest in the core.
Stable isotopes
The carbon stable isotope record of the RR3 core showed little variation in δ13C content, despite the basal AMS 14C date at 74 cm being considerably older than that of the last 210Pb dated horizon at 64.5 cm. These δ13C concentrations of between −22.76 and −23.95 ‰ (a range of 1.19 ‰) are consistent with material derived from C3 plants (−22 to −30‰, e.g. trees) (Maslin and Swann 2006). This indicates that the sedimentary carbon input to the RR3 site has been from the same source for approximately 5,000 years. It is unlikely that the Avicennia mangroves that fringe the study site, a species present in the Richmond River for longer than the time period encapsulated in our core (Hashimoto et al. 2006), have dominated sedimentary carbon inputs over this time period. Mangroves show considerable geographic consistency in their δ13C signature, which often lies between −24.3 and −30‰ (Kieckbush et al. 2004), concentrations which are depleted in comparison to those in this study. Algal species that are associated with mangrove estuaries have been shown to lie between −19.3 and −24.2‰ (Newell et al. 1995; Kieckbush et al. 2004). This provides strong evidence that carbon inputs to sediments at RR3 coring site have been facilitated by algal production, given that all of the δ13C concentrations determined by this study lie between the signatures put forward by Newell et al. (1995) and Kieckbush et al. (2004) for algae associated with estuaries with mangrove populations. However, the minimal variance in the δ13C signatures in core RR3 provides almost no evidence of changes in the source of organic matter used for primary production at this site over the past 5,000 years.
The range for stable nitrogen isotopes (2.27–5‰) show concentrations characteristic of atmospheric inputs, which are generally between 2 and 8‰ (Valiela et al. 2000), however these are also in ranges of known marine and terrestrial inputs (Maksymowska et al. 2000). This makes an accurate interpretation of nitrogen sources to this site difficult. Some alteration and degradation at different depths occurs, which points to some increases in primary productivity, based on the known habits of phytoplankton. During nitrogen assimilation, phytoplankton preferentially use 14N, leaving the overlying waters enriched in 15N (Maslin and Swann 2006). This is then reflected in depleted δ15N concentrations preserved in the sediment record, with the 14N enriched organic matter from the water column settling and contributing to sedimentation. The five groups of samples identified by the mixing diagram (Fig. 6) show minimal variation in their δ13C content, but do form groups due to variation in δ15N content. Group 1 covers four samples between 1 cm and 7 cm at the top of the core, which have some of the higher δ15N concentrations for the core, indicating that in recent times, phytoplankton production may have been on the decrease. This contrasts with Group 5, which comprises of a sample towards the base of the core, and the basal sample. The depleted δ15N signature at these depths points to increases in phytoplankton abundance relative to the younger samples in the core.
Groups 3 and 4 both contain values that indicate 15N enrichment in comparison to the oldest sediments analysed, but generally have δ15N concentrations that are similar to those in Group 1. Thus, apart from two enriched samples at 3–4 cm and 6–7 cm, phytoplankton production inferred from 15N values seems to have been relatively stable over the period, apart from the single sample labeled as Group 2. The 15N of this sample (2.27‰) from a depth of 8–9 cm was the lowest recorded in the core and represents ~1990 AD based on 210Pb dating. This may specify a period of decreased productivity and associated phytoplankton production, although inferences made based on the occurrence of such an outlier may need the support of another more specific geochemical marker or proxy. Overall, nitrogen sources to the Richmond River are dominated by atmospheric inputs, despite the land uses that dominate the catchment.
Multi-proxy inferences
Combination with other paleoenvironmental proxies to aid reconstruction is advised when using stable isotopes (Leng et al. 2006). In this study, data from stable isotope analysis has been amalgamated with diatom assemblages to provide support for the diatom-based inferences. This combination was not always straight forward, and at some depths, sets of results do contrast. While these differences may be attributed to problems often regarded as ‘inherent’ when sampling estuaries, they do warrant further discussion.
Results from differing proxies at a depth of 8–9 cm displayed some consistency in interpretation. Based on the DI-TP result (0.21 mg/L), c:p ratio (0.62) and relative abundance of C. meneghiniana (25.9%), it could be assumed that nutrient enrichment and subsequent eutrophication effects could have intensified around this time. This received some support from the δ15N signature (2.27‰) which was the lowest measured in this study. These correlations may indicate this to be the most productive section of the sedimentary record. These results and this δ15N signature could be expected as phytoplankton would preferentially assimilate the 14N of the nitrite portion during periods of enhanced productivity, then form part of sedimentation, leaving the water column enriched with 15N (Leng et al. 2006).
Results from diatom analysis at other depths (2–3, 3–4, 15–16 cm) in the core with enrichment of 15N exhibit differences in terms of potential inferences that could be made from either diatoms, or stable isotopes, in a single proxy study. Above average DI-TP, along with minimum relative abundances >18% for C. meneghiniana were observed, indicating increased productivity. However, δ15N values were 4.34‰ or greater at these depths, indicative of less assimilation of 14N by phytoplankton, which could mean decreased primary productivity relative to other depths in the core.
Conclusion
This is the first study to use the sedimentary diatom record and geochemical data to reconstruct historical estuarine TP concentrations in Australia. Given the lack of long term data in relation to estuarine water quality in Australia, this paper used a somewhat novel approach, by applying previously under-utilised paleolimnological techniques to retrospectively assess TP concentrations in the Richmond River. While inferences were possible, and in some cases good correlation exists between the diatom and geochemical results, this study also highlighted the need for further work in this field to ensure confidence in reconstructions made through the use of paleoecological techniques in the estuarine environment.
The results of the diatom based environmental reconstruction indicate that the nutrient status of the Richmond River in relation to total phosphorus has a tendency to fluctuate, and that this is more likely a function of climate. Previous research has identified this site to be highly episodic in terms of its freshwater discharge, and this may be exhibiting some control over TP concentrations and subsequent primary production. No clear trend for recent increases in TP could be established, although the lack of a core of sufficient length to predate European settlement in the Richmond River catchment may have had an impact on this. The use of geochemical methods to provide support for this diatom data was moderately successful. It is recommended that in future studies lipids and fatty acids specific to both phytoplankton (C20 and C22 polyunsaturated fatty acids) and vascular plants (stigmasterol, campesterol, β-sitosterol) be used as biomarkers to identify the exact origin of sedimentary inputs. Such investigative work was beyond the scope of this project.
Although paleoecological estuarine research is still relatively novel in Australia, this study has demonstrated the usefulness of paleolimnological techniques for such work. This information can be especially useful when there is a lack of monitoring data, which is the case for the Richmond River estuary. The results of this research, which indicate that nutrient levels in the Richmond River are not increasing over time, but are closely linked to climate, can provide environmental managers involved at the local level with information on which future management decisions regarding nutrient control can be based.
References
Admiraal W, Peletier H (1979) Sulfide tolerance of benthic diatoms in relation to their distribution in an estuary. Brit Phycol J 14:185–196
Anderson NJ (1993) Natural versus anthropogenic change in lakes: the role of the sediment record. Trends Ecol Evol 8:356–361
Andrén E (1999) Changes in composition of the Diatom flora during the last century indicate increased eutrophication of the Oder Estuary, South-western Baltic Sea. Estuar Coast Shelf Sci 48:665–676
Andrén E, Shimmield G, Brand T (1999) Environmental changes of the last three centuries indicated by siliceous microfossil records from the southwestern Baltic Sea. Holocene 9:25–38
Appleby PG, Oldfield F (1983) The assessment of 210Pb data from sites with varying sediment accumulation rates. Hydrobiologia 103:29–35
Bengtsson L, Enell M (1986) Chemical Analysis. In: Berglund BE (ed) Handbook of Holocene palaeoecology and palaeoydrology. Wiley, New York, pp 423–449
Bigler C, von Gunten L, Lotter AF, Hausmann S, Blass A, Ohlendorf C, Sturm M (2007) Quantifying human-induced eutrophication in Swiss mountain lakes since AD 1800 using diatoms. Holocene 17:1141–1154
Bloom AM, Moser KA, Porinchu DF, MacDonald GM (2003) Diatom-inference models for surface-water temperature and salinity developed from a 57-lake calibration set from the Sierre Nevada, California, USA. J Paleolimnol 29:235–255
BOM (2009) Bureau of meteorology. Online Australian climate information. Available at www.bom.gov.au [Date Retrieved 23 September 2009]
Bricker SB, Ferreira JG, Simas T (2003) An integrated methodology for assessment of estuarine trophic status. Ecol Modell 169:39–60
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Colman SM, Baucom PC, Bratton JF, Cronin TM, McGeehin JP, Willard D, Zimmerman AR, Vogt PR (2002) Radiocarbon dating, chronologic framework, and change in accumulation rates of Holocene estuarine sediments from Chesapeake Bay. Quatern Res 57:58–70
Cooper SR, Brush GS (1993) A 2500-year history of anoxia and eutrophication in Chesapeake Bay. Estuaries 16:617–626
Cooper SR, McGlothin SK, Madritch M, Jones DL (2004) Paleoecological evidence of human impacts on the Neuse and Pamlico Estuaries of North Carolina, USA. Estuaries 27:617–633
Daley LT (2001) A short history of the Richmond River. Richmond River Historical Society Inc, Lismore
Davis RJ, Koop K (2006) Eutrophication in Australian rivers, reservoirs and estuaries—a southern hemisphere perspective on the science and its implications. Hydrobiologia 559:23–76
Eyre BD (1997) Water quality changes in an episodically flushed sub-tropical Australian estuary: a 50 year perspective. Mar Chem 59:177–187
Eyre BD (1998) Transport, retention and transformation of material in Australian estuaries. Estuaries 21:540–551
Eyre BD, Pont D (2003) Intra- and inter-annual variability in the different forms of diffuse nitrogen and phosphorus delivered to seven sub-tropical east Australian estuaries. Estuar Coast Shelf Sci 57:137–148
Eyre B, Twigg C (1997) Nutrient behaviour during post-flood recovery of the Richmond River Estuary, northern NSW, Australia. Estuar Coast Shelf Sci 44:311–326
Fluin J, Gell PA, Haynes D, Tibby J, Hancock G (2007) Palaeolimnological evidence for the independent evolution of neighbouring terminal lakes, the Murray Darling Basin, Australia. Hydrobiologia 591:117–134
Foged N (1978) Diatoms in Eastern Australia. Bibliotheca Phycologica, 47
Gabric AJ, Bell PRF (1993) Review of the effects of non-point source nutrient loading on coastal ecosystems. Aust J Mar Freshw Res 44:261–283
Gell PA, Sonneman JA, Reid MA, Illman MA, Sincock AJ (1999) An illustrated key to the common diatom genera from southern Australia—identification Guide No. 26, Cooperative Research Centre for Freshwater Ecology
Gikas GD, Yiannakopoulou T, Tsihrintzis VA (2006) Water quality trends in a coastal lagoon impacted by non-point source pollution after implementation of protective measures. Hydrobiologia 563:385–406
Glibert PM, Heil CA, Oneil JMO, Dennison WC, O’Donohue MJH (2006) Nitrogen, phosphorus, silica and carbon in Moreton Bay, Queensland, Australia; differential limitation of phytoplankton biomass and production. Estuar Coast 29:209–221
Hall RI, Smol JP (1999) Diatoms as indicators of lake eutrophication. In: Stoermer EF, Smol JP (eds) The Diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge
Hashimoto TR, Saintilan N, Haberle SG (2006) Mid-Holocene development of mangrove communities featuring Rhizophoraceae and geomorphic change in the Richmond River estuary, New South Wales, Australia. Geogr Res 44:63–76
Haynes D, Gell PA, Tibby J, Hancock G, Goonan P (2007) Against the tide: the freshening of naturally saline coastal lakes, southeastern South Australia. Hydrobiologia 591:165–183
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Hilgartner WB, Brush GS (2006) Prehistoric habitat stability and post-settlement habitat change in a Chesapeake Bay freshwater tidal wetland, USA. Holocene 16:479–494
Hossain S (1998) Hydrology and suspended sediment transport in the Richmond River catchment and estuary, NSW, Australia. PhD Thesis. Centre for Coastal Management, Southern Cross University, Lismore
Hossain S, Eyre BD, McConchie D (2004) Dry season suspended sediment concentration and sedimentation in the Richmond River estuary, northern NSW, Australia. Aust J Soil Res 42:203–211
Kauppilla P, Meeuwig JJ, Pitkanen H (2003) Predicting oxygen in small estuaries of the Baltic Sea: a comparative approach. Estuar Coast Shelf Sci 57:1115–1126
Kieckbush DK, Koch MS, Serafy JE, Anderson WT (2004) Trophic linkages among primary producers and consumers in fringing mangroves of subtropical lagoons. Bull Mar Sci 74:271–285
Koster D, Pienitz R, Wolfe BB, Barry S, Foster DR, Dixit SS (2005) Paleolimnological assessment of human-induced impacts on Walden Pond (Massachusetts, USA) using diatoms and stable isotopes. Aquat Ecosyst Health Manag 8:117–131
Kronvang B, Grant R, Larsen SE, Svendsen LM, Krsitensen P (1995) Non-point source nutrient losses to the aquatic environment in Denmark: impact of agriculture. Mar Freshw Res 46:167–177
Leng MJ, Lamb AL, Heaton THE, Marshall JD, Wolfe BB, Jones MD, Holmes JA, Arrowsmith C (2006) Isotopes in lake sediments. In: Leng MJ (ed) Isotopes in palaeoenvironmental research. Springer, Berlin, pp 147–184
Leslie C, Hancock GJ (2008) Estimating the date corresponding to the horizon of the first detection of 137Cs and 239 + 240Pu in sediment cores. J Environ Radioact 99:483–490
Logan B, Taffs KH (submitted) The Burrum River estuary: identifying reference sites for Australian subtropical estuarine systems using paleolimnological methods. J Paleolimnol
Maksymowska D, Richard P, Piekarek-Jankowska H, Riera P (2000) Chemical and isotopic composition of the organic matter sources in the Gulf of Gdansk (Southern Baltic Sea). Estuar Coast Shelf Sci 51:585–598
Maslin MA, Swann GEA (2006) Isotopes in marine sediments. In: Leng MJ (ed) Isotopes in palaeoenvironmental research. Springer, Berlin, pp 227–290
McKee L, Eyre B, Hossain S (2000) Intra- and Interannual export of nitrogen and phosphorus in the subtropical Richmond River catchment, Australia. Hydrol Process 14:1787–1809
Miller U, Risberg J (1990) Environmental changes, mainly eutrophication, as recorded by fossil siliceous micro-algae in two cores from the uppermost sediments of the north-western Baltic. Beiheft zur Nova Hedwigia 100:237–253
Natori Y, Haneda A, Suzuki Y (2006) Vertical and seasonal differences in biogenic silica dissolution in natural seawater in Suruga Bay, Japan: effects of temperature and organic matter. Mar Chem 102:230–241
Newell RIE, Marshall N, Sasekumar A, Chong VC (1995) Relative importance of benthic microalgae, phytoplankton, and mangroves as sources of nutrition for penaeid prawns and other coastal invertebrates from Malaysia. Mar Biol 123:595–606
OzCoasts (2008) Australian Online Coastal Information [www.ozcoasts.org.au] Retrieved 30 January 2009
Parr JF, Taffs KH, Lane CM (2004) A microwave digestion technique for the extraction of fossil diatoms from coastal lake and swamp sediments. J Paleolimnol 31:383–390
Reavie ED, Hall RI, Smol JP (1995) An expanded weighted-averaging model for inferring past total phosphorus concentrations from diatom assemblages in eutrophic British Columbia (Canada) lakes. J Paleolimnol 14:49–67
Reid M (2005) Diatom-based models for reconstructing past water quality and productivity in New Zealand lakes. J Paleolimnol 33:13–38
Rosén P, Hall R, Korsman T, Renberg I (2000) Diatom transfer-functions for quantifying past air temperature, pH and total organic carbon concentration from lakes in northern Sweden. J Paleolimnol 24:109–123
Roubeix V, Becquevort S, Lancelot C (2008) Influence of bacteria and salinity on diatom biogenic silica dissolution in estuarine systems. Biogeochemistry 88:47–62
Roy PS, Williams RJ, Jones AR, Yassini I, Gibbs PJ, Coates B, West RJ, Scanes JP, Nichol S (2001) Structure and function of south-east Australian estuaries. Estuar Coast Shelf Sci 53:351–384
Saunders KM (submitted) Development of diatom-based inference models for assessing coastal water quality, natural variability and human impacts in southeast mainland Australia and Tasmania. J Paleolimnol
Saunders KM, Taffs KH (2009) Palaeoecology: a tool to improve the management of estuaries. J Environ Manag 90:2730–2736
Saunders KM, Hodgson DA, Harrison J, McMinn A (2008) Palaeoecological tools for improving the management of coastal ecosystems: a case study from Lake King (Gippsland Lakes) Australia. J Paleolimnol 40:33–47
Scanes P, Coade G, Doherty M, Hill R (2007) Evaluation of the utility of water quality based indicators of estuarine lagoon condition in NSW. Australia. Estuar Coast Shelf Sci 74:306–319
Siver PA (1999) Development of paleolimnological inference models for pH, total nitrogen and specific conductivity based on planktonic diatoms. J Paleolimnol 21:45–59
Smith SDA, Rule MJ, Harrison M, Dalton SJ (2008) Monitoring the sea change: preliminary assessment of the conservation value of nearshore reefs, and existing impacts in a high-growth, coastal region of subtropical eastern Australia. Mar Poll Bull 56:525–534
SoE (2006) State of the environment report. Australian Commonwealth Government Department of Environment and Heritage. Available at: http://www.deh.gov.au/soe/2006/
Stuiver M, Reimer PJ (1993) Extended 14C database and revised CALIB radiocarbon calibration program. Radiocarbon 35:215–230
Taffs K (2005) Diatoms of Northern New South Wales, Australia. Unpublished dataset, Southern Cross University, Australia
Taffs KH, Farago LJ, Heijns H, Jacobsen G (2008) A diatom-based Holocene record of human impact from a coastal environment: Tuckean Swamp, eastern Australia. J Paleolimnol 39:71–82
Tibby J, Reid MA (2004) A model for inferring past conductivity in low salinity waters derived from Murray River (Australia) diatom plankton. Mar Freshw Res 55:597–607
Vaalgamaa S (2004) The effect of urbanisation on Laajalahti Bay, Helsinki City, as reflected by sediment geochemistry. Mar Poll Bull 48:650–662
Valiela I, Geist M, McClelland J, Tomasky G (2000) Nitrogen loading from watersheds to estuaries: verification of the Waquoit Bay Nitrogen Loading Model. Biogeochemistry 49:277–293
Weckström K (2006) Assessing recent eutrophication in coastal waters of the Gulf Of Finland (Baltic Sea) using subfossil diatoms. J Paleolimnol 35:571–592
Whitmore TJ, Brenner M, Curtis JH, Dahlin BH, Leyden BW (1996) Holocene climatic and human influences on lakes of the Yucatan Peninsula, Mexico: an interdisciplinary, palaeolimnological apporach. Holocene 6:273–287
Witkowski A, Lange-Bertelot H, Metzeltin S (2000) Diatom flora of marine coasts 1. In: Lange-Bertelot H (ed) Iconographia Diatomologica, vol 1. Koeltz Scientific Books, Germany
Woodward CA, Shulmeister J (2006) New Zealand chironomids as proxies for human-induced and natural environmental change: transfer functions for temperature and lake production (chlorophyll a). J Paleolimnol 36:407–429
Acknowledgments
We thank Steve Logan, Rod Latimer, Ben Andrews and Peter O’Neill for their assistance with field work; Melissa Bautista for help with stable isotope analyses; Geraldine Jacobsen for assistance with 14C analyses; Damien Maher, Tracey Buss and Dirk Erler for comments on the manuscript. We also thank John Tibby, Krystyna Saunders and an anonymous reviewer whose comments improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Logan, B., Taffs, K.H., Eyre, B.D. et al. Assessing changes in nutrient status in the Richmond River estuary, Australia, using paleolimnological methods. J Paleolimnol 46, 597–611 (2011). https://doi.org/10.1007/s10933-010-9457-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-010-9457-x