Abstract
Fish are particularly sensitive to metabolites produced by Raphidophyte species and these have caused intensive fish kills in several countries. However, the effects on embryos of marine fish are unknown but could probably provoke an important impact on new stock recruitment and hence on fisheries. We evaluated the toxic effects of Chattonella spp. strains from the Gulf of California on three development stages of spotted sand bass (Paralabrax maculatofasciatus): embryo in segmentation stage (ES), embryo (EM), and eleutheroembryo (EL). Embryos (ES) were exposed to different cell concentrations of Chattonella subsalsa, Chattonella marina, Prorocentrum micans, and f/2 medium as control. Also, one set of embryos was tested with cell-free media for C. subsalsa cultures. Incubation lasted until embryos reached apterolarva phase. The ES was the most sensitive stage reaching 98% mortality with C. subsalsa, followed by cell-free media of C. subsalsa cultures, with mortalities close to 90%, whereas EM and EL phases presented mortalities below 60%. This work demonstrates that larval stages of P. maculatofasciatus are highly sensitive to short time exposure to all Chattonella spp. strains tested, that direct physical contact with cells is not required to cause mortality, and that the toxic effect is more pronounced when embryos hatch.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Raphidophyte species are single-celled microalgae that can form harmful algae blooms (HAB) and produce diverse toxic metabolites such as brevetoxin/brevetoxin-like compounds (PbTx) (Khan et al., 1995, 1996; Bourdelais et al., 2002; Marshall et al., 2003; Band-Schmidt et al., 2012), reactive oxygen species (ROS) (Tanaka et al., 1994; Marshall et al., 2002a; Hiroishi et al., 2005; Band-Schmidt et al., 2012), nitric oxide (NO) (Kim et al., 2006, 2008), high concentrations of free fatty acids (FFA) (Marshall et al., 2002b, 2003), hemagglutinin and hemolysin compounds (Fu et al., 2004; Kuroda et al., 2005; de Boer et al., 2009), and possibly some unidentified labile molecular compounds (protein or peptide) (Shen et al., 2010). The mode of action of these diverse toxic metabolites is not clear, but a synergic toxic effect of at least two of these metabolites of these species has been proposed (Marshall et al., 2003; Shen et al., 2010).
Fish are particularly sensitive to these metabolites, Raphidophyte species have caused intensive fish kills in several countries (Australia, Greece, Japan, New Zealand, Norway, Uruguay, and USA), causing important economic losses primarily in fish farming in tropical, subtropical, and temperate regions (Okaichi, 1989; Chang et al., 1990; Horner et al., 1997; Tiffany et al., 2001; Lars-Johan et al., 2002). Furthermore a significant impact has been observed on natural fish populations, but the loss has not been quantified accurately since a great number of organisms settle to the bottom and/or decompose rapidly (Bourdelais et al., 2002; Jugnu & Kripa, 2009).
The presence of Chattonella, Fibrocapsa, and Heterosigma genus has been reported along the Mexican coast (reviewed by Pérez-Morales & Band-Schmidt, 2011). In the Gulf of California, HAB’s of Chattonella have been associated with mortalities of fish, mollusk, crustaceans, and other marine animals (Barraza-Guardado, et al., 2004; Cortés-Altamirano et al., 2006).
Most toxicity studies have focused on the effect of Raphidophyte species on adult fish (Ishimatsu et al., 1996; Hishida et al., 1999; Lee et al., 2003; Marshall et al., 2003; Shen et al., 2011). However, the impact on marine fish embryos has remained unknown, and probably due to the higher sensitive of the early development stages the impact on their survival has important effects on new stock recruitment and hence on the fisheries.
The toxicity was determined in bioassays for study the impact of Chattonella spp. strains from the Gulf of California on the spotted sand bass embryos (Paralabrax maculatofasciatus). P. maculatofasciatus inhabits bays and estuaries in the eastern Pacific coast, from northern San Francisco Bay, California, USA to the coast of Sinaloa, Mexico, including the Gulf of California (Allen et al., 1995). This species was selected as a test organism, since culture conditions have already been established with spontaneous spawning broodstock, which are induced to gonadal maturity by photo-thermal control, without chemicals or manual treatments (Rosales-Velázquez, 1997); this fish has a high commercial value, and cohabits in the same geographical area where Chattonella blooms have been reported (Allen et al., 1995; Álvarez-González et al., 2001; López-Cortés et al., 2011).
Materials and methods
Algal growth
Strains
Strains of the Raphidophyceae Chattonella subsalsa (CSNAV-1), C. marina var. ovata (CMOPAZ-1), and C. marina var. marina (CSPV-3 and CSCV-1), and the dinoflagellate Prorocentrum micans were isolated from Gulf of California, Mexico. For details of Chattonella strains isolation see Band-Schmidt et al. (2012). All strains were maintained in 50 ml culture tubes with 25 ml of media, under controlled conditions at 23 ± 1° C, salinity of 35 ± 1‰, 12:12 light dark cycle and 150 μmol m−2 s−1 of illumination. The seawater was filtered under a low vacuum on Whatman GF/F filters sterilized and enriched with f/2 medium nutrients adding selenium (H2SeO3 to 10−8 M) and lowering the copper concentration (CuSO4 to 10−8 M).
Growth curves
Triplicate 100 ml batch cultures of each strain were grown in 250 ml polycarbonate culture tissue flasks and maintained in conditions described previously. Every second day, a subsample of 2 ml culture was fixed in Lugol’s iodine and counted in 1 ml Sedgewick-Rafter counting slide under an optical microscope (Carl Zeiss) until cultures reached the stationary phase of growth. Cell density was used to calculate exponential growth rates according to Guillard (1973). Cultures of Chattonella spp. and P. micans were doubled stepwise in volume from 25 to 2,500 ml, inoculating 10% of culture in modified f/2 medium (see above).
Fish culture
Capture of fish broodstock
Adult P. maculatofasciatus were collected in Bahía de La Paz using nylon lines and hooks with pieces of giant squid (Dosidicus gigas) as bait. Fish were transported to the laboratory, then were put in a fresh-water bath for 10 min (the change from saline to freshwater allowed to remove any parasites), after which the fish were distributed in four tanks of seawater at 23° C in a Closed System for Spawning Induction (CSSI).
Fish maintenance
Adults of P. maculatofasciatus were quarantined for 2 weeks to adapt them to captivity conditions and to recover from the stress of the fishing effort. They were placed in four tanks with a proportion of six females and four males in the CSSI. The seawater was previously filtered through two cartridge filters (5 and 1 μm pore size), disinfected with eight ultraviolet lamps (30 W), maintained at 23° ± 1° C, and kept under a 13:11 h light–dark cycle. From the second day of captivity the broodstock was fed silver mojarra (Eucinostomus spp.) to satiety as recommended by Rosales-Velázquez (1997).
Water quality
To sustain good water quality the CSSI tanks were cleaned regularly and dissolved oxygen (mg l−1 ± 0.001), temperature (°C ± 0.001), and salinity (‰ ± 0.001) were monitored daily. Ammonia (NH4 +, mg l−1 ± 0.01) and nitrite (NO2, mg l−1 ± 0.001) levels were monitored weekly according to Strickland & Parsons (1972).
Embryos for exposure experiments
The embryos of P. maculatofasciatus were obtained by natural spawning from the broodstock kept in captivity at the CSSI. Before starting the bioassays, embryos quality was verified by observing the morphology of the blastomeres based on the evaluation parameters proposed by Shields et al. (1997).
Embryo development time
The development time of P. maculatofasciatus from embryo to eleutheroembryo in segmentation phase at 23°C was determined, in order to find out the time when the observations would be conducted during the toxicity tests. The identification of each of the development stages was based on previous work done by Ortíz-Galindo (pers. comm.); a sample (30 individuals) was taken from embryos incubated and observed under a light microscope to identify the characteristics of each phase, the sample was returned and another sample was taken for the next observation to avoid drying due to constant exposure of the embryos to the microscope light. Once each phase of embryonic development was identified and the duration in time was registered, data was adjusted to a logistic growth model. The curve was calculated using CurveExpert Pro (data analysis software system, 2012), v. 1.6.5 (http://www.curveexpert.net).
Embryo toxicity test
The sensitivity of P. maculatofasciatus to Chattonella spp. strains was determined in three phases of embryo development: embryo in segmentation (ES), embryo (EM), and eleutheroembryo (EL) ending the exposure when the apterolarva phase was reached (Fig. 1).
Initial development stages of Paralabrax maculatofasciatus embryos used to evaluate the toxicity of Chattonella spp. strains. Full arrows indicate the bioassays with Chattonella subsalsa and cell-free media culture of C. subsalsa, empty arrow indicates the bioassays with C. marina var. ovata and C. marina var. marina
After fertilization, when the segmentation started in the embryos (ES), 1 embryo per well was transferred in 48 polystyrene microdilution well-plates (total of 48 embryos per plate) using a sterile Pasteur pipette, according to the methodology proposed by Panini et al. (2001). In each well 1 ml of different cell concentrations was added of Chattonella subsalsa (2, 4, 6, 8, and 10 × 103 cell ml−1), C. marina var. ovata (2, 4, 6, 8, and 10 × 103 cell ml−1), C. marina var. marina (4, 6, and 8 × 103 cell ml−1), the nontoxic dinoflagellate Prorocentrum micans (4 and 8 × 103 cell ml−1), and modified f/2 medium that was used as control, worth noting that only cell concentrations that were available were used in bioassays. Additionally, one set of embryos was tested with cell-free media of C. subsalsa cultures (4 and 8 × 103 cell ml−1) and modified f/2 medium as a control. For cell-free culture media of C. subsalsa were filtered through glass fiber filters (Whatman GF/F, VWR, Sweden, Ø 25 mm, and pore size of 0.47 μm) with a vacuum pump to 380 mmHg (15 inches Hg) of pressure.
The plates with embryos were incubated in triplicate with continuous light and kept in an acrylic incubator at 23° C. Experiments with EM and EL were evaluated only with C. subsalsa (2, 4, and 8 × 103 cell ml−1), which were placed with 200 μl of sterile seawater, adding their respective cell concentration to achieve the desired exposure phases (8 h and 22 h later, respectively) to reduce handling. The observation of the plates was performed at 8 h (embryo phase), at 22 h (eleutheroembryo phase), and afterward every 12 h. In each observation the survival of embryos was registered. Experiments were completed when the development phase changed from embryo to larvae (apterolarva) at 70 h approximately corresponding to the larval period, at this time the yolk sac is fully absorbed and exogenous feeding initiates (Balon, 2001). The experiments concluded at 70 h because larvae were not feed.
Probit analysis
This test is used to assess the level of stimulus that is necessary to obtain a response in a group of individuals of a population (Song & Lee, 2005; Embry et al., 2010). The Probit test is a regression model specialized of response variables binomial, transforms sigmoid responses in linear. The level of stimulus that causes a response in 50% of individuals from the population under study are characterization parameters for the bioassay denoted as LC50 (median lethal concentration) and LT50 (median lethal time) (Bedaux & Kooijman, 1994). Therefore, the LC50 and LT50 were calculated by Probit analysis.
Statistical analysis
All treatments were repeated at least five times with different batches of embryos, in order to avoid any effect by use only one; with the data set obtained, statistical tests were performed as follows: to detect significant differences in the percentage of embryos survival over time, the normality of the data was calculated using the Kolmogorov–Smirnov test and the homogeneity of variance with the Levin test. When the homogeneity test was accepted the means were compared using one-way or two-way statistical analyses of variance (ANOVA) with Tukey’s post hoc test. The minimum level of statistic significance was set at P < 0.05. Results are reported as average plus the standard error of the mean. Statistical analyses were done using STATISTICA (StatSoft, Inc., 2007) v. 8.0 (http://www.statsoft.com).
Results
Embryo development time
The development time of embryo in segmentation (ES), embryo (EM), and eleutheroembryo (EL) phases were identified in P. maculatofasciatus (Fig. 2). At time 0 of development, the first cleavage started with blastomeric division at 2 cells, this was identified as ES phase and considered until the morula form at 1 h. After 8 h the late gastrula was observed, which was considered as EM phase.
After late gastrula, optic and ootic vesicles, notochord segmentation, somites pigmentation, and the heartbeat were observed; hence hatching occurred, and at 22 h EL phase of development was identified which is characterized by a free embryo with yolk sac. At ~70 h was observed the change of development phase from embryo to larvae (apterolarva) showing the yolk sac fully absorbed, at this time exogenous feeding initiates.
The development curve calculated with the logistic model of growth for P. maculatofasciatus showed three intervals related to the three phases of embryonic development (ES, EM, and EL), these intervals reflect the main morphological and physiological changes observed in fish with indirect ontogeny (Balon, 2001; Falk-Petersen, 2005). These development stages are more suitable for toxicity testing since the evaluation of the effects of chemicals in the embryonic stages of fish are reliable alternatives to the commonly made with juveniles or adults (Belanger et al., 2010; Embry et al., 2010).
Embryo toxicity test with cells of Chattonella subsalsa
Embryo in segmentation
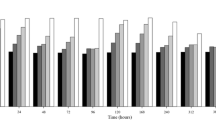
Significant mortality differences (98%) in embryos of P. maculatofasciatus were observed in treatments with C. subsalsa with concentrations from 6 to 10 × 103 cell ml−1 compared with the control (Fig. 3A). Mortality increased markedly from 6 to 91%, after 8 h of exposure, when the embryo is fully formed. Then a slight increase was observed from 34 h toward the end of the test. With the concentration of C. subsalsa of 4 × 103 cell ml−1, a gradual increase with maximum mortalities of 44 ± 8% at 70 h was observed. With the rest of the evaluated treatments of C. subsalsa (2 × 103 cell ml−1) and P. micans (4 and 8 × 103 cell ml−1) mortalities were lower than 25% at 70 h of exposure, presenting no significant difference with control treatments.
Mortality (%) of Paralabrax maculatofasciatus, A embryo in segmentation exposed to Chattonella subsalsa (CSNAV-1) (2, 4, 6, 8, and 10 × 103 cell ml−1), B embryo, and C eleutheroembryo exposed to Chattonella subsalsa (CSNAV-1) (2, 4, and 8 × 103 cell ml−1), as well as Prorocentrum micans (4 and 8 × 103 cell ml−1) and modified f/2 media for the three phases of embryo development as control
Embryo
During the embryo phase, mortality percentage differed at a concentration of C. subsalsa of 8 × 103 cell ml−1 (Fig. 3B) showed a higher mortality at 34 h (37 ± 2%). When eleutheroembryo phase is present, mortality increased until the end of test (47 ± 1%). Prorocentrum micans (8 × 103 cell ml−1) showed the lowest percentage of mortality (9 ± 4%) similar to the control treatment (11 ± 2%). The rest of the cell concentrations tested of C. subsalsa (2 and 4 × 103 cell ml−1) and P. micans (4 × 103 cell ml−1) showed no significant difference in the percentage of mortality compared with the control, with values below 20%.
Eleutheroembryo
During the eleutheroembryo phase the highest mortality (53 ± 5%) of P. maculatofasciatus was observed with the highest concentration of C. subsalsa (8 × 103 cell ml−1), followed by the exposure to 4 × 103 cell ml−1 of C. subsalsa with maximum mortalities of 39 ± 4%, both showing significant mortality differences compared with the control (Fig. 3C). With the rest of the evaluated treatments of C. subsalsa of 2 × 103 cell ml−1 and P. micans of 4 and 8 × 103 cell ml−1 the lowest mortalities (~20%) at the end of test were observed, which did not differ significantly from control treatments with a mortality of 12 ± 3%.
Embryo toxicity test with cell-free media of Chattonella subsalsa cultures
Embryo in segmentation
Significant mortality differences were observed (86 ± 4%) in embryo in segmentation of P. maculatofasciatus in treatments with cell-free media of C. subsalsa cultures with cell densities of 8 × 103 cell ml−1 (Fig. 4A), showing a constant linear increase in mortality from 0 to 70 h, compared with the exposure of cell-free media obtained from C. subsalsa cultures with a lower density (4 × 103 cell ml−1), showing an increase in mortality at 46 h (17 ± 3%) when the eleutheroembryo phase is present, maintaining a gradual increase until the end of test (51 ± 4%). Both treatments showed significant mortality differences compared with the control at 70 h.
Embryo
During the embryo phase the highest mortality (59 ± 6%) of P. maculatofasciatus was observed with cell-free media of C. subsalsa cultures with a cell density of 4 × 103 cell ml−1 (Fig. 4B), showing a linear increase in the mortality from the beginning (8 h) until the end of the test (70 h), followed by the exposure with cell-free media of C. subsalsa cultures of 8 × 103 cell ml−1 with maximum mortalities of 41 ± 6%, showing a slight increase in mortality from 46 h (14 ± 3%) to 70 h (41 ± 6%). Both treatments presented significant differences in the percentage of mortality compared with the control at the end of the test.
Eleutheroembryo
During the eleutheroembryo phase the mortality between treatments with cell-free culture media of C. subsalsa from 4 to 8 × 103 cell ml−1 (Fig. 4C), from the beginning of exposure (22 h) until 58 h, was similar. At the end of the test, the maximum mortalities observed in embryos of P. maculatofasciatus in cell-free media of C. subsalsa cultures of 4 and 8 × 103 cell ml−1, were 49 ± 5% and 35 ± 4%, respectively, which differed significantly from controls.
Embryo toxicity test with cells of Chattonella marina strains
When exposing P. maculatofasciatus from embryo in segmentation to eleutheroembryo, the concentrations of C. marina var. ovata of 4 to 8 × 103 cell ml−1 (Fig. 5A) showed significant increases in mortality (48, 86, and 58%, respectively) from 8 h to 34 h of exposure. The maximum mortality of 96% was obtained with a concentration of C. marina var. ovata of 6 × 103 cell ml−1. Higher concentrations of C. marina var. ovata (10 × 103 cell ml−1) exhibited the lowest mortality (6 ± 2%).
Mortality (%) of Paralabrax maculatofasciatus from embryo in segmentation to apterolarva, exposed to A Chattonella marina var. ovata (CMOPAZ-1) (2, 4, 6, 8, and 10 × 103 cell ml−1), B Chattonella marina var. marina (CSPV-3) (4, 6, and 8 × 103 cell ml−1), C Chattonella marina var. marina (CSCV-1) (4, 6, and 8 × 103 cell ml−1), Prorocentrum micans (4 and 8 × 103 cell ml−11), and modified f/2 media as control
Paralabrax maculatofasciatus exposed to concentrations of C. marina var. marina (CSPV-3) from 4 to 8 × 103 cell ml−1 showed an increase in mortality from 8 h to 34 h (Fig. 5B), obtaining the highest mortality (91 ± 8%) with a concentration of C. marina var. marina (CSPV-3) of 8 × 103 cell ml−1 at the end of test, which shown a significant difference with the control. Concentrations of C. marina var. marina (CSCV-1) from 4 to 8 × 103 cell ml−1 showed no significant difference in relation to the control, with mortality below 15%, which were lower than those obtained with P. micans (4 × 103 cell ml−1) and in the control (24 ± 6% and 23 ± 5%, respectively) (Fig. 5C). The gradual increase in mortality (3–14%,) of P. maculatofasciatus exposed to Chattonella began after 8 h of exposure and lasted the duration of the test.
Probit analysis
Median lethal concentration LC50
Of the three phases of embryonic development assessed (ES, EM, and EL) with C. subsalsa, the period of embryo in segmentation to apterolarva of P. maculatofasciatus was the most sensitive, with a LC50 of 3.2 × 103 cell ml−1 (Table 1). The less sensitive periods were the phases of embryo and eleutheroembryo to apterolarva with a higher LC50 (9.87 and 8.37 × 103 cell ml−1, respectively). With the rest of strains tested from embryo in segmentation to apterolarva, C. marina var. ovata showed the lowest LC50 (1.73 × 103 cell ml−1) while C. marina var. marina (CSPV-3) and C. subsalsa showed a similar LC50 of ~3 × 103 cell ml−1. The strain of C. marina var. marina (CSCV-1) had mortalities below 50%; therefore the LC50 could not be calculated. Test with cell-free media of C. subsalsa cultures was realized with two concentrations; therefore the LC50 could not be calculated.
Median lethal time LT50
The LT50 values in the period of embryo in segmentation (ES) to apterolarva when exposed to C. subsalsa from 6, 8, and 10 × 103 cell ml−1 were similar and lower (~20 h) than those found for EM and EL phases with a LT50 of 59 h 32 min and 54 h 31 min (respectively), at a cell concentration of 8 × 103 cell ml−1 (Table 2). In the test for ES with cell-free media of C. subsalsa culture of 8 × 103 cell ml−1, the LT50 (40 h 01 min) observed was low, whereas for EM and EL phases the LT50 was similar (61 h 45 min and 69 h 13 min, respectively) with cell-free media of C. subsalsa from cultures with a density of 4 × 103 cell ml−1. With C. marina var. ovata the LT50 at cell concentration of 6 × 103 cell ml−1 was nearly 1 day (23 h, 37 min), during the first 24 h development to apterolarva In contrast, with embryos exposed to C. marina var. ovata (2 × 103 cell ml−1) the LT50 was more than doubled (53 h, 37 min). The LT50 of embryos in segmentation phase was inversely proportional to the cell concentration of C. marina var. marina (CSPV-3), with a concentration of 8 × 103 cell ml−1 the LT50 was of 28 h, and with a concentration of 4 × 103 cell ml−1 the LT50 was ~42 h.
Discussion
Raphidophyte species have been reported off the Mexican Pacific and coast of Gulf of Mexico (reviewed by Pérez-Morales & Band-Schmidt, 2011). In this study we tested several Raphidophyte strains from the Gulf of California which have been studied extensively, and which produce brevetoxin-like compounds, superoxide radicals and cause lipid peroxidation (Band-Schmidt et al., 2012).
Worldwide studies (Ishimatsu et al., 1996; Hishida et al., 1999; Lee et al., 2003) reveal the toxic effect of Raphidophyte in juvenile and adult fish. Exposure of Seriola quinqueradiata to Chattonella cells is known to cause severe disturbances in respiratory, cardiovascular, and osmotic functions. It is recently confirmed that the exposure to C. marina could cause a series of alterations in Rhabdosargus sarba, e.g., in respiratory function, including a significant decrease in partial pressure (pO2) of the arterial oxygen, disturbance of gill lamella integrity, increase of plasma lactate, and depletion of plasma glucose (Tang et al., 2005; Shen et al., 2011).
Little is known about the potential impact on fish embryos that are less able to avoid the high cell densities during a HAB of Chattonella. There are no reports of toxicity bioassays to evaluate the toxicity of Raphidophyte on fish embryos. We show that the exposure to C. subsalsa causes significant mortalities on the different stages of the development of P. maculatofasciatus. Embryo in segmentation was the most sensitive development phase with 98% of mortality, whereas the other development phases of the embryo and eleutheroembryo were only 50% or less of mortality. Exposure of embryos to cell-free of C. subsalsa culture gave similar results, showing ES phase the higher mortalities, whereas EM and EL phases showed percentages of mortality <60%. According to the Probit model the LC50 of C. subsalsa is lower for embryo in segmentation (~3.2 × 103 cell ml−1) than for embryo and eleutheroembryo stages (higher to 8 × 103 cell ml−1), additionally the LT50 for the embryo in segmentation bioassays with concentrations from 6 to 10 × 103 cell ml−1, were three times shorter than those determined for EM and EL with a concentration of 8 × 103 cell ml−1. With the cell-free media of cultures at a density of 8 × 103 cell ml−1 of C. subsalsa the LT50 was shorter for ES (~40 h) than for EM and EL stages (~70 h) with cell-free media of cultures with a density of 4 × 103 cell ml−1. Both LC50 and LT50 show that the segmentation phase of embryos of P. maculatofasciatus is the most susceptible to the exposure of C. subsalsa with or without cell contact.
Embryonic development in teleost fish with indirect ontogeny presents physiological, morphological, and biochemical changes between one development phase and the other (Balon, 2001; Falk-Petersen, 2005; Belanger et al., 2010). It is possible that the differences in the mortality between the three phases of embryonic development of P. maculatofasciatus exposed to C. subsalsa (with or without cells), can be explained by the chorion surrounding the unhatched embryo, which is permeable to both salts and water fluxes, as well as to certain molecules (Eddy et al., 1990; Finn, 2007). Thus, such an ion exchange, this permeability will facilitate entry of toxic metabolites produced by C. subsalsa, making embryos more sensitive, resulting in an increase in mortality during the segmentation phase.
In addition to the effect caused by C. subsalsa cells on embryo phases, we have shown that C. subsalsa release under cell disturbance or disruption at least one substance which is toxic to embryos of P. maculatofasciatus, this substance may be related to the release of hemolytic compounds. Tests with cell-free culture media from different toxic marine microalgae have associated the release of hemolytic compounds, which are toxic to fish, as the case of the microalgae Karlodinium micrum, which has been associated with high mortality on Danio rerio, Cyprinodon variegatus, Lepomis macrochirus, Sciaenops ocellatus, Mugil cephalus, Ctenopharyngodon idella, and hybrids of striped bass (Morone saxatilis male × Morone chrysops female) (Deeds et al., 2002; Kempton et al., 2002).
Hemolytic activity has been detected in several Raphidophytes species, including Chattonella sp. (Fu et al., 2004; Kuroda et al., 2005; de Boer et al., 2009). In our work, the mortality percent of fish was variable for the different embryo phases and for both cell-free culture media and cellular concentrations tested. Thus, it is possible that hemolytic compounds released by Chattonella caused different percentages of mortality in embryo phases of P. maculatofasciatus.
Kuroda et al. (2005) observed that only broken cell suspensions of C. marina produce potent hemolytic activity, but intact cells suspension or its cell-free supernatant do not show hemolytic activity, which indicates that the hemolytic agents exist in certain intracellular spaces of C. marina due to cell rupture. These compounds are very light-dependent, because no hemolytic activities were observed in the dark. We perform all bioassays throughout continuous and constant light so that the effect of hemolytic activity could be constant.
Since the main development phase affected by C. subsalsa was from the embryo in segmentation to apterolarva, the remaining Chattonella strains were tested only during this phase of the embryo development. Mortality percentage for Chattonella marina var. ovata, and two strains of C. marina var. marina (CSPV-3 and CSCV-1) differed at each stage of embryonic development. Furthermore, at cell concentrations of Chattonella strains that caused very high mortalities, some embryos died without hatching (data not quantified). Similar effects have been reported for embryos of Oryzias latipes injected with brevetoxins PbTx-1 and -3, which have also been shown to induce developmental abnormalities, cardiovascular disfunction, and low survival (Kimm-Brinson & Ramsdell, 2001; Colman & Ramsdell, 2003). Recently, brevetoxin-like compounds, which have molecular structure similar to neurotoxins (Marshall et al., 2003), were detected in Raphidophyte strains from the Gulf of California (Band-Schmidt et al., 2012); it is possible that these compounds can affect the process of hatching in P. maculatofasciatus embryos.
Strains of C. marina var. ovata followed by C. marina var. marina (CSPV-3) presented the highest mortalities, which were proportional to cell concentration. However, the highest concentration tested of C. marina var. ovata (10 × 103 cell ml−1) gave inconclusive results. Such effects were also observed in studies on two fishes, Acanthochromis polyacanthus and Oryzias melastigma when exposed to various cell concentrations of C. marina, showing the highest toxicity during logarithmic phase of growth and a decrease in toxicity during the stationary phase (Marshall et al., 2003; Shen et al., 2010). Marshall et al. (2003) reported that A. polyacanthus exposed to concentrations of C. marina from 0.25 to 35 × 103 cell ml−1 caused mortality in 143 ± 8 min at concentrations above 8 × 103 cell ml−1 compared to fish exposed to lower cell concentrations (89 ± 6 min). It seems that less toxic compounds are generated at higher cell concentrations. Otherwise, generation of ROS in Chattonella is directly proportional to the cell density; maximum ROS generation has been quantified during the exponential phase of growth, decreasing in stationary phase (from >1.2 to about 0 of O2 − nmol 10−4 cell min−1). For details of Chattonella ROS generation see Kawano et al. (1996).
Is well know that raphidophytes generate superoxide anion (O2 −), hydrogen peroxide (H2O2), and hydroxyl radical (OH−); these compounds have been proposed responsible for their toxicity (Tanaka et al., 1994; Ishimatsu et al., 1996; Oda et al., 1997; Marshall et al., 2002a). However, some workers reject the involvement of ROS as the only cause of death on exposure to marine fish; they report that the concentrations of H2O2 produced by C. marina are not enough to cause lipid peroxidation in gills and erythrocytes of R. sarba for which they require some 50 times more H2O2 than that commonly generated to cause some effect in gills, noting that other mechanisms of toxicity are present in C. marina (Tang et al., 2005; Woo et al., 2006).
Some bioassays showed no negative effects on P. maculatofasciatus embryos since C. marina var. marina (CSCV-1) elicited a low mortality (15%), which is lower than in the controls (25%). Similar results have been observed for different strains of Fibrocapsa japonica where production of ROS and hemolytic compounds differ widely (Hemolytic value EC50 from 0.4 to 1.9 × 104 cell ml−1) (Oda et al., 1997; Fu et al., 2004; de Boer et al., 2009). Chattonella marina var. ovata caused an LC50 of 1.73 × 103 cell ml−1 in ES, lower than that calculated for C. subsalsa (3.2 × 103 cell ml−1). However, the LT50 values for C. marina var. ovata and C. marina var. marina (CSPV-3) were slightly higher than those calculated for C. subsalsa with similar cell concentrations, indicating that C. subsalsa can cause mortality in embryos in a short time. Although differences in toxic effects in this work could be strain specific. It has been reported that C. marina var. ovata is highly toxic to cultured marine fish (Pagrus major, Trachurus japonicus, and Seriola quinqueradiata) and that it produces high amounts of O2 − and H2O2, which are associated with fish kills (Hiroishi et al., 2005).
Toxicity in Chattonella species has also been related to the high production of free polyunsaturated fatty acids and their oxidation products. Marshall et al. (2003) proposed that toxicity of Chattonella could be explained by the production of free fatty acids (FFA). Small amounts of the FFA may be toxic to fish, since 0.2 mg l−1 of the fatty acid 20:5ω3 (eicosapentaenoic acid) in the presence of superoxide (O2 −) presents a LT50 of 83 min which is equivalent to 4 mg l−1 of the eicosapentaenoic acid (EPA) or ~1 × 103 cell ml−1 of a culture of Chattonella with both the EPA concentrations and the Chattonella density being toxic to fish.
HAB events of Chattonella antiqua, C. marina, and H. akashiwo have been associated with high mortalities of farmed fish species (Seriola quinqueradiata, S. rivoliana, Spondyliosoma cantharus, and Pagrus major) with fatty acids 16:4 and 18:4 identified as the main compounds that caused the mortalities (Okaichi, 1989). Recently Band-Schmidt et al. (2012) detected in Chattonella strains from the Gulf of California that the most abundant fatty acids were 18:4 ω-3 (18.2–21.8%) and 20:5 ω-3 (19.8–34.9%); the same FFA are associated with mortalities for A. polyacanthus reported by Marshall et al. (2003) Thus, these compounds may be involved in the mortalities observed in P. maculatofasciatus embryos.
Shen et al. (2010) proposed that the toxins produced by C. marina may be small and labile proteins or peptides, which cannot be extracted by traditional methods. The hypothesis of a synergistic effect between two or more compounds, principally ROS and FFA is perhaps the best option to explain the toxic effect of Chattonella species.
For all bioassays tested in this work, the embryo mortality observed in controls was approximately 20%; this is common under these experimental conditions (Álvarez-González et al., 2001). The effect of bacterial growth or lack of oxygen was not evaluated; however, the embryo mortality in controls was clearly lower than the embryos tested with Raphidophyte strains for the three phases of embryo development.
Last, this study indicates that different strains and cells concentrations of Chattonella from the Gulf of California cause high mortalities of embryos of P. maculatofasciatus and perhaps other larval organisms. Most notably, we determined the high mortality caused by Chattonella strains on the initial development of fish. However, more research is needed to understand the effects of Chattonella and its toxicity and elucidate the trigger factors that cause the high mortality observed in marine fauna.
Conclusion
This work demonstrates that larval stage of Paralabrax maculatofasciatus is highly sensitive to exposure of Chattonella subsalsa and C. marina strains from the Gulf of California, which reflected in high mortality in a short-exposure time. It also shows that direct physical contact with cells is not required to cause mortality, and that the toxic effect is more pronounced when embryos hatch. Paralabrax maculatofasciatus is a useful organism for conducting bioassays to assess the toxic effects of marine microalgae.
References
Allen, L. G., T. E. Hovey, M. Love & T. Smith, 1995. The life history of the spotted sand bass (Paralabrax maculatofasciatus) within the southern California Bight. California Cooperative Oceanic Fisheries investigations. Reports 36: 193–203.
Álvarez-González, C. A., J. L. Ortíz-Galindo, S. Dumas, S. F. Martínez-Díaz, D. E. Hernández-Ceballos, T. Grayeb-Del Alamo, M. Moreno-Legorreta & R. Peña-Martínez, 2001. Effect of stocking density on the growth and survival of spotted sand bass Paralabrax maculatofasciatus larvae in a closed recirculating system. Journal of the World Aquaculture Society 32: 130–137.
Balon, E. K., 2001. Saltatory ontogeny and the life-history model: neglected processes and patterns of evolution. Journal of Bioeconomics 3: 1–26.
Band-Schmidt, C. J., A. Martínez-López, J. J. Bustillos-Guzmán, L. Carreón-Palau, L. Morquecho, N. O. Olguín-Monroy, T. Zenteno-Savín, A. Mendoza-Flores, B. González-Acosta, F. H. Hernández-Sandoval & C. Tomas, 2012. Morphology, biochemistry, and growth of Raphidophyte strains from the Gulf of California. Hydrobiologia 693: 81–97.
Barraza-Guardado, R., R. Cortés-Altamirano & A. Sierra-Beltrán, 2004. Marine die-offs from Chattonella marina and Ch. cf. ovata in Kun Kaak Bay, Sonora in the Gulf of California. Harmful Algae News 25: 7–8.
Bedaux, J. J. M. & S. A. L. M. Kooijman, 1994. Statistical analysis of bioassays, based on hazard modeling. Environmental and Ecological Statistics 1: 303–314.
Belanger, S. E., E. K. Balon & J. M. Rawlings, 2010. Saltatory ontogeny of fish and sensitive early life stages for ecotoxicology tests. Aquatic Toxicology 97: 88–95.
Bourdelais, A. J., C. R. Tomas, J. Naar, J. Kubanek & D. G. Baden, 2002. New fish-killing alga in coastal Delaware produces neurotoxins. Environmental Health Perspectives 110: 465–470.
Chang, F. H., C. Anderson & N. C. Boustead, 1990. First record of a Heterosigma (Raphidophyceae) bloom with associated mortality of cage-reared salmon in Big Glory Bay, New Zealand. New Zealand Journal of Marine & Freshwater Research 24: 461–469.
Colman, J. R. & J. S. Ramsdell, 2003. The type B brevetoxin (Pb Tx-3) adversely affects development, cardiovascular function, and survival in medaka (Oryzias latipes) embryos. Environmental Health Perspectives 111: 1920–1925.
Cortés-Altamirano, R., R. Alonso-Rodríguez & A. Sierra-Beltrán, 2006. Fish mortality associated with Chattonella marina and Chattonella cf. ovata (Raphidophyceae) blooms in Sinaloa (Mexico). Harmful Algae News, IOC Newsletter on Toxic Algae and Algal Blooms 31: 7–8.
CurveExpert Pro, 2012. Version 1.6.5. http://www.curveexpert.net. Accessed 14 August 2012.
de Boer, M. K., M. R. Tyl, M. Fu, G. Kulk, G. Liebezeit, C. R. Tomas, A. Lenzi, J. Naar, E. G. Vrieling & M. van Rijssel, 2009. Haemolytic activity within the species Fibrocapsa japonica (Raphidophyceae). Harmful Algae 8: 699–705.
Deeds, J. R., D. E. Terlizzi, J. E. Adolf, D. K. Stoecker & A. R. Place, 2002. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae)—a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae 1: 169–189.
Eddy, F. B., M. R. Ward, C. Talbot & D. Primmett, 1990. Ionic movements across the chorion in newly shed salmon eggs (Salmo salar L.). Journal of Comparative Physiology. Part B. Biochemical, Systematic, and Environmental Physiology 159: 771–776.
Embry, M. R., S. E. Belanger, T. A. Braunbeck, M. Galay-Burgos, M. Halder, D. E. Hinton, M. A. Léonard, A. Lillicrap, T. Norberg-King & G. Whale, 2010. The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquatic Toxicology 97: 79–87.
Falk-Petersen, I. B., 2005. Comparative organ differentiation during early life stages of marine fish. Fish and Shellfish Immunology 19: 397–412.
Finn, R. N., 2007. The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquatic Toxicology 81: 337–354.
Fu, M., A. Koulman, M. van Rijssel, A. Lützen, M. K. de Boer, M. R. Tyl & G. Liebezeit, 2004. Chemical characterization of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae). Toxicon 43: 355–363.
Guillard, R. R. L., 1973. Methods for microflagellates and nanoplankton. In Stein, J. R. (ed.), Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge: 69–85.
Hiroishi, S., H. Okada, I. Imai & T. Yoshida, 2005. High toxicity of the novel bloom-forming species Chattonella ovata (Raphidophyceae) to cultured fish. Harmful Algae 4: 783–787.
Hishida, Y., A. Ishimatsu & T. Oda, 1999. Effect of environmental hyperoxia on respiration of yellowtail exposed to Chattonella marina. Fisheries Science 65: 84–90.
Horner, R. A., D. L. Garrison & F. G. Plumley, 1997. Harmful algal blooms and red tide problems on the U.S. west coast. Limnology and Oceanography 42(5): 1076–1088.
Ishimatsu, A., T. Oda, M. Yoshida & M. Ozaki, 1996. Oxygen radicals are probably involved in the mortality of yellowtail by Chattonella marina. Fisheries Science 62: 836–837.
Jugnu, R. & V. Kripa, 2009. Effect of Chattonella marina [(Subrahmanyan) Hara et Chihara 1982] bloom on the coastal fishery resources along Kerala coast, India. Indian Journal of Marine Sciences 38: 77–88.
Kawano, I., T. Oda, A. Ishimatsu & T. Muramatsu, 1996. Inhibitory effect of the iron chelator Desferrioxamine (Desferal) on the generation of activated oxygen species by Chattonella marina. Marine Biology 126: 765–771.
Kempton, J. W., A. J. Lewitus, J. R. Deeds, J. M. Law & A. R. Place, 2002. Toxicity of Karlodinium micrum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae 1: 233–241.
Khan, S., M. S. Ahmed, O. Arakawa & Y. Onoue, 1995. Properties of neurotoxins separated from a harmful red tide organism Chattonella marina. Israeli Journal of Aquaculture Bamidgeh 47: 137–141.
Khan, S., O. Arakawa & Y. Onoue, 1996. A toxicological study of the marine phytoflagellate, Chattonella antiqua (Raphidophyceae). Phycologia 35: 239–244.
Kim, D., K. Yamaguchi & T. Oda, 2006. Nitric oxide synthase-like enzyme mediated nitric oxide generation by harmful red tide phytoplankton, Chattonella marina. Journal of Plankton Research 28: 613–620.
Kim, D., Y. S. Kang, Y. Lee, K. Yamaguchi, K. Matsuoka, K. W. Lee, K. S. Choi & T. Oda, 2008. Detection of nitric oxide (NO) in marine phytoplankters. Journal of Biosciences and Bioengineering 105: 414–417.
Kimm-Brinson, K. L. & J. S. Ramsdell, 2001. The red tide toxin, brevetoxin, induces embryo toxicity and developmental abnormalities. Environmental Health Perspectives 109(4): 377–381.
Kuroda, A., T. Nakashima, K. Yamaguchi & T. Oda, 2005. Isolation and characterization of light-dependent hemolytic cytotoxin from harmful red tide phytoplankton Chattonella marina. Comparative Biochemistry and Physiology. C. Pharmacology, Toxicology and Endocrinology 141: 297–305.
Lars-Johan, N., D. Einar & D. Didrik, 2002. A new bloom of Chattonella in Norwegian waters. Harmful Algae News 23: 3–5.
Lee, K. S., A. Ishimatsu, H. Sakaguchi & T. Oda, 2003. Cardiac output during exposure to Chattonella marina and environmental hypoxia in yellowtail (Seriola quinqueradiata). Marine Biology 142: 391–397.
López-Cortés, D. J., C. J. Band-Schmidt, I. Gárate-Lizárraga, J. J. Bustillos-Guzmán, F. E. Hernández-Sandoval & E. J. Núñez-Vázquez, 2011. Co-ocurrence of Chattonella marina and Gymnodinium catenatum in Bahía de La Paz, Gulf of California (Spring 2009). Hidrobiológica 2: 185–196.
Marshall, J. A., M. Hovenden, T. Oda & G. M. Hallegraeff, 2002a. Photosynthesis does influence superoxide production in the ichthyotoxic alga Chattonella marina (Raphidophyceae). Journal of Plankton Research 24: 1231–1236.
Marshall, J. A., P. D. Nichols & G. M. Hallegraeff, 2002b. Chemotaxonomic survey of sterols and fatty acids in six marine raphidophyte algae. Journal of Applied Phycology 14: 255–265.
Marshall, J. A., P. D. Nichols, B. Hamilton, R. J. Lewis & G. M. Hallegraeff, 2003. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polyacanthus): the synergistic role of reactive oxygen species and free fatty acids. Harmful Algae 2: 273–281.
Oda, T., A. Nakamura, M. Shikayama, I. Kawano, A. Ishimatsu & T. Muramatsu, 1997. Generation of reactive oxygen species by raphidophycean phytoplankton. Bioscience, Biotechnology, and Biochemistry 61: 1658–1662.
Okaichi, T., 1989. Red tide problems in the Seto Inland Sea, Japan, 137-142. In Okaichi, T., D. M. Anderson & T. Nemoto (eds), 1st International symposium on red tides, 1987. Conference Proceedings, Takamatsu, Prefecture of Kagawa, Japan: 489.
Panini, E. B., C. C. Mylonas, S. Zanuy, M. Carrillo, J. Ramos & M. P. Bruce, 2001. Incubation of embryos and larvae of marine fish using microtiter plates. Aquaculture International 9: 189–195.
Pérez-Morales, A. & C. J. Band-Schmidt, 2011. Brevetoxins off the coasts of Mexico: potential effects on public health. CICIMAR Oceánides 26: 59–68.
Rosales-Velázquez, M. O., 1997. Effect of feeding on the spawning of the spotted sand bass Paralabrax maculatofasciatus (Teleostei: Serranidae) maintained in captivity. Thesis (MA.SC), Centro Interdisciplinario de Ciencias Marinas-IPN, La Paz, B.C.S. Mexico: 62 (in Spanish).
Shen, M., J. Xu, T. Y. Tsang & D. W. T. Au, 2010. Toxicity comparison between Chattonella marina and Karenia brevis using marine medaka (Oryzias melastigma): evidence against the suspected ichthyotoxins of Chattonella marina. Chemosphere 80: 585–591.
Shen, M., J. Xu, M. W. L. Chiang & D. W. T. Au, 2011. Unravelling the pathway of respiratory toxicity in goldlined seabream (Rhabdosargus sarba) induced by the harmful alga Chattonella marina. Aquatic Toxicology 104: 185–191.
Shields, R. J., N. P. Brown & N. R. Bromage, 1997. Blastomere morphology as predictive measure of fish egg viability. Aquaculture 155: 1–12.
Song, X. Y. & S. Y. Lee, 2005. A multivariate probit latent variable model for analyzing dichotomous responses. Statistica Sinica 15: 645–664.
StatSoft, Inc., 2007. STATISTICA (data analysis software system), version 8.0. http://www.statsoft.com. Accessed 20 October 2010.
Strickland, J. D. H. & T. R. Parsons, 1972. A Practical Handbook of Seawater Analysis. 2nd ed. Bull. 167. Journal of Fisheries Research Board of Canada, Ottawa: 310.
Tanaka, K., Y. Muto & M. Shimada, 1994. Generation of superoxide anion radicals by the marine phytoplankton organism, Chattonella antiqua. Journal of Plankton Research 16: 161–169.
Tang, J. Y. M., D. M. Anderson & D. W. T. Au, 2005. Hydrogen peroxide is not the cause of fish kills associated with Chattonella marina: cytological and physiological evidence. Aquatic Toxicology 72: 351–360.
Tiffany, M. A., S. B. Barlow, V. E. Matey & S. H. Hurlbert, 2001. Chattonella marina (Raphidophyceae), a potentially toxic alga in the Salton Sea, California. Hydrobiologia 466: 187–194.
Woo, S. P. S., W. Liu, D. W. T. Au, D. M. Anderson & R. S. S. Wu, 2006. Antioxidant responses and lipid peroxidation in gills and erythrocytes of fish (Rhabdosargus sarba) upon exposure to Chattonella marina and hydrogen peroxide: implications on the cause of fish kills. Journal of Experimental Marine Biology and Ecology 336: 230–241.
Acknowledgments
This research was supported by several institutional projects (SIP 2012-1152, 2013-0942), and by Consejo Nacional de Ciencia y Tecnología (CONACYT projects SEP 61226). APM is fellow of Dirección General de Asuntos de Personal Académico (DGAPA-UNAM); CJBS is fellow of the Comisión de Operación y Fomento de Actividades Académicas (COFFA) and the Programa de Estímulos al Desempeño de los Investigadores (EDI) of the Instituto Politécnico Nacional. Authors thank M. R. Pacheco-Chávez, J. M. Martínez-Brown, and J. I. Velázquez-Abunader for technical assistance. We also thank Dr. D. W. Johnson for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Rights and permissions
About this article
Cite this article
Pérez-Morales, A., Band-Schmidt, C.J., Ortíz-Galindo, J.L. et al. Mortality in the initial ontogeny of Paralabrax maculatofasciatus (Actinopterygii, Perciformes, Serranidae) caused by Chattonella spp. (Raphidophyceae). Hydrobiologia 722, 247–261 (2014). https://doi.org/10.1007/s10750-013-1707-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1707-2