Abstract
Poor larval survival is a bottleneck to commercial hatchery production of the tropical black-lip rock oyster, Saccostrea echinata. This study investigated the synergistic effects of water temperature and salinity on embryonic and larval development across each major larval life stage. Results showed that water temperature and salinity have a significant effect on embryonic development of S. echinata and that embryos did not develop below 17 °C and 14 psu. Survival was high (55–100%) across all treatments and larval stages, and shell size was used primarily to determine larval response to treatments. Larval shell size increased as water temperature and salinity increased, reaching optima at 32 °C and 23 psu for D-veligers (mean DVM 78.18± 0.85 μm), at 32 °C and 26 psu for umbonate larvae (mean DVM 183.40± 2.60 μm), and at 32 °C and 29 psu for eyed larvae (mean DVM 249.64± 3.22 μm). It is recommended that S. echinata embryos and larvae are cultured within 28–32 °C and at salinity optima for each larval stage: embryo development at 32 psu, D-veligers and umbonate larvae between 23 and 26 psu, and eyed larvae between 28 and 30 psu. This is the first investigation of the combined effects of water temperature and salinity on S. echinata and provides valuable information to accelerate commercial aquaculture of this tropical species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tropical black-lip rock oyster, Saccostrea echinata, has been identified as a species with potential for aquaculture because of its large size, local consumption, and valued flavour (Glude 1984; Fleming 2015). During the 1970s, several Pacific island countries trialled farming of this species based on wild spat supply (Coeroli et al. 1984), with significant programs in the Palau Islands and New Caledonia (Glude 1984). These trials ultimately failed because of insufficient recruitment of wild spat (Glude 1984) and, despite hatchery culture of S. echinata having been shown possible (Southgate and Lee 1998), hatchery output was poor compared to that of other commercial oyster species. Today, the success of pilot-scale S. echinata farms in northern and eastern Australia is drawing fresh attention to this underutilised tropical oyster.
Relatively poor survival in the hatchery is the key bottleneck to commercialisation efforts for S. echinata. Southgate and Lee (1998) reported low survival of 4.2–5.2% from D-veliger to competent pediveliger larvae. More recently, Nowland et al. (2018) reported low spat yield across three hatchery production runs, with 0.25–0.49% survival of pediveliger larvae to spat. There is a clear need for further research to determine optimal culture conditions for S. echinata larvae, as a basis for developing more appropriate hatchery culture protocols that will improve the success and reliability of hatchery production of this species.
The effects of water temperature and salinity on bivalve ontogeny have been described for many species (e.g. Doroudi et al. 1999; Nair and Appukuttan 2003; Huo et al. 2014; O’Connor et al. 2015). However, these variables do not influence ontogeny in isolation (Kinne 1964; Lough 1975), and it is therefore imperative to understand their synergistic effects on larval development. Information on the interactive effects of water temperature and salinity is available for some temperate oyster species. For example, His et al. (1989) investigated the interactive effect of water temperature and salinity on Crassostrea gigas larvae and reported high survival across treatments but significantly superior growth at 30 °C and a salinity of 30. Similarly, Dove and O'Connor (2007) investigated the combined effects of water temperature and salinity on Saccostrea glomerata during early ontogeny, reporting ontogenetic shifts in optima from 26 °C and salinity of 35 for embryonic development, to 30 °C and salinity of 34 for D-veliger, and 30 °C and salinity of 26 for umbonate larvae.
The broad distributions of temperate oyster species cover latitudinal distances that result in large variations in water temperature. Crassostrea virginica, for example, experiences water temperatures from 10 to 33 °C across major oyster breeding areas in coastal Louisiana, USA (Rybovich et al. 2016). In contrast, tropical oyster larvae live in comparatively temperature-stable marine environments. Peck et al. (2014) demonstrated that tropical invertebrates were less resilient to elevated temperatures than temperate species. This may result in narrower ranges of water temperature tolerance for tropical oysters, when compared to temperate species.
In contrast to the relatively stable water temperature regimes experienced by tropical rock oysters, they are often exposed to large and rapid salinity changes during the monsoonal wet season. This period of heavy freshwater run-off is often associated with increased phytoplankton abundance and is thought to drive reproduction in tropical oysters (Angell 1986). Rao (1950) demonstrated that Ostrea madrasensis do not spawn until salinity falls below 26, which is also the optimal salinity for embryonic development of this species. Southgate and Lee (1998) similarly established that reduced salinity is a critical spawning stimulus for S. echinata in northern Australia, where wet season inundation can reduce coastal salinity to 12, giving a summer range of 12–39 (Lindsay 1994). Such variability makes it impossible to predict salinity optima for hatchery production of many tropical oyster species, including S. echinata.
Studies into the interactive effects of water temperature and salinity on the larvae of tropical oyster species are limited. Lemos et al. (1994) recommend combined optima of 30 °C and salinity of 25–35 for greater larval growth of Crassostrea rhizophorae. Other studies have investigated the independent effects of water temperature and salinity on the larvae of tropical rock oysters (Dos Santos and Nascimento 1985; Tan and Wong 1996; Xu et al. 2011; Huo et al. 2014), including S. echinata (Coeroli et al. 1984). Coeroli et al. (1984) reported optima of 29 °C and salinity of 20–30, but advised that their results be considered with caution because of discrepancies; furthermore, the optimal salinity range reported is too wide to be useful for hatchery production. More information is required on the culture requirements of S. echinata as a basis for developing more appropriate hatchery culture protocols and improved hatchery production. This study addresses this knowledge gap by investigating the synergistic effects of water temperature and salinity on the embryonic and larval development of S. echinata across each major larval development stage.
Materials and methods
Experimental set-up and general methodology
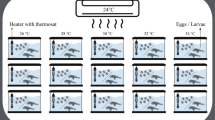
Aquaria containing S. echinata embryos and larvae used in the experiments were held within a series of individual, insulated (AIR-CELL Insulbreak 65, Australia) 20 L water baths whose water temperatures were maintained using thermostatically controlled immersion heaters (± 0.5 °C) (EHEIM thermocontrol 25, Germany). Water in each water bath was continually mixed using aeration. Hyposaline solutions (< 36 psu) were generated by dilution of seawater with demineralised freshwater. Replicate (n = 4) sets of 100 mL aquaria were maintained at 54 treatment crosses, six water temperatures (17, 20, 23, 26, 29, and 32 °C), and nine salinities (11, 14, 17, 20, 23, 26, 29, 32, and 36 psu). Replicate treatments were assigned to one of 4 blocks in a randomised complete block design. These experiment treatments were repeated for developing embryos (experiment 1), D-veliger larvae (2 days post hatch (dph); experiment 2), umbonate larvae (13 dph; experiment 3), and eyed larvae (20 dph; experiment 4).
Oyster spawning and larval culture
Saccostrea echinata broodstock (n = 49) were collected from South Goulburn Island, Northern Territory, Australia (11° 38′ 46″ S 133° 25′ 14″ E), and mass spawned following methods described by Nowland et al. (2018). The eggs from six females and sperm from 13 males were each pooled, and incubated in 10 L of 1 μm filtered sea water (FSW) at ambient temperature (28 °C) and salinity (36 psu) for 30 min. Two millilitres of sperm suspension was added to the egg incubation vessel for each litre of egg suspension (Utting and Spencer 1991), which was sufficient to ensure that at least one sperm was visible at the periphery of each egg. Fertilisation was confirmed by cell division (observing a cleavage plane) at 1 h post fertilisation (hpf), when fertilised eggs were washed over a 15-μm nylon mesh screen to remove excess sperm (O'Connor et al. 2008). Embryos were then counted and either stocked into experimental aquaria for experiment 1, or transferred into four 1000-L tanks filled with 1 μm FSW at a stocking density of 10 eggs mL−1, with gentle aeration. Larvae were maintained in four white, open top 1000-L tanks (OzPoly, Australia) through to the settlement so that they were available for use in later experiments (experiments 2–4). These larvae were cultured using the methods described by Nowland et al. (2018), in ambient FSW, with water temperature of 28± 1 °C and salinity of 36 psu.
Experiment 1: effects of varying water temperature and salinity on embryos
Embryos were transferred into each 100-mL aquaria (sterile plastic specimen containers) across all temperature/salinity treatments at a concentration of 10 eggs mL−1, where they were left for 48 h to develop to the D-veliger stage. After 48 h, further development was stopped by the addition of 10 mL of 5% buffered formalised seawater. The number of embryos that reached the D-veliger stage, the number of abnormal D-veligers (irregularity in velums or shell shape), and the dorsoventral measurements (DVM) and anteroposterior measurements (APM) of the shells of 30 randomly selected D-veligers in each replicate, was recorded using a Sedgwick-Rafter slide and a compound light microscope.
Experiments 2–4: effects of varying water temperature and salinity on larvae
Larvae of different life stages (D-veliger larvae (2 dph; experiment 2), umbonate larvae (13 dph; experiment 3), and eyed larvae (20 dph; experiment 4)) were collected at the appropriate time from the main larval culture tanks and transferred into experimental aquaria as detailed for experiment 1. Larvae were selected with the appropriate sized mesh screens (O'Connor et al. 2008) to ensure uniform stocking size at each developmental stage. The mean DVM (± SE) for larvae in each experiment was 65.50± 1.60 μm (experiment 2), 151.00± 2.60 μm (experiment 3), and 209.50± 4.50 μm (experiment 4), and rearing densities were 5 larvae mL−1 (experiment 2), 2.5 larvae mL−1 (experiment 3), and 2 larvae mL−1 (experiment 4). Water in each aquarium in all experiments was changed every 2 days. Larvae in experiment 2 were fed daily at a density of 15,000 cells mL−1 with an algae ration comprised of a 2:1:1 mixture of Chaetoceros calcitrans (Takano 1968), Tisochrysis lutea (Bendif et al. 2013), and Pavlova spp. (CS50, CISRO catalogue code) (O'Connor et al. 2008). Larvae in experiment 3 and experiment 4 were fed daily at a density of 25,000 cells mL−1 with 3:2:2:3 and 1:1:1:2 mixtures of C. calcitrans, T. lutea, Pavlova spp., and Chaetoceros muelleri (Lemmermann 1898), respectively. Experiments 2, 3 and, 4 were stopped after 4 days by the addition of 10 mL of 5% buffered formalised seawater. Shell dimensions (DVM and APM), abnormality and mortality (the number of empty shells) of 30 randomly selected larvae, in each replicate were measured and recorded as detailed above.
Statistical analyses
Transformation of the percentage development from embryo to D-veliger stage and survival data across all larval stages could not correct for heterogeneity of variance. Therefore, a Bray-Curtis similarity resemblance matrix was created, replacing the large number of zeros with a one. Then, the PERMANOVA function in PRIMER-E version 7 (Clarke and Gorley 2015) was used to test development and survival in a two-fixed-factor design (water temperature, salinity).
Shell dimension data across all experiments were pooled within treatments and a randomly selected subset of data generated for each treatment to provide sufficient power for analysis: D-veligers at 48 hpf n > 14 (experiment 1), D-veligers at 6 dph n > 107 (experiment 2), umbonate larvae at 17 dph n > 88 (experiment 3), pediveligers at 24 dph n > 110 (experiment 4). Data were subsequently analysed using a two-fixed-factor design (water temperature, salinity) ANOVA. Comparisons of DVM and APM from 20,806 larvae were made to determine their relationship and provide a correlation equation for the larvae of S. echinata. Analyses were completed and plots generated using R (R Core Team 2017).
Results
Effects of water temperature and salinity on embryonic development and larval survival
There was a significant interaction of water temperature and salinity on the development of S. echinata embryos (Pseudo-F = 14.45; df 40/215; P (perm) < 0.001). In general, development success (% development to D-veligers) significantly increased as water temperature and salinity increased (Fig. 1a). Optimum development occurred at 32 °C and 32 psu with 82.5% (± 6.14) of embryos developing to D-veligers, whilst embryos failed to develop below 17 °C and 14 psu (Fig. 1a). The interaction of water temperature and salinity was not significant for the survival of S. echinata D-veligers from 2 to 6 dph (Pseudo-F = 1.23; df 40/215; P (perm) > 0.05) or for umbonate larvae from 13 to 17 dph (Pseudo-F = 1.43; df 40/215; P (perm) > 0.05). Although not shown in Fig. 1 (because of software limitations), survival was high for D-veligers and umbonate larvae, > 72.5%± 8.54 and > 55% ± 25.11, respectively, across all treatment crosses. There was a significant effect of water temperature and salinity on the survival of eyed S. echinata larvae from 20 to 24 dph (Pseudo-F = 1.63; df 40/215; P (perm) < 0.05), where survival was above 80% (± 4.51) across all treatment crosses and highest (100%) at 32 °C and 36 psu (Fig. 1d).
The effect of water temperature and salinity on S. echinata development and survival. Contour figures indicate a the percent of embryos that developed to D-veligers 48 h post fertilisation, b percent survival of D-veliger 2–6 days post hatch (dph), c percent survival of umbonate larvae 13–17 dph, and d percent survival of eyed larvae 20–24 dph
The number of abnormalities in shell development was generally low with no discernible trend relating to variation in water temperature and/or salinity. Of the embryos that developed into 48 h D-veligers, abnormalities occurred across all treatments and ranged from 0.83% (± 0.96) to 10.83% (± 3.44). The occurrence of abnormalities was highest in D-veligers at 6 dph and ranged from 5% (± 0.96) to 36.67% (± 15.22). Umbonate and eyed larvae showed few abnormalities, occurring only in seven of the 54 treatment crosses, with a maximum of 4.16% (± 4.16) abnormality, and in 17 of the 54 treatment crosses, with a maximum of 5.83% (± 1.59) abnormality, respectively. It was notable that larvae cultured in the extreme treatment ranges (temperatures < 20 °C and salinities < 14 and > 36 psu) in this study appeared physiologically stressed and moribund and had poorly pigmented digestive systems.
The effects of water temperature and salinity on larval size
The independent effects of water temperature and salinity on the DVM of 48 h D-veligers were significant (F = 21.71; df 4/363; P < 0.001 and F = 6.94; df 5/363; P < 0.001, respectively); however, there was no significant interaction of water temperature and salinity on these larvae (F = 1.42; df 16/363; P > 0.05). There was a trend of increasing shell size for 48 h D-veligers as water temperature and salinity increased, with optima at 32 °C and 32 psu, corresponding with a mean larval DVM of 63.29 (± 0.78) μm (Fig. 2a). There was a significant result for the interaction of water temperature and salinity on the shell size of D-veligers from 2 to 6 dph (F = 16.91; df 40/5777; P < 0.001); umbonate larvae from 13 to 17 dph (F = 5.07; df 40/4751; P < 0.001); and eyed larvae from 20 to 24 dph (F = 10.47; df 40/5939; P < 0.001). The size of larvae increased as water temperature and salinity increased, reaching optima at 32 °C and 23 psu for D-veligers, with mean DVM of 78.18± 0.85 μm; 32 °C and 26 psu for umbonate larvae, with mean DVM of 183.40± 2.60 μm; and 32 °C and 29 psu for eyed larvae, with mean DVM of 249.64± 3.22 μm, before subsequently decreasing with further increases in water temperature and salinity (Fig. 2b, c, and d). Mean DVM and APM showed a positive linear correlation with a conversion equation of y = 0.926x + 15.272, y = DVM and x = APM (Fig. 3).
Discussion
Our results show that water temperature and salinity play significant synergistic roles in embryonic development of S. echinata. Development to D-veliger stage was less than 50% below 23 °C and 23 psu, and embryos failed to develop altogether below 17 °C and 14 psu. The highest rate of embryonic development to D-veliger stage of S. echinata was 82.50% (± 6.14) at 32 °C and 32 psu. Helm et al. (2004) reported a “normal” expected development rate of 30–85% to D-veliger stage in large-scale rearing of temperate bivalves, although O'Connor et al. (2008) expected development rates above 90% during hatchery production of S. glomerata. To achieve a commercially acceptable development yield above 80%, our results indicate that S. echinata hatcheries should incubate embryos at the reported optima of 32 °C and 32 psu. It is also notable that the results of this study were generated using very small experimental vessels (100 mL) and that improved development rates would be expected when culturing embryos and early larvae at a larger scale. For example, O'Connor and Lawler (2004) reported a maximum yield of 60% when culturing larvae of the Akoya pearl oyster, Pinctada imbricata, in experimental vessels (100 mL) compared to yields of around 95% commonly achieved in the hatchery.
High survival (55–100%) was recorded for all larval stages across treatments in this study, which contrasts with low survival of S. echinata larvae previously reported during hatchery culture (Southgate and Lee 1998; Nowland et al. 2018). In the current study, larvae that were cultured at extreme treatment ranges showed signs of physiological stress and appeared moribund (Helm et al. 2004). It is likely that these larvae were exposed to short-term, sub-lethal conditions, which can result in poor growth rates, reduced resilience, and decreased post-metamorphic survival (Davis and Calabrese 1964; Lucas 2008; Xu et al. 2011; Wang and Li 2018). Longer periods of exposure to these sub-lethal conditions may have long-term and possibly detrimental effects on S. echinata larval fitness and survival, and is a subject for further research.
Our results suggest an ontogenetic shift in optimal rearing conditions for S. echinata larvae. Maximal mean shell DVM (63.29± 0.78 μm) of 48 h D-veligers occurred at a water temperature of 32 °C and salinity of 32 psu; this was followed by a salinity shift to an optimum range of 23–26 psu that supported the greatest mean DVM of 78.18± 0.85 μm and 183.40± 2.60 μm, for older D-veliger (2–6 dph) and umbonate larvae, respectively, whilst eyed larvae obtained maximal mean DVM (249.64± 3.22 μm) in slightly higher salinities of 28–30 psu. Tan and Wong (1996) reported a similar ontogenetic shift in salinity optima of embryos and larvae of the tropical oyster, Crassostrea belcheri, where the optimal salinity ranges for embryos, larval development, and larvae settlement were 24–30, 12–24, and 12–18, respectively. This shifting in salinity optima during larval development, reported for both S. echinata and C. belcheri, contrasts to results reported from similar research with embryos and larvae of a tropical pearl oyster (Doroudi et al. 1999), where salinity optima did not differ greatly throughout larval development. Furthermore, O’Connor et al. (2015) reported that larvae of the edible oyster, Ostrea angasi, can be reared over a broad range of water temperatures but require a more defined salinity range. Water temperature and salinity optima reported for pearl oysters or temperate oyster larvae are not, therefore, a reliable basis for establishing hatchery protocols for tropical rock oyster species.
Seasonal freshwater run-off in the tropics results in strong vertical stratification that persists for weeks in many coastal areas (Williams et al. 2006; Hopley et al. 2007), and broad seasonal fluctuations in salinity characterise tropical inshore zones. For example, a salinity range of 4–36 was recorded at South Goulburn Island, in Australia’s Northern Territory from November 2014 to January 2016 (S. Nowland, unpublished data). This is the collection site for the broodstock used in the present study and indicates the broad range of salinities that embryos and larvae of S. echinata would be exposed to in this area. Lindsay (1994) reported that S. echinata in tropical north Queensland, Australia, reproduce during the rainy season, from December to February. The ontogenetic shift in salinity optima from embryos/early larvae to older larvae of S. echinata, reported here, supports a pattern of inshore, wet season spawning, followed by exposure of older larvae to higher salinity further offshore. This assumption holds when considering the defined salinity preference of the tropical pearl oyster, Pinctada margaritifera (Doroudi et al. 1999), which is a subtidal offshore species, compared to the shifting salinity optima of intertidal tropical rock oysters.
In the present study, larval size was positively correlated with increasing salinity up to a point (23 psu for D-veligers, 26 psu for umbonate larvae, and 29 psu for eyed larvae) from which further salinity increases resulted in declining larval shell size. However, no reduction in size was observed for the upper limits of water temperature in this study (32 °C) which was preferable across all life stages, and suggests that the extreme upper limit of thermal tolerance of S. echinata embryos and larvae was not tested. However, additional experimentation to determine the upper limits of thermal tolerance for this species is unlikely to be useful from a hatchery perspective. Higher water temperatures during larval culture may stimulate bacterial proliferation and conditions such as vibriosis, which are well known to cause mass mortality in bivalve hatcheries (Tubiash et al. 1965; Camacho et al. 2011). Additionally, whilst culture of S. echinata larvae at temperatures higher than 32 °C may increase growth rates, these conditions are also likely to increase larval food demands and decrease response time should there be a bacterial bloom (Helm et al. 2004; Lucas 2008). Hatcheries need to consider the trade-offs between optimising culture conditions for larvae growth, managing bacteria load and water quality, and the economics of production.
Culture parameters used previously in this laboratory for hatchery culture of S. echinata were 28 °C and 36 psu (Nowland et al. 2018), based on the early work of Coeroli et al. (1984) and Southgate and Lee (1998). The results of the present study have shown that lowering salinity during larval culture had significant positive effects on larval development. Other tropical rock oyster larvae that display a preference for low salinities include C. rhizophorae, where larval survival and total biomass gain was highest at salinities of 20–30 (Lemos et al. 1994); and Crassostrea hongkongensis, where low salinities, 15–23, were recommended to increase hatchery yields (Huo et al. 2014). The optimal culture parameters determined in the current study were used in a recent hatchery production run of S. echinata at the Darwin Aquaculture Centre, Australia, and the percentage of settled pediveliger larvae increased from a prior level of 0.49% (Nowland et al. 2018) to 4.26%. Furthermore, metamorphosis occurred 3 days earlier (18 dph) and was spontaneous in culture tanks. These demonstrable gains highlight the importance of species-specific optimisation of culture conditions to maximise larval performance and hatchery production.
Abnormalities during embryonic development of S. echinata in the current study were low, occurred across most treatment groups with no discernible treatment-based trend, and were within acceptable levels for hatchery culture of oysters (Gosling 2003; Helm et al. 2004). In contrast to our results, a high proportion of embryos of the tropical mangrove oyster, C. rhizophorae, incubated at salinities lower than 19 were reported to show abnormal development (97.6%), such as irregular or incompletely formed shells, at the D-veliger stage (Dos Santos and Nascimento 1985). Other factors may increase the occurrence of abnormalities when they interact with temperature. In a study on the interactive effects of ocean acidification and water temperature on the ontogeny of S. glomerata, Parker et al. (2009) reported abnormalities in D-veligers ranging from 4 to 90%, where the greatest percentage of larval abnormalities occurred at elevated partial pressure of carbon dioxide (pCO2) in seawater across all temperature treatments.
Mesh screens are arguably the most important tool for larval rearing of bivalve molluscs; they are fundamental for retaining and grading larvae (Gosling 2003; Lucas 2012). The relationship between mesh aperture, or pore size, and the minimum larval size retained, is species-specific and depends on larval shape (Helm et al. 2004). This study provided an opportunity to accurately determine shell dimensions of S. echinata throughout larval development, by examining the relationship between DVM and APM across 20,806 larvae. This provides hatcheries with a conversion equation and, more importantly, clearly defines appropriate screen mesh sizes for this species.
This is the first study to investigate the interactive effects of water temperature and salinity on embryonic and larval development of S. echinata, and to provide a correlation equation for DVM and APM for the larvae of this species. A revised culture protocol for S. echinata incorporating the results of this study is shown in Table 1. It is recommended that S. echinata embryos and larvae be cultured at a water temperature within the range 28–32 °C, but that salinity be varied according to the stage of larval development to maximise larval production; embryo development should be conducted at 32 psu, D-veligers and umbonate larvae should be cultured between 23 and 26 psu, and eyed larvae between 28 and 30 psu. Alternatively, a salinity range of 26–28 psu may be adopted in situations where phased salinity manipulation is not practical. Table 1 also includes recommended mesh screen sizes for D-veligers, umbonate larvae, and eyed larvae of S. echinata based on larval shell morphometric relationships determined in this study.
The results of this study provide valuable new information that will assist in developing improved commercial hatchery production protocols for S. echinata. However, further research is required to fine-tune other aspects of larviculture, such as larval stocking density, food ration, and diet composition, to further optimise hatchery culture protocols for S. echinata.
References
Angell CL (1986) The biology and culture of tropical oysters. International Centre for Living Aquatic Resources Management, Manila
Bendif EM, Probert I, Schroeder DC, Vargas C (2013) On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J Appl Phycol 25:1763–1776
Camacho EG, Dominguez GR, Armenta OOZ, Haws M, Supan J, Bajo LJA, Llamas GH, Valenzuela JEV, Juárez RNP (2011) Developing hatchery methods for the mangrove oyster, Crassostrea corteziensis for the Pacific Coast of Mexico. Final reports: investigations 2009–2011. Autonomous University of Sinaloa, Mexico, p 288
Clarke KR, Gorley RN (2015) PRIMER v7: user manual/tutorial. PRIMER-E, Plymouth
Coeroli M, De Gaillande D, Landret JP (1984) Recent innovations in cultivation of molluscs in French Polynesia. Aquaculture 39:45–67
Davis HC, Calabrese A (1964) Combined effects of temperature and salinity on development of eggs and growth of larvae of M. mercenaria and C. virginica. US Fish Wild Serv Fish Bull 63:393–404
Doroudi MS, Southgate PC, Mayer RJ (1999) The combined effects of temperature and salinity on embryos and larvae of the black-lip pearl oyster, Pinctada margaritifera (L.). Aquac Res 30:271–277
Dos Santos AE, Nascimento IA (1985) Influence of gamete density, salinity and temperature on the normal embryonic development of the mangrove oyster Crassostrea rhizophorae. Aquaculture 47:335–352
Dove MC, O'Connor WA (2007) Salinity and temperature tolerance of Sydney rock oysters Saccostrea glomerata during early ontogeny. J Shellfish Res 26:939–947
Fleming AE (2015) Improving business investment confidence in culture-aligned indigenous economies in remote Australian communities: a business support framework to better inform government programs. IIPJ 6(3):1–36
Glude JB (1984) The applicability of recent innovations to mollusc culture in the western Pacific islands. Aquaculture 39:29–43
Gosling E (2003) Bivalve molluscs biology, ecology and culture. Fishing News Books, Oxford
Helm MM, Bourne N, Lovatelli A (2004) Hatchery culture of bivalves. FAO Fisheries Technical Paper 471. FAO, Rome, p 203
His E, Robert R, Dinet A (1989) Combined effects of temperature and salinity on fed and starved larvae of the Mediterranean mussel Mytilus galloprovincialis and the Japanese oyster Crassostrea gigas. J Mar Biol 100:455–463
Hopley D, Smithers S, Parnell K (2007) The geomorphology of the great barrier reef. Cambridge University Press, New York
Huo Z, Wang Z, Liang J, Zhang Y, Shen J, Yao T, Su J, Yu R (2014) Effects of salinity on embryonic development, survival, and growth of Crassostrea hongkongensis. J Ocean Univ China 13:666–670
Kinne O (1964) The effects of temperature and salinity on marine and brackish water animals: II. Salinity and temperature-salinity combinations. Oceanogr Mar Biol Annu Rev 2:281–339
Lemmermann E (1898) Der grosse Waterneverstorfer Binnensee. Eine biologische Studie. Forschungsber Biol Stat Plön 6:166–205
Lemos MBN, Nascimento IA, Araujo MMS, Pereira SA, Bahia I, Smith DH (1994) The combined effect of salinity, temperature, antibiotic and aeration on larval growth and survival of the mangrove oyster, Crassostrea rhizophorae. J Shellfish Res 13:187–192
Lindsay SR (1994) The reproductive seasonality of two tropical rock oysters, Cassostrea echinata (Quoy and Gaimard) and C. sedea (Iredale), from North Queensland, Australia. Honours Dissertation, James Cook University
Lough RG (1975) A revaluation of the combined effects of temperature and salinity on survival and growth of bivalve larvae using response surface techniques. Fish Bull 73:86–94
Lucas JS (2008) Feeding and metabolism. In: Southgate PC, Lucas JS (eds) The pearl oyster. Elsevier, Oxford
Lucas JS (2012) Bivalve molluscs. In: Lucas JS, Southgate PC (eds) Aquaculture: farming aquatic animals and plants second edition. Blackwell Publishing, Sussex
Nair MR, Appukuttan KK (2003) Effect of temperature on the development, growth, survival and settlement of green mussel Perna viridis (Linnaeus, 1758). Aquac Res 34:1037–1045
Nowland SJ, O’Connor WA, Southgate PC (2018) Embyonic, larval, and early postlarval development of the tropical black-lip rock oyster Saccostrea echinata. J Shellfish Res 37:73–77
O’Connor S, Moltschaniwskyj N, Bolch CJS, O’Connor WA (2015) Assessment of temperature or salinity effects on larval development by catecholamine-induced metamorphosis of hatchery-reared flat oyster, Ostrea angasi (Sowerby 1871) larvae. Aquac Res 46:2501–2511
O'Connor WA, Lawler NF (2004) Salinity and temperature tolerance of embryos and juveniles of the pearl oyster, Pinctada imbricata Röding. Aquaculture 229:493–506
O'Connor WA, Dove M, Finn B, O'Connor S (2008) Manual for hatchery production of Sydney rock oysters (Saccostrea glomerata). NSW Department of Primary Industries, Port Stephens
Parker LM, Ross PM, O'Connor WA (2009) The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Glob Chang Biol 15:2123–2136
Peck LS, Morley SA, Richard J, Clark MS (2014) Acclimation and thermal tolerance in Antarctic marine ectotherms. J Exp Biol 217:16–22
R Core Team (2017) R: a language and environment for statistical computing. The R Foundation, Vienna
Rao KV (1950) Observations on the probable effects of salinity on the spawning, development and setting of the Indian backwater oyster, Ostrea madrasensis Preston. In: Proceedings of the Indian Academy of Science, p 231–254
Rybovich M, La Peyre MK, Hall SG, La Peyre JF (2016) Increased temperatures combined with lowered salinities differentially impact oyster size class growth and mortality. J Shellfish Res 35:101–113
Southgate PC, Lee PS (1998) Hatchery rearing of the tropical blacklip oyster Saccostrea echinata (Quoy and Gaimard). Aquaculture 169:275–281
Takano H (1968) On the diatom Chaetoceros calcitrans (Paulsen) emend. and its dwarf form pumilus forma nov. Bull Tokai Reg Fish Res Lab 55:1–7
Tan S, Wong T (1996) Effect of salinity on hatching, larval growth, survival and settling in the tropical oyster Crassostrea belcheri (Sowerby). Aquaculture 145:129–139
Tubiash HS, Chanley PE, Leifson E (1965) Bacillary necrosis, a disease of larval and juvenile bivalve mollusks. J Bacteriol 90:1036–1044
Utting SD, Spencer BE (1991) Laboratory leaflet 68: the hatchery culture of bivalve mollusc larvae and juveniles. Ministry of Agriculture, Fisheries and Food Directorate of Fisheries Research, Lowestoft
Wang T, Li Q (2018) Effects of salinity and temperature on growth and survival of juvenile Iwagaki oyster Crassostrea nippona. J Ocean Univ China 17:1–6
Williams D, Wolanski E, Spagnol S (2006) Hydrodynamics of Darwin harbour. In: Wolanski E (ed) The environment in Asia Pacific harbours. Springer, Dordrecht
Xu F, Guo X, Li L, Zhang G (2011) Effects of salinity on larvae of the oysters Crassostrea ariakensis, C. sikameaand the hybrid cross. Mar Biol Res 7:796–803
Acknowledgements
This study was conducted within the Northern Territory Governments “Tropical Rock Oyster Aboriginal Economic Development Program”. We thank the Warruwi community of Goulburn Island for supporting this project with the supply of broodstock. We also recognise the significant support provided by the Darwin Aquaculture Centre staff, in particular we thank Cameron Hartley, Paul Armstrong, Shannon Burchert, and Ella-Monique Mason for their contribution during hatchery culture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nowland, S.J., O’Connor, W.A., Penny, S.S. et al. Water temperature and salinity synergistically affect embryonic and larval development of the tropical black-lip rock oyster Saccostrea echinata. Aquacult Int 27, 1239–1250 (2019). https://doi.org/10.1007/s10499-019-00381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00381-7