Abstract
Diatoms have been routinely used to monitor the ecological status of French rivers. Since 2000, this monitoring has been carried out to comply with the Water Framework Directive. Ecoregions corresponding to the aquatic fauna and flora of rivers were originally defined on the basis of physical descriptors, designated “hydro-ecoregions” in France. Since, they have been simplified into diato-ecoregions for diatoms. In this study, data sets for benthic diatoms from rivers in north-eastern France were used to identify four main groups of diatom assemblages. The first such assemblage is found in small rivers with crystalline geologies in mountainous massifs with neutral to slightly acidic pH. The second assemblage occurs in small rivers flowing on limestone in lowland regions with an alkaline pH. The third corresponds to the assemblage found in large rivers in limestone lowland regions, and the fourth in highly mineralized rivers with high levels of chloride. Within each of these main assemblages, several communities have been characterized corresponding to different levels of pollution: from pristine to highly polluted. Multivariate analysis showed that the underlying geology was the main factor structuring the diatom assemblages, followed by the pollution level. River size had little impact on diatoms. Statistical tests showed that diato-ecoregions provided little information about diatom assemblages in the studied region, whereas there was a close correlation between diatoms and hydro-ecoregions. Nevertheless, these hydro-ecoregions did not account for the diatom assemblages in the highly mineralized rivers. These ecoregions could be further improved by merging the hydro-ecoregions of the crystalline mountainous massifs, all of which shared the same diatom assemblages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assemblages of benthic diatoms in rivers are influenced both by environmental descriptors that are not affected by human activities, such as the dominant geology of the river basin (Cantonati, 1998; Tison et al., 2004; Rimet et al., 2007), the altitude of the sampling site (Ndiritu et al., 2006; Rimet et al., 2007), the distance from the source (Potapova & Charles, 2002), and by others that can be affected by human activities, such as the organic load and nutrient concentration of the water (e.g., Van Dam et al., 1994; Kelly & Whitton, 1998; Kovács et al.; 2006). Within this framework, benthic diatoms have been recommended in the last several decades as an appropriate tool for pollution assessment in rivers (e.g., Round, 1991; Coste et al., 1991; Whitton, 1991; Whitton & Kelly, 1995), and have been routinely used throughout France since the late 1990s to indicate pollution levels of watercourses. Several diatom indices, such as the Specific Pollution-sensitivity Index (Cemagref, 1982) and the standardized Biological Diatom Index (Lenoir & Coste, 1996; Afnor, 2000, 2007), are routinely used for this purpose. The number of sites sampled has increased gradually year by year, especially in response to the strong impetus from the European Water Framework Directive (European Commission, 2000). Since the year 2000, this Directive has required an assessment of the ecological status of rivers using benthic diatoms.
Sampling benthic diatoms is an administrative obligation to comply with the requirements of the Water Framework Directive, but it also has the advantage of providing a mass of samples that has made it possible to develop large-scale diatom databases (e.g., Gosselain et al., 2005). These databases can be used to elucidate the ecology and macroecology (Passy, 2007) of benthic diatoms, and to compare the pollution assessments obtained using diatoms in different European countries (Kelly et al., 2008). Moreover, such databases provide a new way to explore the large-scale geographical distribution of diatom assemblages, as has been done for France (Tison et al., 2005b), Finland (Soininen, 2004a), United States (Potapova & Charles, 2002; Pan et al., 2004), and even a combination of several European countries (e.g., Kovács et al., 2006; Rimet et al., 2007). Most of the studies investigating the biogeographical distribution of diatom assemblages demonstrate the importance of taking into account the regional distribution of diatoms, as summarized in the review of Soininen (2007): the dispersal pattern of diatoms is far not ubiquitous.
Since 2000, the Water Framework Directive (European Commission, 2000) has taken on board this variable distribution, which had already been demonstrated for other aquatic organisms, such as macroinvertebrates (Illies, 1978), and now requires the ecological status to be evaluated on the basis of ecoregions. To do this, ecoregions have been defined in various European countries. Ecoregions reflecting the aquatic fauna and flora are designated as “hydro-ecoregions” in France (Wasson et al., 2002). The methodology for the definition of these “hydro-ecoregions” was based on the classification system B proposed in the Water Framework Directive. This system requires obligatory factors (altitude, geology, and geographical coordinates) and optional parameters (morphology, hydrology, and climatic parameters). In that framework, a total of 22 hydro-ecoregions of first level were defined in mainland France. In order to take into account more local particularities, 54 hydro-ecoregions of second level were defined from the first level hydro-ecoregions using the same methodology. A supplementary work was carried out to test the adequacy of the first level hydro-ecoregions to diatom assemblages: based on diatom samplings carried out on the entire national river network, 11 diatom communities in France have been identified using artificial neural network models and were geographically located (Tison et al., 2005a). The 22 hydro-ecoregions have been related to these 11 benthic diatom assemblages, and were simplified to provide five regions (Tison et al., 2005a), known as “diato-ecoregions.” These hydro-ecoregions and diato-ecoregions have already been tested at a national scale, but testing their effectiveness at a regional scale is also of major interest for water managers (Rimet et al., 2006), because this could provide greater detail at a regional scale. This has been done in a particular ecoregion of the United States (Weilhoefer & Pan, 2006), and has led to a critique and subsequent improvements of the ecoregional classification previously in use there. This approach is being considered by the water managers of the Meuse, Moselle, and Sarre basins, which are located in north-eastern France.
The study reported here had three objectives. The first was to identify and describe the diatom assemblages in the Meuse, Moselle, and Sarre basins. The second objective was to define the environmental parameters that structure diatom assemblages to the greatest extent, and the third was to assess the match between the proposed ecoregions (hydro-ecoregions and diato-ecoregions) and the diatom assemblages in this region. Several clustering techniques, simple descriptive statistics and multivariate analyses were used for this purpose.
Materials and methods
Study area
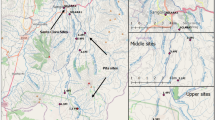
The sampling area is situated in north-eastern France (Fig. 1), and measures approximately 230 km east/west and 250 km north/south. It encompasses three major river basins. First, the Meuse basin, which is largely dominated by agriculture; the River Meuse flows for about 340 km in France, and then continues its course in Belgium. Second, the Moselle basin, which is mostly dominated by industrial activities; the River Moselle flows for 310 km in France; and then continues its course along the border of Luxembourg and Germany. Third, the Sarre basin is occupied by forestry in its upper part, but industrial and agricultural activities in its lower part. The River Sarre flows for 130 km in France, and then continues its course in Germany.
Location of the study area. (A) Main rivers, (B) first level hydro-ecoregions: Ardennes mountains (crystalline geology; 1), Limestone plateaus (2), Vosges mountains (crystalline geology; 3). Diato-ecoregions: region of low conductivities (1 and 3), region of high conductivities (2), (C) second level hydro-ecoregions
From 2000 to 2005, diatoms were sampled as part of the biomonitoring program for national river networks (RNB—Réseau National Bassin and Rref—Réseau de Référence). The samplings followed the Biological Diatom Index standard (Afnor, 2007), which incorporates the European standard for diatom sampling (Afnor, 2003), and were carried out once a year during the summer period (June–September). Benthic diatoms were collected from at least five stones from the lotic parts of the sampling sites. The upper surface of the stones was scrubbed with a toothbrush. The samples were fixed in 4% formaldehyde. In the laboratory, the diatom valves were cleaned using 40% hydrogen peroxide to eliminate organic matter, and with hydrochloric acid to dissolve calcium carbonate. Clean diatom frustules were mounted in synthetic resin (Naphrax©). At least 400 valves were counted and identified in each sample using a light microscope with a 1,000× magnification. Abundances of all observed taxa were expressed as relative counts. The identifications and counts were carried out by Krammer & Lange-Bertalot (1986, 1988, 1991a, b) and Krammer (2000, 2001, 2002).
The counts were carried out by different people, but to ensure a taxonomic homogeneity between them, the counts were systematically checked and, if necessary, identified and counted again by the author of this article.
Physical and chemical analyses were also carried out at the same sampling sites each month. Water temperature, dissolved oxygen, conductivity, and pH were measured in the field. For NO3 −, NO2 − NH4 +, Kjeldahl nitrogen (NKJ, quantification of organic nitrogen), total nitrogen (quantification of organic and mineral nitrogen), total phosphorus, PO4 3−, Na+, Ca2+, Cl−, K+, Mg2+, SO4 2−, biological oxygen demand (BOD), chemical oxygen demand (COD), dissolved organic carbon (DOC), suspended matter, and carbonates and bicarbonates, water samples were collected and analyzed in laboratory according to standard procedures (APHA, 1995). Since diatom samplings were carried out between June and September (low flow season), an average was calculated for each parameter between June and September to have a representative value for the site corresponding to the diatom sampling period. River width was measured in the field. For each sampling site, the Strahler rank was defined according to Strahler (1957). This rank gives a first approximation of the size of the river, and it was used for this purpose in this study. Strahler ranks were calculated using a geographical information system (GIS).
Data analysis
A cluster analysis was used to define groups of samplings with similar diatom assemblages; the Two Way Indicator Species Analysis (Twinspan analysis; Hill, 1979) was computed from the diatom counts (expressed as percentages) with the PcOrd software (McCune & Mefford, 2006). The pseudospecies cut-off levels used were those suggested by the software: 0, 2, 5, 10, and 20.
Several Multi Response Permutation Procedures (MRPPs; Biondini et al., 1985) were computed with the PcOrd software (McCune & Mefford, 2006) to select the best number of Twinspan groups. This analysis calculates an A-statistic, which is a descriptor of within-group homogeneity. This statistic varies between −1 and 1: if the A-statistic approaches 1, the groups are completely different; if the A-statistic approaches 0, the heterogeneity within groups equals what would be expected by chance; if the A-statistic approaches −1 the groups are homogeneous.
A geographical information system (MapInfo©) was used to locate the groups on the studied area. Average diatom assemblages were calculated for each Twinspan group by calculating the average abundance of each taxon in the group. Then, the indicator taxa were defined for each group using the Indicator Species analysis of Dufrêne & Legendre (1997) with the PcOrd software (McCune & Mefford, 2006). This analysis gives an idea of the “consistency” of each taxon for each group. This analysis yields an index calculated on the basis of the abundance and the faithfulness of each taxon in each group. If a taxon has a high Indicator Species analysis index in a group, then the group will be indicated by this taxon. Moreover, this index is tested by mean of a Monte-Carlo test; the indices presenting a significant P value (<5%) are shown for the corresponding taxa. This analysis calculates the indicator taxa of each group on the basis of its abundance and faithfulness in the group.
In order to determine the most structuring parameters for the 16 diatom assemblages, a forward selection and a discriminant analysis were carried out on 22 environmental parameters (bicarbonates, Ca, chlorides, conductivity, K+, Mg2+, pH, Na+, sulfate, NH4 +, NKJ, dissolved organic carbon, chemical oxygen demand, biological oxygen demand, NO2 −, O2, total nitrogen, NO3 −, PO4 2−, total phosphorus, river width, and altitude) and on the 16 groups. The Ginkgo program (De Caceres et al., 2007) was used to compute these analyses. The 22 environmental parameters were standardized before computing the analyses. The standardization was carried out by dividing the difference between the considered value and the average value of the parameter by its standard deviation. Finally, the groups were characterized using physical and chemical parameters, and box plot graphs.
In order to comply with the requirements of the WFD, a typological system has been developed in France, leading to the definition of several hydro-ecoregions on the basis of various parameters (altitude, geology, river basin size, river morphology, substrate, and climatology; Wasson et al., 2001, 2002). Several precision levels have been defined in these hydro-ecoregions: France includes 22 first-level hydro-ecoregions, which are sub-divided into 54 second-level hydro-ecoregions. These hydro-ecoregions were then simplified into 5 diato-ecoregions to match the benthic diatoms found in rivers by Tison et al. (2005a, b). The match between diatom assemblages and these various ecoregional classifications were assessed by means of MRPP.
For each sample, based on its diatom composition, the diatom index of water quality, known as the Specific Pollution-Sensitivity Index (IPS; Cemagref, 1982), was calculated. This index assesses the water quality according to five classes (IPS values: very bad quality < 5 ≤ bad < 9 ≤ intermediate quality < 13 ≤ good < 17 ≤ very good). The rivers in the studied area had Strahler ranks from 1 to 6; rivers with Strahler ranks of 1 and 2 were grouped together, as were rivers of ranks 5 and 6, also according to Chandesris et al. (2006). The water quality classes and the river size classes were also compared to the ecoregional classifications by means of MRPP.
Results
Floristic inventories
A total of 744 samplings were carried out from 2000 to 2005, and 567 taxa were identified. For the following statistical analyses and due to software limitations (size of the database which could be used), a selection of the most abundant taxa was carried out: the sum of the abundance of each taxon was calculated over the 744 samplings, and the 220 taxa with the highest sum values were selected.
Twinspan analysis and geographical location of the assemblages
The results of the MRPP computed on the Twinspan classification are shown in Fig. 2. There is a gradual increase in the A-statistic as the number of groups increases. However, the increase in the A-statistic value was weak above 16 groups, and therefore a total of 16 final groups was chosen. Figure 3 shows the results of the Twinspan classification (Table 1).
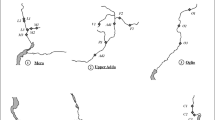
Groups 2 and 3 are plotted on a geographical map as shown in Fig. 4. A clear separation between these two groups can be observed: group 2 corresponds to the lowland limestone region, and group 3 to the crystalline and mountainous area. The final 16 groups are plotted on geographical maps as shown in Fig. 5, and can be recombined to form the two former groups: group 2 “diatom assemblages of limestone regions,” and group 3 “diatom assemblages of crystalline mountainous regions.”
Diatom assemblages on limestone geology
Diatom assemblages numbers 16–23 constitute this set; their average diatom assemblages and indicator taxa are shown in Annex 1 (Supplementary Material).
Only two of them can be considered to be of good and very good ecological quality, respectively. The first (group 16) correspond to small streams (Strahler rank of 3) near the source (median of 12 km), and its most indicator taxa are Achnanthidium biasolettianum, Denticula tenuis, and Encyonopsis microcephala. Several of these rivers are karst resurgences, with low PO4 3− and NH4 + concentrations (Fig. 6). The second (group 17) correspond to large river sites (Strahler rank of 5) rather far from the source (median distance of 218 km), and most of the sampling sites are located in the Meuse River. Achnanthidium cf. straubianum and Cymbella excisa are its most indicative taxa. Phosphorus concentrations at these sampling sites are relatively low (Fig. 6).
Four communities are characteristic of various specific levels of pollution (groups 18–21). The communities of groups 18 and 19 correspond to sampling sites with relatively high organic pollution (median DOC of 3.8 mg l−1 and 3.5 mg l−1 for groups 18 and 19, respectively). The river sizes vary in group 18, whereas they are mostly small to middle sized for group 19 (Strahler rank of 3 and 4). The indicator taxon for group 18 is Nitzschia sociabilis. The most indicator taxa of group 19 are Navicula tripunctata and Nitzschia dissipata.
The communities of groups 20 and 21 are exposed to a higher level of organic pollution (see NH4 + and PO4 3− in Fig. 6). Indicator taxon of group 20 are Platessa conspicua and Psammothidium lauenburgianum and Navicula veneta is the indicator taxon for group 21.
Finally, diatom communities of groups 22 and 23 correspond to rivers characterized by high electrolyte contents (see conductivity in Fig. 6), and in particular, high concentrations of Cl− (medians of 43 and 371 mg l−1 for groups 22 and 23, respectively) and of SO4 2− (medians of 105 and 133 mg l−1). The indicator taxa of these groups are Cyclotella atomus (group 22), Nitzschia frustulum, N. inconspicua, Navicula recens (group 23). Three kinds of rivers can be identified in these groups. The first kind, which in fact includes most of them, are large rivers (Strahler rank of 5) impacted by chloride discharges from soda extraction plants; the second are small rivers (Strahler rank of 3 and 4) with naturally high electrolyte contents, and the third are rivers downstream of coal mines discharging water with a high SO4 2− content.
Diatom assemblages on crystalline geology
The average diatom assemblages and the indicator taxa of this set of assemblages are shown in Annex 1 (Supplementary Material).
The communities of groups 24 and 25 correspond to organically polluted and lightly polluted rivers, respectively (median values for PO4 3− 0.13 and 0.03 mg l−1 for groups 24 and 25, respectively). The indicator taxa of these groups are Eolimna minima, Fistulifera saprophila, Sellaphora seminulum (group 24), and Fragilaria capucina var. vaucheriae (group 25).
The communities of groups 26 and 27 are present in transition geologies: these rivers have limestone bedrock, but their conductivities are low (median conductivity 182 μS cm−1), because they are influenced by their catchment areas, which are largely dominated by a crystalline geology. The rivers in group 26 are more polluted than those in group 25 (e.g., PO4 3− concentrations of 0.10 and 0.19 mg l−1 for groups 26 and 27, respectively). Nitzschia fonticola is the most indicative taxon for group 26 and Achnanthes subhudsonis is the most indicative taxon for group 27.
The last four communities (groups 28–31) correspond to very small to small rivers with low pollution levels (Strahler rank of 1–3). One of them (community 29) is constituted by a single sampling site with a low electrolyte (52 μS cm−1) content and pH (6.5). Its community is dominated by acidophilic taxa of the genus Eunotia (E. intermedia and E. tenella).
The communities of groups 28 and 30 correspond to small rivers (Strahler rank of 3), but can be distinguished in terms of pollution level, even if both of them are very lightly polluted. The sampling sites of group 30 are more polluted than those of group 28 (median of PO4 3− 0.05 and 0.1 mg l−1, respectively, for groups 28 and 30). The most indicator taxa of community 28 are Psammothidium oblongellum and P. subatomoides. Achnanthidium subatomus and Cocconeis placentula var. euglypta are the most indicator taxon of group 30.
Group 31 corresponds to very small, unpolluted rivers (Strahler ranks 1 and 2). Taxa such as Fragilaria gracilis, Gomphonema micropus, and Diatoma mesodon are the most indicative species of this assemblage.
Comparison of the classifications and of the importance of environmental parameters for diatom assemblages
Comparisons of the importance of different classifications (water quality assessed using the IPS diatom index, the diato-ecoregions, the hydro-ecoregions, and the river size assessed with the Strahler rank) for diatom assemblages are shown in Fig. 7. The hydro-ecoregion classification is the most informative for diatom assemblages; that of water quality is weaker but comparable. River size and diato-ecoregions are much less informative in explaining variability in diatom assemblages.
The results of the forward selection and the discriminant analysis are shown in Fig. 8. Fifteen parameters out of 22 were retained by the forward selection to compute the discriminant analysis. The results of the discriminant analysis show clearly that conductivity, pH, and carbonates are the most structuring parameters for all 16 diatom assemblages. Parameters related to pollution (NH4 +, total phosphorus, dissolved oxygen, and NKJ) are secondary, as are altitude and river width. Box plot graphs of the most important parameters are shown in Fig. 6.
Discriminant analysis carried out on 22 environmental parameters and the 16 final groups. A forward selection was realized to remove seven redundant parameters. The canonical coefficient of the environmental parameters on the first axis of the discriminant analysis (absolute value) are represented for the 15 lasting parameters
Discussion
Diatom assemblages of the north-eastern France
Diatom assemblage of the studied river basins (Meuse, Moselle, and Sarre basins) can be divided into two main diatom biotypes. The first one corresponds to rivers flowing on limestone regions and the second to rivers flowing on crystalline geology. Inside each type, several particular communities can be described and typified.
Groups 16–23 belong to these diatom assemblages of limestone region, some corresponding to low polluted situations (groups 16 and 17). Watercourses of group 16 are mainly small karstic unpolluted rivers and its most indicator taxa are regularly referenced as characteristic of pristine waters as Achnanthidium biasolettianum (Rimet et al., 2004) and Encyonopsis microcephala (Reichardt, 1997). Group 17 encompassed large rivers relatively weakly polluted and flowing on limestone in regions of low population density; its most indicator taxa Achnanthidium cf. straubianum and Cymbella excisa are considered as indicator of good water quality by diatom indices (e.g., Cemagref, 1982; Lenoir & Coste, 1996).
Four communities (18–21) correspond to rivers with various pollution levels and their indicator taxa are characteristic of polluted waters. For instance, Nitzschia sociabilis for group 18, Navicula tripunctata, Nitzschia dissipata for group 19 are considered as mesosaprobic by Van Dam et al. (1994); Navicula veneta for group 21 is considered as polysaprobic by Van Dam et al. (1994). Group 20 clusters small polluted rivers and its indicator taxa, Platessa conspicua and Psammothidium lauenburgianum, were also recorded in the same kind of small polluted streams in Luxembourg (Rimet et al., 2004).
Groups 22 and 23 gather rivers with high electrolyte contents, some of these rivers being naturally salty (salt rock in the river basin), some being impacted by soda industries or coal mines. The indicator taxa of these groups, Nitzschia frustulum, N. inconspicua, and Navicula recens are often observed in brackish waters (Witkowski et al., 2000). Several rare taxa observed in these rivers, such as Entomoneis paludosa, Pleurosira laevis, and Haslea spicula, clearly indicate high levels of Cl−, since they are species found in brackish water or in seawater (Witkowski et al., 2000).
Communities of groups 24–31 are present in rivers flowing on crystalline geology, or with a hydro-graphical basin largely dominated by such kind of geology. Some of them correspond to polluted situations as group 24 with indicator taxa (e.g., Eolimna minima and Sellaphora seminulum) considered as pollution tolerant according to various diatom indices (Cemagref, 1982; Lenoir & Coste, 1996).
Groups 26 and 27 regroup sampling sites on limestone bedrock, but with a basin dominated by crystalline geology. The water conductivity of these rivers is, therefore, quite low when compared to rivers flowing on limestone geology since the beginning of their course. The most indicator taxa of these groups are Nitzschia fonticola, a quite widespread taxon considered as mesosaprobic (Van Dam et al., 1994) and an invasive taxon (Coste & Ector, 2000) Achnanthes subhudsonis recorded in base poor rivers in Himalaya (Juttner et al., 2003).
Communities of groups 28 and 30 gather small rivers characterized by low pollution levels and most of their indicator taxa are referenced in waters of low conductivities, Psammothidium oblongellum, P. subatomoides, and Achnanthidium subatomus (Rimet et al., 2004), Cocconeis placentula var. lineata (Monnier et al., 2007), Gomphonema rhombicum (Iserentant & Ector, 1996).
Structuring importance of environmental descriptors for diatoms assemblages
The geology of the bedrock was an important environmental descriptor in explaining the heterogeneity and geographical distribution of diatom assemblages in the rivers of the studied area. This descriptor has also been shown to be determinant in other rivers: in Luxembourg (Rimet et al., 2004), in France (Tison et al., 2004), in European mountains (Cantonati, 1998; Rimet et al., 2007), and in the rivers of Oregon USA (Weilhoefer & Pan, 2006). Water conductivity, pH, and mineral content such as bicarbonate are directly linked to the composition of the dominant geology in the river basin. They were determinant in our study in explaining diatom communities, as already observed elsewhere (Potapova & Charles, 2003; Soininen, 2004b). In his review covering more than 12 studies of stream diatom assemblages, Soininen (2007) showed that major ion concentration is a primary structuring factor at the scale of a wide geographical range.
Nevertheless, several studies have found geology to be secondary when compared to pollution, as is the case in Spanish rivers (e.g., Leira & Sabater, 2005; Tornes et al., 2007), or in mid-Atlantic streams in the US (Pan et al., 1999). The reason given for the relative importance of the effects of geology and pollution on diatoms is that the human impact masks that of geology (Tornes et al., 2007). In our study, human impact appeared to be secondary even though land use pressures are very varied in north-eastern France: some areas are dominated by industrial activities and mining (the north of the region), others are agricultural (the west of the region, especially in the Meuse Basin), and others again are dominated by forestry (the east of the region, mainly in the north of the Vosges hydro-ecoregion). The most important environmental descriptors that represented pollution in the present study were NH4 + and total phosphorus. Diatom composition in polluted areas differed depending on the geological substrate of the basin. For instance assemblage 19, present in small to medium size polluted rivers on limestone bedrock, was dominated by Amphora pediculus, and had Navicula tripunctata and Nitzschia dissipata as indicator taxa. These two indicator taxa were never present at high abundances on crystalline geologies. Diatom assemblage 24, which is also present in small to medium size polluted rivers but on crystalline geology, was dominated by Eolimna minima and had Naviculadicata seminulum, Craticula molestiformis as indicator taxa. This means that the impact of geology can also be observed in polluted areas, whereas it is usually only under reference conditions that the diatom assemblages are clearly related to geology (Tison et al., 2005; Rimet et al., 2007). This means that our findings are quite in disagreement with the common idea that pollution homogenizes diatom assemblages (Pan et al., 2000). However, perhaps, we need to consider the possibility that geology could determine land use, and that this in turn influences water quality. A detailed study of the agricultural practices should be made, and related to the different diatom assemblages.
Some of the rivers in the studied area have naturally high conductivities. We found that these rivers regularly reached conductivities of 3,000–4,000 μS cm−1, and had chloride concentrations of 500 mg l−1 (e.g., River Seille and River Sanon). The taxonomic composition of their diatom assemblages is very different from that found in all the other rivers. These naturally salty rivers (dissolved salt in rivers comes from geological layers containing rock salt) were assigned to diatom assemblage 23, together with rivers impacted by mining activities for sodium exploitation. Mining activities discharge large quantities of CaCl2 and NaCl into these rivers (River Meurthe), artificially increasing conductivity over 2,500 μS cm−1, with a chloride concentration over 600 mg l−1, and a Na+ concentration of over 220 mg l−1. Upstream from these mining activities, conductivities are below 500 μS cm−1, the chloride concentration is below 20 mg l−1, and the Na+ below 12 mg l−1. Indicator taxa of this assemblage are typical of such high conductivities with Navicula recens and Nitzschia frustulum considered to be indicators of high electrolyte contents by Van Dam et al. (1994) and Krammer & Lange-Bertalot (1986, 1988). Some taxa, such as Pleurosira laevis, Haslea spicula, and Entomoneis paludosa, can be found in these rivers, but more rarely, and are usually considered as brackish and even marine taxa (Witkowski et al., 2000). A second diatom assemblage (No. 22) is found in water with high electrolyte contents, but is only present in rivers exposed to artificial saline pollution.
The impact of conductivity on freshwater rivers has already been thoroughly investigated, for instance, in Italy (Torrisi & Dell’Uomo, 2006; Torrisi et al., 2008), and a similar diatom flora was found. A diatom index assessing organic pollution and the chloride content of water has been developed in this context in Italy (Dell’Uomo, 2004) and also in Germany (Ziemann, 1971, 1982, 1999). Mining activities in the studied area were located within a precise, restricted zone, but they impacted on long stretches of river (see Fig. 1 for the locations of the mining activities). From the Na+ extraction facilities on the River Meurthe to the country’s boundary with Germany and Luxembourg (more than 100 km downstream), the water of the river is impacted by these high chloride concentrations. The extent of this impact in our study area explains why the structuring effect of Na+ on diatom assemblages was comparable to that of other environmental descriptors, such as the organic and trophic pollution.
Correspondence between ecoregions and diatom assemblages
Several ecoregional classifications have been proposed to describe the natural variety of diatom assemblages in France. The first classification was the hydro-ecoregional typology, which was constructed on the basis of geology, geomorphology, and climate Wasson et al. (2001). A total of three main hydro-ecoregions (first-order hydro-ecoregions) were present in the studied area (Vosges, Ardennes, and Limestone plateaus) plus seven second level hydro-ecoregions. These seven second level hydro-ecoregions were simplified and adapted to benthic diatoms by Tison et al. (2005a, b). Two diato-ecoregions were present in the studied area (regions of low conductivities and regions of high conductivities). The results clearly show that the seven hydro-ecoregions describe the diatom assemblage diversity well, even better than the water quality classes given by the IPS diatom index (Cemagref, 1982). These hydro-ecoregions also give a better description of diatom diversity than the river size (Strahler ranks). In other studies, river size is often observed to be the most structuring environmental descriptor for diatom assemblages (e.g., Pan et al., 2000; Potapova & Charles, 2002). This has led some authors to establish diatom biotypologies for bioassessment purposes (Descy & Coste, 1991). This complex descriptor does not have any direct influence on diatoms, but it is a proxy for several correlated parameters, such as slope or elevation, which directly influence water turbulence or temperature for instance. These last two parameters do have direct effects on specific diatom composition.
Even though these hydro-ecoregions seem to be appropriate, they do not account for the diatom assemblages of the naturally salty rivers (e.g., River Seille and River Sanon). In the hydro-ecoregions of Wasson et al. (2002), these particular rivers are classified as the “Limestone Plateaus” hydro-ecoregion, even though they show completely different diatom taxa, from these, found in the typical diatom assemblages of limestone plateaus. Therefore, a particular river type should be defined for these rivers to improve the hydro-ecoregion classification of the diatom assemblages in the Meuse, Moselle, and Sarre river basins.
A second comment can be made about separating the hydro-ecoregion of “Vosges” from that of “Ardennes”. Both these hydro-ecoregions are characterized by crystalline geologies (schist or granite), which confer low conductivities on the water of the rivers. These two mountainous regions display similar diatom assemblages, with indicator taxa characterizing low conductivities, such as Achnanthidium subatomus (Rimet et al., 2004), Gomphonema rhombicum (Iserentant & Ector, 1996), or slightly acidic pH, such as Psammothidium subatomoides (Manoylov, 2007). Therefore, to improve the ecoregional classification for diatom assemblages, these two regional entities could be merged.
The second ecoregional classification tested, with two diato-ecoregions, is a simplification of the seven hydro-ecoregions. Their accuracy for explaining the diatom assemblage diversity of the studied area was poor, and, indeed, performed least well of the four classifications tested. The diato-ecoregions are probably more suitable for explaining diatom assemblages at larger scale, such as the national scale.
Diatom assemblages in the area studied display considerable diversity. This diversity and the structure of these assemblages were mainly explained by the dominant geology of the catchments area. The structuring effect of geology on diatoms was greater than that of pollution, since diatom assemblages in polluted rivers still reflected the dominant geology of the basin. This finding contrasted with that of other studies showing increasing uniformity of diatom assemblages as the pollution level increases (e.g., Pan et al., 1999; Leira & Sabater, 2005; Tornes et al., 2007). A point to consider in further studies is whether geology influences land use, which in turn could influence the water quality and finally diatom assemblages of polluted areas. In several studies, river size appeared to be a determinant factor controlling the structure if the diatom assemblages (e.g., Descy & Coste, 1991; Molloy, 1992), and the downstream effect is one of the most structuring gradients on river diatoms (Potapova & Charles, 2002). In our study, this gradient did not appear to have greater structuring effect than geology or anthropic pressure. In our study, river size was assessed from the riverbed width and the Strahler rank, and detected rather weak impacts. A possible explanation for these disagreements between our findings and the other studies cited is the wide variety of geologies encountered in the area studied (sedimentary and crystalline geologies).
The step, in which the most important structuring parameters and the main diatom assemblages were identified, was necessary to understand the tests of the different ecoregional classifications. The diato-ecoregions tested gave rather poor concordance with the diatom assemblages of the studied area. They only matched the first two groups defined in the Twinspan analysis. The diato-ecoregions are probably more suitable for describing diatom assemblages at the national scale (France) than at a more local scale. The hydro-ecoregions provided good results, and gave a good idea of the distribution of diatom assemblages in the Meuse, Moselle, and Sarre catchment basins. Nevertheless, they could be improved by merging the hydro-ecoregions of the Ardennes and the Vosges massifs, which have the same geologies and river morphologies, and the same reference diatom assemblages. In contrast, another hydro-ecoregion should be created to take into account the rivers with high conductivities flowing over naturally salted geologies, which have very distinctive diatom floras, which are known and have been thoroughly investigated (Pierre, 1968, 1970, 2001).
References
Afnor, 2000. Norme Française NF T 90-354: Qualité de l’eau. Détermination de l’Indice Biologique Diatomées (IBD). Afnor: 1–53.
Afnor, 2003. NF EN 13946. Qualité de l’eau - Guide pour l’échantillonnage en routine et le prétraitement des diatomées benthiques de rivières. Afnor: 1–18.
Afnor, 2007. NF T90-354. Qualité de l’eau - Détermination de l’Indice Biologique Diatomées (IBD). Afnor: 1–79.
APHA, 1995. Standard Methods for Examination of Water and Wastewater, 19th ed. American Public Health Association, Washington, DC.
Biondini, M. E., C. D. Bonham & E. F. Redente, 1985. Secondary successional patterns in a sagebrush (Artemisia tridentata) community as they relate to soil disturbance and soil biological activity. Vegetatio 60: 25–36.
Cantonati, M., 1998. Diatom communities of springs in the Southern Alps. Diatom Research 13: 201–220.
Cemagref, 1982. Etude des méthodes biologiques quantitative d’appréciation de la qualité des eaux. Rapport Q.E.Lyon-A.F.Bassin Rhône-Méditerranée-Corse: 218 pp.
Chandesris, A., J. G. Wasson, H. Pella, H. Sauquet & N. Mengin, 2006. Typologie des cours d’eau de France métropolitaine. Cemagref BEA/LHQ, Lyon: 62.
Coste, M. & L. Ector, 2000. Diatomées invasives exotiques ou rares en France: principales observation effectuées au cours des dernières décennies. Systematic and Geography of Plants 70: 373–400.
Coste, M., C. Bosca & A. Dauta, 1991. Use of algae for monitoring rivers in France. In Whitton, B. A., E. Rott & G. Fridriech (eds), Use of algae for monitoring rivers. Institut für Botanik, Universität Innsbruck, Innsbruck: 75–83.
De Caceres, M., F. Oliva, X. Font & S. Vives, 2007. Ginkgo, a program for non-standard multivariate fuzzy analysis. Advances in Fuzzy Sets and Systems 2: 41–56.
Dell’Uomo, A., 2004. L’indice diatomico di eutrofizzazione/polluzione (EPI-D) nel monitoraggio delle acque correnti - linee guida. Agenzia Regional per la Protezione dell’Aambiente e per i servizi Tecnici; Agenzia Proviciale dell’Ambiente e per i servizi Tecnici: 101 pp.
Descy, J. P. & M. Coste, 1991. A test of methods for assessing water quality based on diatoms. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 24: 2112–2116.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
European Commission, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000 establishing a framework for Community action in the field of water policy. Official Journal of the European Communities 327: 1–72.
Gosselain, V., M. Coste, S. Campeau, L. Ector, C. Fauville, F. Delmas, M. Knoflacher, M. Licursi, F. Rimet, J. Tison, L. Tudesque & J. P. Descy, 2005. A large-scale stream benthic diatom database. Hydrobiologia 542: 151–163.
Hill, M. O., 1979. TWINSPAN: a FORTRAN Program for Arranging Multivariate Data in an Ordered Two-way Table by Classification of the Individuals and Attributes. Ecology and Systematics, Cornell University, Ithaca, NY.
Illies, J., 1978. Limnofauna europea, a checklist of the animals inhabiting European inland waters, with accounts to their distribution and ecology (except protozoa). G. Fischer Verlag, Stuttgart: 532 pp.
Iserentant, R. & L. Ector, 1996. Gomphonema rhombicum M. Schmidt (Bacillariophyta): typification et description en micrscopie optique. Bulletin Français de Peche et de Pisciculture 341(342): 115–124.
Juttner, J., S. Sharma, B. M. Dahal, S. J. Ormerod, P. J. Chimonides & E. J. Cox, 2003. Diatoms as indicators of stream quality in the Kathmandu Valley and Middle Hills of Nepal and India. Freshwater Biology 48: 2065–2084.
Kelly, M. G. & B. A. Whitton, 1998. Biological monitoring of eutrophication in rivers. Hydrobiologia 384: 55–67.
Kelly, M. G., C. Bennett, M. Coste, C. Delgado, F. Delmas, L. Denys, L. Ector, C. Fauville, M. Ferréol, M. Golub, A. Jarlman, M. Kahlert, J. Lucey, B. Ni Chathain, I. Pardo, P. Pfister, J. Picinska-Faltynowicz, J. Rosebery, C. Schranz, J. Schaumburg, H. Van Dam & J. Vilbaste, 2008. A comparison of national approaches to setting ecological status boundaries in phytobenthos assessment for the European Water Framework Directive: results of an intercalibration exercise. Hydrobiologia 621: 169–182.
Kovács, C., M. Kahlert & J. Padisák, 2006. Benthic diatom communities along pH and TP gradients in Hungarian and Swedish streams. Journal of Applied Phycology 18: 105–117.
Krammer, K., 2000. Diatoms of the European Inland Waters and Comparable Habitats. Volume 1. The genus Pinnularia. Gantner Verlag, Ruggell, Germany: 703 pp.
Krammer, K., 2001. Diatoms of the European Inland Waters and Comparable Habitats, Volume 2. Navicula sensu stricto, 10 Genera Separated from Navicula sensu stricto, Frustulia. Gantner Verlag, Ruggell, Germany: 526 pp.
Krammer, K., 2002. Diatoms of the European Inland Waters and Comparable Habitats, Vol. 3. Cymbella. Gantner Verlag, Ruggell, Germany: 584 pp.
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae 1. Teil: Naviculaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart: 876 pp.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart: 596 pp.
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae. 3. Teil: Centrales Fragilariaceae, Eunotiaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Die Süßwasserflora von Mitteleuropa, Bd. 2. Gustav Fischer Verlag, Stuttgart, Jena: 576 pp.
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gesamtliteraturverzeichnis Teil 1–4. In Ettl, H., G. Gärtner, J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart: 437 pp.
Leira, M. & S. Sabater, 2005. Diatom assemblages distribution in catalan rivers, NE Spain, in relation to chemical and physiographical factors. Water Research 39: 73–82.
Lenoir, A. & M. Coste, 1996. Development of a practical diatom index of overall water quality applicable to the French national water board network. In Whitton, B. A. & E. Rott (eds), Proceedings of Use of Algae for Monitoring Rivers II. Institut für Botanik, Universität Innsbruck, Innsbruck: 29–43.
Manoylov, K. M., 2007. On some Psammothidium species from rivers in the United States. Diatom Research 22: 317–327.
McCune, B. & J. Mefford, 2006. PC-ORD. Multivariate Analysis of Ecological Data, Version 5.18. MjM Software, Gleneden Beach, OR, USA.
Molloy, J. M., 1992. Diatom communities along stream longitudinal gradients. Freshwater Biology 28: 59–69.
Monnier, O., F. Rimet, M. Bey, R. Chavaux & L. Ector, 2007. Sur l’identité de Cocconeis euglypta Ehrenberg 1854 et C. lineata Ehrenberg–Une approche par les sources historiques. Diatomania 11: 30–45.
Ndiritu, G. G., N. N. Gichuki & L. Triest, 2006. Distribution of epilithic diatoms in response to environmental conditions in an urban tropical stream, Central Kenya. Biodiversity and Conservation 15: 3267–3293.
Pan, Y., R. J. Stevenson, B. H. Hill, P. Kaufmann & A. Herlihy, 1999. Spatial patterns and ecological determinants of benthic algal assemblages in mid-Atlantic streams, USA. Journal of Phycology 35: 460–468.
Pan, Y., R. J. Stevenson, B. H. Hill & A. T. Herlihy, 2000. Ecoregions and benthic diatom assemblages in Mid-Atlantic Highlands streams, USA. Journal of the North American Benthological Society 19: 518–540.
Pan, Y., A. Herlihy, P. Kaufmann, J. Wigington, J. van Sickle & T. Moser, 2004. Linkage among land-use, water quality, physical habitat conditions and lotic assemblages: a multi-spatial scale assessment. Hydrobiologia 515: 59–73.
Passy, S. I., 2007. Differential cell size optimization strategies produce distinct diatom richness-body size relationships in stream benthos and plankton. Journal of Ecology 95: 745–754.
Pierre, J. F., 1968. Etude hydrobiologique de la Meurthe. Contribution à l’écologie des populations algales. Bulletin de l’Académie Lorraine des Sciences 7: 261–412.
Pierre, J. F., 1970. Hydrobiologie du Sanon: contribution à l’étude des affluents de la Meurthe. Bulletin de l’Académie Lorraine des Sciences 9: 534–542.
Pierre, J. F., 2001. Catalogue des algues du Nord-est de la France et des régions attenantes 1959–2001. Bulletin de l’Académie Lorraine des Sciences 40: 1–100.
Potapova, M. & D. F. Charles, 2002. Benthic diatoms in USA rivers: distribution along spatial and environmental gradients. Journal of Biogeography 29: 167–187.
Potapova, M. & D. F. Charles, 2003. Distribution of benthic diatoms in US rivers in relation to conductivity and ionic composition. Freshwater Biology 48: 1311–1328.
Reichardt, E., 1997. Bermerkenswerte Diatomeenfunde aus Bayern. IV. Zwei neue Arten aus den Kleinen Ammerquellen. Berichte der Bayerischen Botanischen Gesellschaft zur Erforschung der Heimischen Flora 68: 61–66.
Rimet, F., L. Ector, H. M. Cauchie & L. Hoffmann, 2004. Regional distribution of diatom assemblages in the headwater streams of Luxembourg. Hydrobiologia 520: 105–117.
Rimet, F., D. Heudre, J. L. Matte, & P. Mazuer, 2006. Communautés de diatomées des bassins Moselle, Meuse et Sarre - Correspondance avec les Hydro-Ecorégions. Direction Régionale de l’Environnement – Lorraine, Metz, France: 51 pp.
Rimet, F., J. Goma, J. Cambra, E. Bertuzzi, M. Cantonati, C. Cappelletti, F. Ciutti, A. Cordonier, M. Coste, F. Delmas, J. Tison, L. Tudesque, H. Vidal & L. Ector, 2007. Benthic diatoms in western European streams with altitudes above 800 M: characterisation of the main assemblages and correspondence with ecoregions. Diatom Research 22: 147–188.
Round, F. E., 1991. Use of diatoms for monitoring rivers. In Whitton, B. A., E. Rott & G. Friedriech (eds), Proceeding of Use of algae for monitoring rivers I. STUDIA Studentenf orderungs-Ges.m.b.H, Innsbruck: 25–32.
Soininen, J., 2004a. Benthic diatom community structure in boreal streams. Distribution patterns along environmental and spatial gradients. Thesis, University of Helsinki, Finland: 46 pp.
Soininen, J., 2004b. Determinants of benthic diatom community structure in boreal streams: the role of environmental and spatial factors at different scales. International Review of Hydrobiology 89: 139–150.
Soininen, J., 2007. Environmental and spatial control of freshwater diatoms - A review. Diatom Research 22: 473–490.
Strahler, A. N., 1957. Quantitative analysis of watershed geomorphology. Transactions of the American Geophysical Union 38: 913–920.
Tison, J., J. L. Giraudel, M. Coste, Y. S. Park & F. Delmas, 2004. Use of unsupervised neural networks for ecoregional zoning of hydrosystems through diatom communities: case study of Adour-Garonne watershed (France). Archiv für Hydrobiologie 159: 409–422.
Tison, J., M. Coste, & F. Delmas, 2005a. Flores diatomiques des cours d’eau: proposition de valeurs limites du « Bon Etat » pour l’IPS et l’IBD. Ministère de l’Environnement et du Développement Durable, Direction de l’Eau: 20 pp.
Tison, J., Y. S. Park, M. Coste, J. G. Wasson, L. Ector, F. Rimet & F. Delmas, 2005b. Typology of diatom communities and the influence of hydro-ecoregions: a study on the French hydrosystem scale. Water Research 39: 3177–3188.
Tornes, E., J. Cambra, J. Goma, M. Leira, R. Ortiz & S. Sabater, 2007. Indicator taxa of benthic diatom communities: a case study in Mediterranean streams. Annales de Limnologie-International Journal of Limnology 43: 1–11.
Torrisi, M. C. & A. Dell’Uomo, 2006. Biological monitoring of some Apennine rivers (central Italy) using the diatom-based Eutrophication/Pollution Index (EPI-D) compared to other European diatom indices. Diatom Research 21: 159–174.
Torrisi, M. C., A. Dell’Uomo & L. Ector, 2008. Assessment of quality of the Apennine rivers (Italy) using the diatom indices: the River Foglia. Cryptogamie Algologie 29: 45–61.
Van Dam, H., A. Mertens & J. Sinkeldam, 1994. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherlands Journal of Aquatic Ecology 28: 117–133.
Wasson, J. G., A. Chandesris, H. Pella & Y. Souchon, 2001. Défintion des hydroécorégions françaises. Méthodologie de détermination des conditions de référence au sens de la Directive cadre pour la gestion des eaux. Cemagref report, Lyon, France: 35 pp.
Wasson, J. G., A. Chandesris, H. Pella & L. Blanc, 2002. Les hydro-écorégions de France métropolitaine. Approche régionale de la typologie des eaux courantes et éléments pour la définition des peuplements de référence. Cemagref report, Lyon, France: 190 pp.
Weilhoefer, C. L. & Y. D. Pan, 2006. Diatom assemblages and their associations with environmental variables in Oregon Coast Range streams, USA. Hydrobiologia 561: 207–219.
Whitton, B. A., 1991. Use of algae for monitoring rivers. Journal of Applied Phycology 3: 287.
Whitton, B. A. & M. G. Kelly, 1995. Use of algae and other plants for monitoring rivers. Australian Journal of Ecology 20: 45–56.
Witkowski, A., H. Lange-Bertalot & D. Metzeltin, 2000. Diatom flora of marine coast I. Iconographia Diatomologica 7: 1–925.
Ziemann, H., 1971. Die Wirkung des Salzgehaltes auf die Diatomeenflora als Grundlage für eine biologische Analyse und Klassifikation der Binnengewässer. Limnologica 8: 505–525.
Ziemann, H., 1982. Indikatoren für den Salzgehalt der Binnengewässer - Halobiensystem. In Breitig, G. & W. von Tümpling (eds), Ausgewählte Methoden der Gewässeruntersuchung; II: Biologische, Microbiologische und Toxikologische Methoden. Jena, Germany.
Ziemann, H., 1999. Salzgehalt. Bestimmung des Halobienindex. In Tümpling, V., W. Friedrich & G. Friedrich (eds), Biologische Gewässeruntersuchung. Jena, Germany: 309–313 pp.
Acknowledgments
The author thanks G. Demortier of the Rhin-Meuse Water Agency, and P. Mazuer, J. L. Matte, D. Heudre, and L. Moreau of the Diren-Lorraine (Regional Environmental Protection Agency of the French Ministry in charge of Environmental Protection). The English has been checked by Monika Ghosh. The article was improved by constructive remarks by two anonymous reviewers and Pr. Padisak Judit.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rimet, F. Benthic diatom assemblages and their correspondence with ecoregional classifications: case study of rivers in north-eastern France. Hydrobiologia 636, 137–151 (2009). https://doi.org/10.1007/s10750-009-9943-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9943-1