Abstract
The present study is based on the identification of benthic diatoms sampled along the Coastal Central Constantine in northeastern of Algeria. Our work aims to address relationships between environmental variables and characterize the spatial and temporal distribution of the diatom flora. Diatoms and samples for physicochemical analysis were collected from 26 sampling sites between May 2017 and August 2018. The number of species accounted for was 109 species in the Safsaf watershed, 117 taxa in the Kebir and 129 species in the watershed of Guebli which was the most diversified catchment. The great majority of diatom taxa were cosmopolitan species. Naviculaceae were the most abundant (47.87%). Our results demonstrated the main environmental variables controlling the diatom distribution in all the watersheds, including conductivity, and pollution gradients highlighted in Canonical Correspondence Analyses (CCA). The results of the Shannon diversity index (H’) showed that the species diversity observed in the sampled sites during summer is higher than that observed in spring and winter. During this season, mean Shannon diversity values were around H’ = 4.49, 3.98, and 4.17, respectively, while species richness varied between 13 and 34 across the three watersheds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diatoms are unicellular microalgae belonging to the phylum Bacillariophyta. They proliferate in rivers, colonizing nearly all suitable habitats, and have been widely used in studies related to water quality monitoring (Round 1991). They are the most common and diverse group of organisms found in many rivers and streams, making them vital components of these ecological systems. River benthic communities are influenced by a variety of factors, particularly ionic content, pH, dissolved organic matter, and nutrients, with populations responding differently depending on their particular environmental tolerances (Potapova and Charles 2003, 2005; Blanco and Bécares 2010).
Benthic diatom communities respond quickly to water disturbances, such as changing physicochemical conditions or pollution-related stressors. Due to their ability to respond to environmental changes, benthic diatoms have been used in aquatic bio-assessment over the years (Round 1991; Szczepocka and Szulc 2009; Solak and Àcs 2011; Taş et al. 2019), and for example they are widely used as indicators of water quality in lotic ecosystems (Pandey et al. 2017; Tan et al. 2017; Ozer et al. 2018). In various regions, numerous diatom indices have been developed as a tool to assess the ecological status of rivers (Prygiel and Coste 1996; Prygiel et al. 2002; Lavoie et al. 2006; Tison et al. 2008).
Most research on benthic diatoms and their relationships with ecological factors has been conducted in Europe and the United States (Potapova and Charles 2003; Rimet 2009; Blanco et al. 2013; Oğuz et al. 2020). To our knowledge studies on benthic diatoms in Algeria are limited. To date Baudrimont (1974) made significant contributions to the diatom flora from fresh and brackish waters of Algeria.
Further, to our knowledge, Algerian hydrosystems have been very little studied to date, particularly with regard to their ecological aspects using benthic diatoms. Studies by Chaïb et al. (2011), Chaïb and Tison-Rosebery (2012), and Nehar et al. (2015) who mainly focused on taxonomy and/or water quality assessment of rivers from northern Algeria, constitute a significant contribution to the diatom database of Algeria.
Chaïb et al (2011) described numerous diatom taxa in El Kebir-east watershed in the eastern coastal area of Constantine (northeast Algeria), reaching 322 identified species sampled over a span of 3 years at 23 sampling stations, where a marked longitudinal gradient was the key determinant of the distribution of these diatom communities. In addition, two more factors that affected the shape of diatom assemblages were related to water conductivity and also the man-made impact. In comparison, Nehar et al. (2015) reported 56 taxa collected from El-hammam stream at Mascara and estuary of Cheliff River at Mostaganem (northwest Algeria), most of them cosmopolitan species but with 10 taxa being recorded for the first time in the country. In their papers, those authors suggest a revision of autecological values for taxa because of the peculiar water quality context of the Algerian hydrosystems.
Recent works conducted on diatoms of some Algerian wetlands have recorded few numbers of diatom taxa compared to other studies, e.g., El Haouati et al. (2015) recorded 24 species in Reghaia lake in northern Algeria, and Chabaca et al (2020) reported 18 genera in Oubeira lake (northeast Algeria). The diatom flora of Chott-Chergui and Ain Dalia dam (northwest and northeast Algeria) was composed of 36 and 72 species, respectively (Heramza et al. 2021; Negadi et al. 2021), while the study of Draredja et al. (2019), carried out in Mellah lagoon (northeast of Algeria), reported 160 diatom taxa including 52 centric species and 108 pennate species. All of those authors focused their studies on monitoring the spatiotemporal evolution of diatom communities and on defining the main abiotic factors contributing to these dynamics.
The present study is a contribution to complete the Algerian database of diatoms inventoried, set up to date by the aforementioned works and the determinant factors of their distribution. Our investigation is the first of its kind to be carried out with a large sample size in space and time in the watershed of the Coastal Central Constantine, divided into three main watersheds (Safsaf, Kebir, and Guebli) which belongs to the Mediterranean region, characterized by a humid to sub-humid (subtropical) climate (Titi Benrabah et al. 2013).
The present paper aims to: (a) Evaluate the contribution of the environmental variables in the distribution of diatom species, and (b) Set up a checklist of the diatom species identified in all sampling sites of the Coastal Central of Constantine.
Materials and methods
Study area
As aforementioned our study was conducted throughout the Coastal Central Constantine, divided into three main watersheds (Safsaf, Kebir, and Guebli). In each watershed, samples were taken upstream and downstream of the main watercourse and its tributaries (Table 1).
The Coastal Central Constantine watershed is situated in the northern part of Algeria and encompasses the entire city of Skikda. The cities of Constantine and Guelma border the south, Annaba city borders the east, and Jijel and Mila cities border the west. The watershed belongs to the Mediterranean region, which is defined by a humid to sub-humid climate (Titi Benrabah et al. 2013), with rainfall varying from 650 to 1800 mm. The riverbed substratum includes pebbles, stones, silt, sand, gravel, and blocks. All three watersheds are characterized by various anthropogenic pressures: the Safsaf watershed receives the discharges of the industrial zone of Skikda city (location of a petrochemical complex), and urban domestic water inflows (Khelfaoui and Zouini 2010). The Kebir watershed is subject to significant anthropogenic activities, including deforestation, misuse of agricultural fertilizers, population growth, untreated discharges of industrial activities, and urban wastewaters (Bouleknafet and Derradji 2017). In the Guebli watershed, the pressures from agglomerations and urban sewage threaten also its water quality (Mecibah et al. 2019).
Diatom sampling
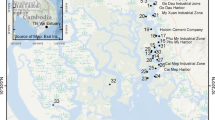
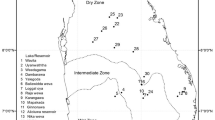
The sampling sites were selected according to their accessibility, the presence of suitable substrata, and the shade. The samples were collected across the three seasons: winter, spring, summer which encompasses the fall. A total of 26 sampling sites were monitored upstream and downstream of thirteen tributaries between May 2017 and August 2018 (Fig. 1).
Map of the study area indicating the location of the sampling sites from the Coastal Central Constantine. See Table 1 for sampling sites codes
Diatoms were collected together with water samples for physicochemical analyses. The samples were collected and treated according to the standardized (Frensh) method NF T90-354 (AFNOR 2007) by scraping a total area of 100 cm2 of the upper substrate surface (rocks, pebbles, and stones) using a small brush. At least 5 stones or small blocks were scrapped, then the diatom suspensions were poured and preserved in bottles containing 5% formaldehyde. In the laboratory, samples were digested by heating in a sandbox using a few drops of hydrogen peroxide (H2O2 30%) to remove any existing organic matter, in addition to hydrochloric acid to eliminate carbonate matter. For samples with high organic content, few ml of nitric and/or sulfuric acids are added to improve the mineralization of our samples. This step is followed by a series of centrifugations and rinsing until a neutral pH was obtained. Permanent slides were prepared from cleaned diatom frustules, mounted, and conserved using Canada Balsam (IR = 1.55). Diatoms were identified under light microscopy (Optika® BX60, × 1000, Ponteranica - BG, Italy). From each permanent slide, a minimum of 400 valves were identified and counted. Taxa identification was accomplished at the species-level in accordance with the works of Hofmann et al. (2011), Blanco et al. (2010), and Krammer and Lange-Bertalot (1988–1991).
Physicochemical and environmental parameters
Water samples were collected at mid-depth, against the stream, and well away from the river bank. Three samples were collected for each sampling site to obtain a representative sample of water quality. Water temperature, flow velocity, and water depth were measured in situ, together with dissolved oxygen (DO, HI 9146 oxymeter, HANNA, Rhode Island, USA), and water conductivity (ORION 3-STAR conductimeter Thermo Scientific, Waltham, Massachusetts, USA). Total nitrogen (Nt) and phosphates (Pt) were subsequently determined in the laboratory using a photometer (UV-1700 pharmaSpec, SHIMADZU, Tokyo, Japan), biochemical oxygen demand (BOD5, Oxi Top Box®BOD-meter type, Xylem Inc., Washington, D.C., USA), whereas chlorides and chemical oxygen demand (COD) were measured using volumetric dosage methods according to the American Society for Testing Materials (ASTM).
Data analyses
Statistical analyses
A Corrplot analysis was carried out in OriginPro2021 (Seifert 2014) to identify highly significant Pearson correlations (p < 0.001) between environmental variables including temperature, velocity, altitude, dissolved oxygen (DO), water conductivity, total nitrogen (Nt), phosphates (Pt), biochemical oxygen demand (BOD5), chlorides (Cl), and chemical oxygen demand (COD).
Agglomerative Hierarchical Clustering was carried out using the R package pvclust (Suzuki and Shimodaira 2006) version i386 4.1.1 (R Development Core Team 2020) with the Ward method (Ward.D2, Murtagh and Legendre 2014, 2011). This method shows groups of sampling sites, each resulting in a cluster including a group of sites based on their indicator species.
This is a method of arranging factors into homogeneous groups which share the same information and are strongly related to each other (Chavent et al. 2012). By summing up the clusters at various levels, and looking for similarities and differences, each object or data point is treated as a single individual cluster at the very beginning (Vijaya et al. 2019). The algorithm uses comparison metrics (for example, distance metrics) to compare the clusters and determine their similarities and differences, and the two most similar clusters or data points are merged together (Vijaya et al. 2019). P values were calculated using the IndVal Analysis (Monte Carlo permutation tests McCune and Grace 2002) under R using the package Indicspecies (De Cáceres and Legendre 2009) to obtain diatom indicator species for each group of sampling sites resulting from the cluster analysis.
A preliminary CA showed that gradient length is > 2 and thus an unimodal response of species is assumed and that is why we chose the unimodal CCA method. Canonical correspondence analysis (CCA) is a multivariate method of determining the relationships between biotic assemblages and abiotic variables. The method is intended for the extraction of ecological gradients from environmental data. The gradients serve as the foundation for describing and visualizing differential habitat preferences among taxa using an ordination diagram (ter Braak and Verdonschot 1995). CCA was performed on the environmental parameters cited above using PAST v. 4.03 (Hammer et al. 2001). Variables were log-transformed (log (1 + x)) for species abundances to avoid log (0) values. Two environmental variables were considered for CCA analysis as independent factors: ‘seasons’ and ‘pollution’ are expressed as multistate variables. ‘Seasons’ were converted into numeric values in R using combined functions as.character/as.numeric (spring = 1, summer = 2, winter = 3). The factor ‘Pollution’ was first calculated as an average value of the variables Nt, Pt, BOD5, and COD, then classified as a numeric value ranging from heavily impacted sites (100) to reference sites (10), with higher values meaning higher degree of pollution. Five (5) classes were defined (heavily impacted = 100, bad quality = 80, moderately impacted = 50, slightly impacted = 30, no impact = 10). Rare species (less than five individuals per site or in less than 3 sites) were eliminated for multivariate analyses.
Diatom indices and BDI
Diversity indices were computed also for each sampling site (Shannon’s diversity index (H’'), Evenness (E), species richness), besides the diatom-based water quality index BDI calculated using Omnidia v. 5.3 (Lecointe et al. 1993). Shannon Weaver Diversity Index (H’) is the most commonly and widely used method to compute the biotic diversity of a given assemblage, determined by the following Eq. (1) (Shannon and Weaver 1949).
where pi (the relative abundance of speciei) = (ni/N).
Species evenness (E) is the ratio of observed (H’) to maximum diversity (Hmax) given its specific richness following Eq. (2):
where H max = log2 S (Ramade 2009).
The Biological Diatom Index (BDI) (Lenoir and Coste 1996) is a standardized method developed and used in France for monitoring the quality of watercourses. Prygiel and Coste (2000) provide detailed descriptions of field sampling, laboratory processes, taxonomic determinations, and calculation methods in their manual (Coste et al. 2009). The BDI has values ranging from 0 to 20 with 5 classes: poor, low, moderate, good and very good. The higher the value of the index, the better the ecological status of the water (Szulc and Szulc 2013). For a given site, BDI score is the average of pollution sensitivity values of the diatom species present, multiplied by their relative abundances, and weighted by the ecological amplitude of each taxon following Eq. (3):
where Ai, Si and Vi denote, respectively, the abundance, pollution sensitivity and ecological amplitude of the ith taxon in the diatom assemblage.
The identified taxa with their abundance are listed in Appendix 1.
Results
Diatoms identification and ecology
The diatom species identified belong to seven families. Naviculaceae and Nitzschiaceae have shown the highest species abundance (47.87 and 31.86%, respectively). The total number of species taken into account for all multivariate analyses was 109 species in Safsaf watershed, and 117 species in Kebir watershed. Guebli watershed was the most diversified catchment with 129 species. The three watersheds share 61 common taxa (see Appendix 1).
During spring, the taxa Achnanthidium minutissimum (3.80%), Nitzschia umbonata (3.41%), and Navicula phyllepta (2.88%) were the most dominant among all the species, respectively, in Guebli, Kebir and safsaf watersheds. During summer, the most dominant taxa were Mayamaea permitis (2.44%) at Kebir watershed, and Nitzschia palea (9.91%) in Guebli and Safsaf watersheds, being dominant during winter at the three watersheds.
Nitzschia capitellata and Achnanthidium minutissimum were frequent in Guebli and Kebir watersheds during the spring, while Navicula phyllepta and Gomphonema parvulum were frequent in Kebir and Safsaf watersheds. Navicula species were also common in the spring including Navicula phyllepta in Safsaf and Kebir watersheds and Navicula veneta in Guebli. While Mayamaea permitis was frequent in Kebir and Guebli watersheds during the summer period.
The mean values of the environmental variables calculated for the 3 seasons are summarized in Appendix 2. Temperature maximum values have been recorded in summer and ranged between 26.35 and 31.5 C°, conductivity varied from 300 to 2646 µS cm−1, and maximum value of total nitrogen (Nt) was recorded in summer at Guebli watershed estimated at 943.27 mg.L−1 while in phosphates, at Kebir watershed it was recorded as 37.26 mg.L−1. Maximum velocity and dissolved oxygen values have been recorded in winter; velocity varied between 0.04 and 4.25 cm.s−1 whereas dissolved oxygen ranged from 0.88 to 16.97 mg.L−1. Concerning pollution, the sampling sites ranged from slightly to heavily impacted, which are the most downstream sampling points. In general, samples sampled in summer were characterized by high values of temperature, conductivity, and nutrients compared to those sampled in spring, while samples sampled in winter presented high current velocity and dissolved oxygen (see Appendix 2).
Correlations between environmental variables
A global correlation analysis was performed on 10 environmental parameters. In the Safsaf watershed (Fig. 2a), chlorides are correlated strongly and positively to water conductivity (Pearson coefficient (r) = 0.9; p < 0.001). BOD5 and COD are associated negatively to DO (r = 0.64 and 0.69, respectively; p < 0.01). For Kebir watershed (Fig. 2b), phosphates showed a positive correlation with total nitrogen (Nt) (r = 0.61; p < 0.001). DO demonstrated a negative correlation with temperature (r = 0.79; p < 0.001). In the Guebli watershed (Fig. 2c), conductivity showed a significant positive correlation with chloride concentrations (r = 0.64; p < 0.001). DO is associated negatively to total nitrogen (Nt) (r = 0.91; p < 0.001).
Environmental variables corrplot of Safsaf, Kebir and Guebli watersheds. The letters indicate the watersheds: (a) for Safsaf, (b) for Kebir and (c) for Guebli. The color of the squares is proportional to the Pearson coefficient (red for positive correlation, blue for negative correlation, *p < 0.05 shows significant correlation, **p < 0.01 shows good correlation, ***p < 0.001 for excellent correlation). The numbers inside the squares reflect the precise Pearson value
Indicator species
The dendrograms in Fig. 3 show the results of the Agglomerative Hierarchical Cluster Analysis, grouping of sampling sites. Each cluster includes a group of sites based on their indicator species.
Hierarchical clustering dendrogram of diatom species of Safsaf, Kebir, and Guebli watersheds. The letters indicate the watersheds: (a) for Safsaf, (b) for Kebir and (c) for Guebli. Capital letters indicate the seasons: W for winter, Sm for summer, and S for spring. The colored frames represent the groups: blue = group1, green = group2, red = group3, orange = group4, black = group5
Indicator species of each group of sites were obtained by IndVal analysis using Monte Carlo permutation tests, and resulting P values are shown in Table 2. We have obtained 16 common indicator species that are considered good indicators in European diatom indices included in Omnidia (https://hydrobio-dce.irstea.fr/wp-content/uploads/2014/05/OMNIDIAFILE.xls) with Indexed Parameter Value (IPV) = 3.
The cluster sites were classified based on the indicator species present, and the sampling sites in each cluster shared the same environmental conditions. Five clusters were determined for Safsaf and Kebir watersheds (Figs. 3a, b), while four groups appeared in the Guebli watershed dendrogram (Fig. 3c). For Safsaf watershed, the first cluster grouping sites of the streams Safsaf and Zeramna sampled in spring, is characterized by high Nt concentrations ranging from 73.01 to 747.74 mg.L−1, and COD (99.55 to 163.97 mg.L−1) including two species of the genus Nitzschia. The second cluster is less diversified with only Craticula subminuscula. While the third group is the most diversified among all the clusters with five species. The sampling sites regrouped into these two clusters are sampled in winter, and showed maximal values of current velocity (4.25 cm.s−1), dissolved oxygen (13.44 cm.s−1), and chlorides (414.90 mg.L−1). The 4th Cluster, comprised all sites sampled in summer characterized by high temperature ranging between 24.1 and 29.99 °C, and low current velocity (0.52 cm.s−1). The last cluster includes the two remaining sites SAD-S and SAU-S with high altitude (85 and 89 m, respectively) and grouped three species.
The first cluster of Kebir watershed included sites almost slightly impacted with low water conductivity (300 to 1140.5 µS.cm−1) and comprised three taxa. The second group which comprised sites with slightly high conductivity surpassing 1650 µS.cm−1 was the most diversified with six species. The third cluster comprising the two impacted sites of Fendek stream showed the highest conductivity values with two taxa Craticula accomoda and Nitzschia umbonata followed by the fourth group which included nutrient-rich sites with high BOD and COD loads and maximal values on chlorides (521.85 mg.L−1), which incorporated five taxa. Coming later the last group comprising sites slightly to moderately impacted showing the highest dissolved oxygen values (16.97 mg.L−1) while current velocity ranged from 0.16 to 0.29 cm.s−1 with four diatoms.
In Guebli watershed, the first cluster included sites with low water conductivity (from 463 to 873.5 µS.cm−1) and high current velocity (maximum 0.33 cm.s−1) with three taxa. The second group comprised sites with high dissolved oxygen concentrations showing the lowest diversity with only one specie of the family Surerellaceae. The third cluster included sites with strong water conductivity compared to the stations previously cited, with high altitude with three diatom species. The last cluster was composed of the most impacted sites with high concentrations of BOD, COD, chlorides, and nutrients (Nt and phosphates) and showed the highest diversity with five taxa.
Environmental variables affecting diatom’s distribution
CCA analysis triplots highlighted the main drivers among abiotic variables explaining the distribution of the sampling sites for each watershed (Fig. 4). Species names are given in Appendix 1.
Canonical correspondence analysis (CCA) triplots showing ordination of diatom species, sites and environmental variables studied in Safsaf, Kebir and Guebli watersheds. The letters indicate the watersheds: (a) for Safsaf, (b) for Kebir and (c) for Guebli. Environmental variables are represented by arrows in green. Sites names are written in black with capital letters indicating the seasons: W for winter, Sm for summer, and S for spring. Species codes are shown in blue. Some taxa codes are hidden. See Table 1 for sites codes and Appendix 1 for species codes
For the Safsaf watershed, the first two axes explained 44.51% of the total variance (Fig. 4a). Along the first axis, the sampling sites were arranged according to a seasonal gradient, sites sampled in spring being plotted on the right positive part of the axis, as opposed to samples taken in summer and winter that are plotted on the left side of axis 1 (Fig. 4a).
The sampling sites in summer and winter were plotted along the second axis. The sites sampled in winter and associated with high conductivity, current velocity, and dissolved oxygen values, were characterized by the presence of Nitzschia frustulum (p = 0.013), Amphora copulata (p = 0.024), Nitzschia clausii (p = 0.034), Hantzschia abundans (p = 0.047), and Nitzschia tryblionella (p = 0.039) on the positive part of the axis, whereas Craticula subminuscula (p = 0.031) was plotted on the negative part of the axis. Sites sampled in summer, characterized by the lowest current velocity, hosted Navicula erifuga (p = 0.004), Navicula cryptocephala (p = 0.044), and Nitzschia umbonata (p = 0.048). In the upper quadrant appear diatoms such as Cymatopleura elliptica (p = 0.001), Navicula tripunctata (p = 0.006), and Navicula cryptotenella (p = 0.029). The pollution vector was observable in the second quarter on the right of CCA1. Species associated with this parameter included Nitzschia fonticola (p = 0.033), and Nitzschia soratensis (p = 0.016).
The first two axes in the CCA carried out on Kebir watershed explained 25.95 and 20.03%, respectively, of the total inertia. The first axis segregated sites with low water conductivity from polluted sites, characterized by the presence of species such as Nitzschia frustulum (p = 0.007), Cocconeis euglypta (p = 0.016), and Craticula subminuscula (p = 0.006). The sampling sites sampled in the winter and summer seasons were plotted on the upper quadrant while those sampled in spring were clustered on the lower quadrant. Stations with high current velocity and dissolved oxygen values were plotted in the upper quadrant, characterized by the presence of Diadesmis confervacea (p = 0.003), Sellaphora pupula (p = 0.001), Fallacia arvensis (p = 0.001), and Sellaphora difficilima (p = 0.013). On the opposite side, sampling sites showing strong water conductivity were plotted and characterized by the presence of Cocconeis pediculus (p = 0.001), Cymbella excisa (p = 0.006), Encyonopsis microcephala (0.015), Bacillaria paxillifera (p = 0.031), Gyrosigma acuminatum (p = 0.030), and Rhoicosphenia abbreviata (p = 0.034). The contaminated sites were clustered around the pollution vector: diatoms taxa showing affinity to this type of habitat were Craticula accomoda (p = 0.016) and Nitzschia umbonata (p = 0.001), while Navicula gregaria (p = 0.047), Planothidium frequentissimum (p = 0.002), Achnanthidium minutissimum (p = 0.008), Tryblionella constricta (p = 0.032), and Navicula rostellata (p = 0.008) were plotted on the right part of the CCA plot (Fig. 4b, Table 2).
Finally, in the Guebli watershed (Fig. 4c), the first CCA axis accounted for 20.76% and the second for 19.62% of the total inertia. A seasonal gradient was visible along the first axis separating sites sampled in spring from those sampled in summer and winter periods. The pollution gradient appeared along the second axis which distinguished the stations with high altitude and chlorides, and was characterized by the presence of Cymatopleura solea (p = 0.001), Cymatopleura elliptica (p = 0.001), Caloneis amphisbaena (p = 0.001), Tryblionella levidensis (p = 0.001) and Caloneis amphisbaena f. subsalina (p = 0.003), from the stations with high velocity and oxygen values and lower conductivity levels. The most indicative species were Navicula radiosa (p = 0.001), Geissleria decussis (p = 0.002), Achnanthes exigua (p = 0.048), and Surirella angusta (p = 0.001), whereas Nitzschia capitellata (p = 0.001), Navicula phyllepta (p = 0.031), and Gomphonema tergestinum (p = 0.006) were the most representative species recorded in Fessa wadi sampling sites.
Diatom indices and BDI
Shannon index values showed that the specific diversity observed in the sampled sites across the three watersheds during summer season is higher than in spring and winter. Mean values of Shannon diversity during summer were around H’ = 4.49, 3.98, and 4.17, respectively, at the three watersheds Safsaf, Kebir, and Guebli, while species richness varied between 13 and 34 across the three watersheds (Table 3).
The average diversity (H’) and evenness (E) values for Kebir watershed in winter and spring were similar, recording H’ = 3.86 and E = 0.88, respectively, in winter and 3.64 and 0.85, respectively, in spring, while in summer the values were H’ = 3.98 and E = 0.91, respectively. In Guebli watershed, the mean values of diversity (H’) and evenness (E) during spring were H’ = 4.02 and E = 0.90, respectively, which are very close to those recorded in the summer (H’ = 4.17 and E = 0.91). At the three watersheds, mean values of diversity and evenness in summer were greater than those obtained in winter and spring.
BDI scores ranged from 1 to 16.5. At Safsaf watershed the highest BDI score (11.05) was obtained in station 5 sampled in spring, while in Guebli watershed the highest value was at station 20 sampled in spring. In Kebir watershed, four stations situated upstream and downstream of the Mougger watercourse sampled in spring and summer recorded the highest BDI scores (16.5, 14.53, 16.5, and 13.63, respectively). The lowest diversity, evenness, and BDI values that were observed at station 13 sampled in spring in Kebir watershed recorded 2.35, 0.62, and 1, respectively (Table 3).
Discussion
Diatom communities
Spatial and temporal relationships between environmental variables and diatom communities were investigated throughout the present study, and illustrated by the hierarchical clusters, corrplots, and CCA analyses for each watershed separately. Our species diversity findings are coherent with those of Baudrimont (1974), who studied taxa from Algerian fresh and brackish waters in arid and semi-arid regions; which has demonstrated also that these species are comparable to those in European freshwaters with noticeable saline and alkaline composition. Most of our identified taxa have been already illustrated in the book by Lange-Bertalot et al. (2017), and are known to be cosmopolitan, indicating high conductivity and nutrient-rich waters. The diatom taxa identified in our sampling sites are common and typically found in the Kebir East, El Hammam, and Cheliff Rivers (northeastern and northwest of Algeria) as reported in the papers of Chaïb et al. (2011), Chaïb and Tison-Rosebery (2012) and Nehar et al. (2015).
Most of these species were identified recently also in the study of El Haouati et al. (2015) who recorded 24 taxa in Reghaia Lake. Chabaca et al. (2020) recorded over 18 genera in the Oubeira lake, and the study by Hermaza et al. (2021) identified 72 species in Ain Dalia dam.
The current study is the first attempt to determine the species composition of the Coastal Central Constantine, Northeastern Algeria, where no research has been conducted before. It contributes to the completion of the Algerian database of diatoms inventoried thus far by the aforementioned works, as well as the determinants of their distribution. Furthermore, our study demonstrates that the key drivers can differ between geographically close watersheds.
Dominant taxa and diversity
Over the three watersheds, our floristic results revealed the dominance of Naviculaceae, which showed the highest abundance (47.87%). Similar results were demonstrated at El Kebir-East River (Chaïb et al. 2011), and at the streams of Chott Chergui wetland with a percentage of 25.71% (Negadi et al. 2021).
Among the identified diatoms, the taxa Gomphonema parvulum, Achnanthidium minutissimum, Navicula gregaria, Nitzschia frustulum, Craticula subminuscula, Amphora pediculus and Nitzschia palea are common in studies conducted in running waters in northern Algeria (Chaïb and Tison-Rosebery 2012; Nehar et al. 2015; Negadi et al. 2021).
Diatoms and water quality
Nitzschia umbonata was most abundant in site 13 (KFU_S) sampled in spring, with an abundance of 70.41%, which decreased largely diversity and evenness values (H’ = 1.62, E = 0.54) thus reflecting the poor water quality of this station (BDI = 1). The presence of this species is related to pollution (Duong et al. 2007). Chaïb et al. (2011) observed the proliferation of this diatom at sites associated to pollution in Kebir East, commonly associated with Nitzschia palea and Navicula veneta. In Turkey, Çetin et al. (2021) recorded also a high abundance of this species (15.6%) in a polluted river in the Kızılırmak basin. In terms of water quality, as inferred from diatom-based indices, Chaïb and Tison-Rosebery (2012) have already tested the Biological Diatom Index (BDI) in the Kebir-East River, despite the dominance of the indifferent Achnanthidium minutissimum (75%) at Ain Assel station (that becomes a temporary pool during the dry periods) with nutrient (PO43− and NH4+) levels attaining 0.5 and 1 mg.L−1 respectively, this station recorded the highest BDI score (18/20), which did not reflect the actual poor water quality of the site.
Effect of environmental variables on diatoms distribution
The results of the CCA triplots demonstrated three environmental gradients controlling the diatom distribution in all the watersheds including conductivity, temperature, and pollution. Some studies have revealed the links between the environmental variables and the diatom community throughout Co-Inertia Analysis (CIA) and Canonical Correlation Analysis (CCorA), e.g., the diatom communities of the Kebir-East River are controlled by environmental conditions, including a high seasonal gradient between floods and drought, acidic to neutral waters, high conductivities and pollution (Chaïb et al. 2011; Chaïb and Tison-Rosebery 2012) while the distribution of diatom assemblages of rivers from Chott Chergui wetland are mainly influenced by temperature and conductivity (Negadi et al. 2021). Kivrak and Uygun (2012) reported that the distribution and the composition of the diatom flora in the Turkish stream Akarçay are affected by the physic-chemistry of water including conductivity, PO43−, and BOD.
According to Nehar et al. (2015), the diatom species composition and distribution are linked to anthropogenic pressures and driven by various environmental factors such as chlorides, pH, and temperature. In the same way, Hermaza et al. (2021) studied the spatial and temporal distribution of diatoms of the Aïn Dalia dam and emphasized the impact of nutrients (NO2− and PO43-), suspended matter, temperature, and dissolved oxygen on the diatom distribution. Furthermore, El Haouati et al. (2015) highlighted the relationship between the environmental characteristics of Reghaia Lake and the diatoms, reporting the direct impact of nutrients (PO43− and NH4+), temperature, conductivity, and oxygen on the occurrence of different diatom taxa.
The waters of the three watersheds were characterized by high conductivities (> 1000 μS.cm−1), and high chloride contents, as confirmed by correlation analyses which revealed a strong correlation between the concentration of chlorides and water conductivity. In particular, conductivity is one of the main environmental factors influencing the structure and the distribution of diatom communities (Rimet 2009).
Indicator species and their ecological preferences
The clustering and Indval analysis revealed the presence of the genus Navicula such as N. erifuga and N. cryptocephala (Table 2) showing their preference for high temperatures in Safsaf watershed during summer (group 4) (Fig. 3a); the mean value of temperature being around 29.14 °C. According to Hermaza et al. (2021), this genus prefers high temperatures in the summer and slightly polluted environments.
According to Indval analysis (Table 2), the presence of the indicator species Nitzschia fonticola in group 1 (Fig. 3a, Table 2) was related to high Nt concentrations and COD. This diatom was recorded also in polluted rivers in north-eastern France (Rimet 2009).
The species Navicula gregaria (Fig. 3b, Table 2) showed its preference to nutrient-rich sites in Kebir watershed, which indicates the trophic status of these locations. This species has been already noted in Noga et al. (2014) as tolerant to eutrophic waters. Blanco and Bécares (2010) confirmed that pollution and the trophic state of water affect the structure and the distribution of such diatom communities.
The taxa Navicula cryptotenella in Safsaf watershed, Navicula rostellata and Achnanthidium minutissimum from Kebir watershed, as well as Cymatopleura solea from the watershed of Guebli are related to high loads on Nt and phosphates, which explained their presence in the sampling sites located close to agricultural lands exposed to soil drainage. These taxa colonized nutrient-rich rivers with high N and P loads, as demonstrated in the works of Chaïb et al. (2011), Chaïb and Tison-Rosebery (2012), El Haouati et al. (2015), and Negadi et al. (2021).
Craticula subminuscula was recorded in stations showing the greatest water conductivity in Kebir watershed, this species is known by its tolerance to high conductivity levels, which confirms the results and conclusions of Chaïb and Tison-Rosebery (2012) and Nehar et al. (2015).
Seasonal variation, diversity, and diatom assemblages
The specific diversity observed in the sampled sites across the three watersheds during summer is above that observed in spring and winter.
This can be explained by the high level recorded in summer of the main variables controlling the distribution of diatoms including temperature, conductivity, and pollution (availability of nutrients). According to Bussard (2015), temperature, light, nutrient concentration, movement of water masses, and even salinity would all influence algae growth and distribution.
Similar findings have been noted in other studies, at Mellah lagoon (H’ = 4.56 in Draredja et al. (2019), and in Aïn Dalia dam (H’ = 3 in Hermaza et al. (2021). In contrast to these results, diatom assemblages at Oubeïra Lake (Chabaca et al. 2020) were less diversified compared to those found in our studied basins, with a diversity score of H’ = 2.35. On the other hand, the wet season was the most diverse at Chott Chergui wetland (Negadi et al. 2021), while in Reghaia lake, diatom assemblages were more diversified during spring and fall (El Haouati et al. 2015).
Achnanthidium minutissimum (ADMI) was abundant in the Kebir watershed during the spring and summer seasons exceeding 17% in station 10 sampled in spring, whereas in Guebli watershed this specie was the most dominant during the spring period reaching 17.91% in relative abundance. Other studies reported also the dominance of this diatom reaching abundances of up to 75% at Kebir East River (Chaïb and Tison-Rosebery 2012), and 55% at El Hammam stream (Nehar et al. 2015) in summer, with a mean diversity and evenness scores of H’ = 2.5, E = 0.8 and H’ = 1.9, E = 0.6, respectively, also subdominant (21.26%) in spring at Mina watershed (Negadi et al. 2018) with an average of 2.5 and 0.8, respectively, in diversity and evenness. In Chabaca et al. (2020), the mean diversity values were 2.34, and 2.22 respectively at two stations in Oubeïra Lake, northeastern Algeria.
Diatom populations in relation to pollution
Populations exposed to impaired waters harbor species tolerant to organic pollution (Castillejo et al. 2018; Karaouzas et al. 2018). In our case, high levels of organic and chemical pollution (related to sewage and industrial effluents) explained the presence of the tolerant taxa Craticula accomoda and Nitzschia umbonata in stations 13 and 14 sampled in winter. Çetin et al. (2021) reported that those species were also predominant in winter with rates of 12.5% for C. accomoda, and 15.6% for N. umbonata.
Other pollution-tolerant species were also identified from impacted sampling sites (mostly all downstream locations in all the watersheds) such as Nitzschia palea, Nitzschia capitellata, Navicula veneta, Gomphonema parvulum, and Mayamaea permitis. These species, considered pollution indicators, have been already found at polluted sampling sites in a number of studies such as (Cochero et al. 2017; Kheiri et al. 2018; Rybak et al. 2019; Bezzeghoud and Bouhameur 2021) besides the monograph by Lange-Bertalot (2017). The significant dominance of Craticula subminuscula, Gomphonema parvulum, Nitzschia palea, and the species complex formed by Nitzschia inconspicua and Nitzschia frustulum indicate the polluted status of these waters, as shown in a study conducted on Hassar stream in Morroco by Fawzi et al. (2011).
Conclusion
The current study is the first attempt to characterize the benthic diatom communities of Coastal Central Constantine, including three watersheds (Safsaf, Kebir and Guebli) in Northeastern Algeria. Data analyses allowed us to highlight the relationships between the environmental variables and the diatom community composition and distribution. The examined sampling sites in Safsaf, Kebir, and Guebli watersheds are driven by fluctuations in water quality, characterized by high conductivity and pollution levels. A seasonal gradient was also evident, segregating clearly species dominant in spring from those most common in summer or winter. The main variables controlling the distribution of diatom communities across the three watersheds were temperature, conductivity, and pollution. There was a seasonal variation across the three watersheds; summer was the most diversified; the specific diversity observed in the sampled sites during summer is above that observed in spring and winter.
Our results are congruent with all studies carried out on benthic diatoms in Algerian streams and highlight the distribution particularities of our species, compared to the ecological profiles elaborated during surveys in France, notably for the development of the BDI.
Data Availability
Not applicable.
References
AFNOR (2007) (Association Française de Normalisation).Qualité écologique des milieux. Norme Française NFT 90–354 - Qualité de l ‘ eau - Détermination de l ‘ Indice Biologique Diatomées (IBD). Paris
Baudrimont R (1974) .Recherches sur les Diatomées des eaux continentales de l’ Algérie. Ecologie et paléo-écologie.Mémoire, Soc. Hist. Nat. Afr. Nord, nouvelle série – 12,265. (in French)
Bezzeghoud B, Bouhameur M (2021) Bioindication par les diatomées epilithiques dans deux bassins hydrographiques (chelif et cotiers oranais) nord-ouest algérien. 11:2579–2590. (in French)
Blanco S, Bécares E (2010) Are biotic indices sensitive to river toxicants? A comparison of metrics based on diatoms and macro-invertebrates. Chemosphere 79:18–25. https://doi.org/10.1016/j.chemosphere.2010.01.059
Blanco S, Cejudo-Figueiras C, Álvarez-Blanco I et al (2010) Atlas de las Diatomeas de la cuenca del Duero/Diatom Atlas of the Duero Basin. Área de Publicaciones. Universidad de León, León, p 386
Blanco S, Cejudo-Figueiras C, Álvarez-Blanco I et al (2013) Epiphytic diatoms along environmental gradients in Western European shallow lakes. Clean - Soil, Air, Water 42:229–235. https://doi.org/10.1002/clen.201200630
Bouleknafet Z, Derradji E (2017) Hydrogéologie Et Vulnérabilité à La Pollution Des Ressources En Eau Dans La Plaine Du Kebir Ouest. Hydrogeology and Vulnerability to Pollution of the Water Resources in the Kebir West Plain 94:85–94 ((in French))
Bussard A (2015) Capacités d’acclimatation des diatomées aux contraintes environnementales. Museum National d’histoireNaturelle, Paris France, p 302
Castillejo P, Chamorro S, Paz L et al (2018) Response of epilithic diatom communities to environmental gradients along an Ecuadorian Andean River. Comptes Rendus - Biol 341:256–263. https://doi.org/10.1016/j.crvi.2018.03.008
Çetin T, Solak CN, Yılmaz E (2021) Testing the performance of European diatom indices for evaluating the ecological status in the Kızılırmak basin, Turkey: flowing waters. Environ Sci Pollut Res 28:43567–43578. https://doi.org/10.1007/s11356-021-13282-1
Chabaca H, Marniche F, Tadjine A (2020) Diatoms diversity in Oubeïra Lake, northeastern Algeria. Biodivers J 11:573–580. https://doi.org/10.31396/biodiv.jour.2020.11.2.573.580
Chaïb N, Tison-Rosebery J (2012) Water quality assessment and application of the biological diatom index in the Kebir-East wadi Algeria. African J Aquatic Sci. https://doi.org/10.2989/16085914.2011.6368
Chaïb N, Alfarhan AH, Al-Rasheid KAS, Samraoui B (2011) Environmental determinants of diatom assemblages along a North African wadi, the Kebir-East, North-East Algeria. J Limnol 70:33–40. https://doi.org/10.3274/JL11-70-1-06
Chavent M, Kuentz-Simonet V, Liquet B, Saracco J (2012) ClustOfVar: An R package for the clustering of variables. J Stat Softw 50:1–16. https://doi.org/10.18637/jss.v050.i13
Cochero J, Licursi M, Gómez N (2017) Effects of pulse and press additions of salt on biofilms of nutrient-rich streams. Sci Total Environ 579:1496–1503. https://doi.org/10.1016/j.scitotenv.2016.11.152
Coste M, Boutry S, Tison-Rosebery J, Delmas F (2009) Improvements of the Biological Diatom Index (BDI): description and efficiency of the new version (BDI-2006). Ecol Indic 9:621–650. https://doi.org/10.1016/j.ecolind.2008.06.003
De Cáceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. https://doi.org/10.1890/08-1823.1
Draredja MA, Barour C, Frihi H et al (2019) Diatoms diversity and dynamics in a southern Mediterranean lagoon (Mellah, Algeria). Biodivers J 10:127–140. https://doi.org/10.31396/biodiv.jour.2019.10.2.127.140
Duong TT, Feurtet-Mazel A, Coste M et al (2007) Dynamics of diatom colonization process in some rivers influenced by urban pollution (Hanoi, Vietnam). Ecol Indic 7:839–851. https://doi.org/10.1016/j.ecolind.2006.10.003
El Haouati H, Arab A, Tudesque L et al (2015) Study of The Diatoms of Reghaia Lake Northern Algeria. Rev d’écologie, UK
Fawzi B, Chlaida M, Oubraim S, et al (2011) Application de certains indices diatomiques à un cours d’eau marocain : Oued HassarApplication of some diatom indices to a Moroccan water course: Hassar stream. Rev des Sci l’eau 14:73. (in French). Doi: https://doi.org/10.7202/705410ar
Hammer Ø, Harper DA, Ryan P (2001) PAST: Paleontological Statistics software package for education and data analysis. Palaeontol Electron 4(1):99
Heramza K, Barour C, Djabourabi A et al (2021) Environmental parameters and diversity of diatoms in the Aïn Dalia dam, Northeast of Algeria. Biodiversitas 22:3633–3644. https://doi.org/10.13057/biodiv/d220901
Hofmann G, Werum M, Lange-Bertalot H (2011) Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die ökologische Praxis; über 700 der häufigsten Arten und ihrer Ökologie. Gantner.
Karaouzas I, Smeti E, Vourka A et al (2018) Assessing the ecological effects of water stress and pollution in a temporary river - Implications for water management. Sci Total Environ 618:1591–1604. https://doi.org/10.1016/j.scitotenv.2017.09.323
Kheiri S, Solak CN, Edlund MB et al (2018) Biodiversity of diatoms in the Karaj River in the Central Alborz. Iran Diatom Res 33:355–380. https://doi.org/10.1080/0269249X.2018.1557747
Khelfaoui F, Zouini D (2010) Gestion intégrée et qualité des eaux dans le bassin versant du Saf-Saf ( wilaya de Skikda , nord-est algérien ). d:50–56. (in French).
Kivrak E, Uygun A (2012) The structure and diversity of the epipelic diatom community in a heavily polluted stream (the akarçay, Turkey) and their relationship with environmental variables. J Freshw Ecol 27:443–457. https://doi.org/10.1080/02705060.2012.671147
Krammer K, Lange-Bertalot H (1988–1991) .Bacillariophyceae. In: Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (Eds.), Süsswasserflora von Mitteleuropa, Band 2(2–4). Gustav Fischer Verlag, Stuttgart/Jena.
Lange-Bertalot H, Hofmann G, Werum M, Cantonati M (2017) Freshwater benthic diatoms of Central Europe: over 800 common species used in ecological assessment. English Edition with updated taxonomy and added species, Koeltz Botanical Books, Schmitten- Oberreifenberg, Germany, pp 1–942
Lavoie I, Campeau S, Grenier M, Dillon PJ (2006) A diatom-based index for the biological assessment of eastern Canadian rivers: An application of correspondence analysis (CA). Can J Fish Aquat Sci 63:1793–1811. https://doi.org/10.1139/F06-084
Lecointe C, Coste M, Prygiel J (1993) “Omnidia”: software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia 269–270:509–513. https://doi.org/10.1007/BF00028048
Lenoir A, Coste M (1996) Development of a practical diatom index of overall water quality applicable to the French National Water Board network. In: Whitton BA, Rott E (eds) Use of Algae for Monitoring Rivers II. Universität Innsbruck, Innsbruck, pp 29–45
McCune B, Grace J (2002) Analysis of Ecological Communities. J Exp Mar Bio Ecol 289:303–305. https://doi.org/10.1016/S0022-0981(03)00091-1
Mecibah I, Medjani F, Djidel M (2019) Assessment of surface water quality in the Guebli River watershed (Northeast Assessment of surface water quality in the Guebli River watershed (Northeast of Algeria )
Murtagh F, Legendre P (2011) Ward’s hierarchical clustering method: clustering criterion and agglomerative algorithm. J Classif 31:1–20. https://doi.org/10.1007/s00357-014-9161-z
Murtagh F, Legendre P (2014) Ward’s Hierarchical agglomerative clustering method: which algorithms implement ward’s criterion? J Classif 31:274–295. https://doi.org/10.1007/s00357-014-9161-z
Negadi M, Hassani A, Ait Hammou M et al (2018) Diversity of Diatom epilithons and quality of water from the subbasin of Oued Mina (district of Tiaret, Algeria) M. Ukr J Ecol 8:41–50. https://doi.org/10.15421/2018
Negadi M, Ait Hammou M, Miara MD, et al (2021) Floristic diversity and Ecology of EpilithicDiatoms of the ChottChergui wetland (North-West of Algeria)
Nehar B, Blanco S, Hadjadj-Aoul S (2015) Diversity and ecology of diatoms in northwest of Algeria: Case of El-hammam stream and estuary of Cheliff river. Appl Ecol Environ Res 13:37–52. https://doi.org/10.15666/aeer/1301_037052
Noga T, Kochman N, Peszek Ł et al (2014) Diatoms (Bacillariophyceae) in rivers and streams and on cultivated soils of the podkarpacie region in the years 2007–2011. J Ecol Eng 15:6–25. https://doi.org/10.12911/22998993.1084168
Oğuz Çam A, Kaleli MA, Akçaalan R et al (2020) Composition and Distribution of Benthic Diatoms in Different Habitats of Burdur River Basin. Turkish J Water Sci Manag 4:31–57. https://doi.org/10.31807/tjwsm.658001
OriginPro, (2021) Version 2021. OriginLab Corporation, Northampton, MA, USA
Ozer T, Erkaya IA, Solak CN, Udoh AU (2018) Diversity and Ecology of Algae from Melen River (Western Black Sea River Catchment) in Turkey. Turkish J Fish Aquat Sci 18:81–90. https://doi.org/10.4194/1303-2712-v18
Pandey, Lavoie I, Morin S, et al (2017) River water quality assessment based on a multi-descriptor approach including chemistry, diatom assemblage structure, and non-taxonomical diatom metrics. 1–2
Potapova M, Charles DF (2003) Distribution of benthic diatoms in U.S. rivers in relation to conductivity and ionic composition. Freshw Biol 48:1311–1328. https://doi.org/10.1046/j.1365-2427.2003.01080.x
Potapova M, Charles DF (2005) Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol Indic 7:48–70. https://doi.org/10.1016/j.ecolind.2005.10.001
Prygiel J, Coste M (1996) Les diatomées et les indices diatomiques dans les réseaux de mesure de la qualité des cours d’eau Français : Historique et avenir. BFPP - Bull Fr La Pech La Prot Des Milieux Aquat 69:65–79. https://doi.org/10.1051/kmae:1996006
Prygiel J, Carpentier P, Almeida S et al (2002) Determination of the biological diatom index (IBD NF T 90–354): Results of an intercomparison exercise. J Appl Phycol 14:27–39. https://doi.org/10.1023/A:1015277207328
Prygiel J, Coste M (2000) Guide méthodologique pour la mise en oeuvre de l’Indice Biologique Diatomées NF T90–354
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramade F (2009) Eléments d’écologie : Ecologie fondamentale, 4ème. Dunod, Paris, p 689p
Rimet F (2009) Benthic diatom assemblages and their correspondence with ecoregional classifications: Case study of rivers in North-Eastern France. Hydrobiologia 636:137–151. https://doi.org/10.1007/s10750-009-9943-1
Round FE (1991) Diatoms in river water-monitoring studies. J Appl Phycol 3:129–145. https://doi.org/10.1007/BF00003695
Rybak M, Noga T, Poradowska A (2019) Diversity in anthropogenic environment-Permanent puddle as a place for development of diatoms. J Ecol Eng 20:165–174. https://doi.org/10.12911/22998993/111463
Seifert E (2014) OriginPro 9.1: Scientific data analysis and graphing software - Software review. J Chem Inf Model 54:1552. https://doi.org/10.1021/ci500161d
Shannon CE, Weaver W (1949) The mathematical theory of communication. The University of Illinois. Urbana, Chicago, London, pp 3–24
Solak CN, Àcs É (2011) Water quality monitoring in European and Turkish rivers using diatoms. Turkish J Fish Aquat Sci 11:329–337. https://doi.org/10.4194/trjfas.2011.0218
Suzuki R, Shimodaira H (2006) Pvclust: An R package for assessing the uncertainty in hierarchical clusteringuncertainty in hierarchical clustering. Bioinformatics 22(12):1540–1542. https://doi.org/10.1093/bioinformatics/btl117
Szczepocka E, Szulc B (2009) The use of benthic diatoms in estimating water quality of variously polluted rivers. Oceanol Hydrobiol Stud 38:17–26. https://doi.org/10.2478/v10009-009-0012-x
Szulc B, Szulc K (2013) The use of the Biological Diatom Index (BDI) for the assessment of water quality in the Pilica River. Poland. https://doi.org/10.2478/s13545-013-0073-z
Tan X, Zhang Q, Burford MA et al (2017) Benthic diatom based indices for water quality assessment in two subtropical streams. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00601
Taş B, Tepe Y, Ustaoğlu F, Alptekin S (2019) Benthic algal diversity and water quality evaluation by biological approach of turnasuyu creek, ne turkey. Desalin Water Treat 155:402–415. https://doi.org/10.5004/dwt.2019.24225
ter Braak CJF, Verdonschot PFM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–289. https://doi.org/10.1007/BF00877430
Tison J, Giraudel JL, Coste M (2008) Evaluating the ecological status of rivers using an index of ecological distance: an application to diatom communities. Ecol Indic 8:285–291. https://doi.org/10.1016/j.ecolind.2007.02.006
Titi Benrabah S, Kherici Bousnoubra H, Kherici N, Cote M (2013) Assessment and management of water resources in Northeastern Algeria: case of watersheds Kebir West Safsaf and Guebli rivers, Skikda. Appl Water Sci 3:351–357. https://doi.org/10.1007/s13201-013-0085-2
Vijaya V, Sharma S, Batra N (2019) Comparative study of single linkage, complete linkage, and ward method of agglomerative clustering. In: International conference on machine learning, big data, cloud parallel comput trends, prespectives Parallel Computing pp 568–573. https://doi.org/10.1109/COMITCon.2019.8862232
Acknowledgements
The present work is funded by the Algerian Ministry of Higher Education and Scientific Research and the University of 20 August 1955 (Algeria), and it fits into the university training research project (PRFU) carrying the code A16N01UN210120180002. The authors would like to thank María BORREGO RAMOS and the other members of the Diatom Lab (University of León, Spain) for their assistance in identifying the diatom species. We are extremely grateful to Dr. Azzedine HADEF for the elaboration of the map of the studied area.
Funding
This study is funded by the Algerian Ministry of Higher Education and Scientific Research, and the University of 20 August 1955 (Algeria), in the frame of a University Training Research Project (PRFU) carrying the code A16N01UN210120180002 under the aegis of Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT) Algeria.
Author information
Authors and Affiliations
Contributions
Sampling was carried out by Hadjer Kaddeche. Treatment of samples, physical-chemical analyses and species identification were conducted by Hadjer Kaddeche, Faïza Noune and Sabrina Dzizi. Hadjer Kaddeche and Nadjla Chaïb collected data, analyzed the results and wrote the first draft of the manuscript. Nadjla Chaïb and Zine Eddine Boudjellab are thanked for realization and conceptualization of the graphs. Saúl Blanco-Lanza identified species, supervised the projet and reviewed the results. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This study did not involve any animals or human participants.
Financial and Non-financial interests
The authors have no relevant financial or non-financial interests to disclose.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Additional information
Handling Editor: Dr. Man Xiao
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaddeche, H., Noune, F., Dzizi, S. et al. Determinant factors of diatom assemblage’s distribution along the Coastal Central Constantine (Northeastern Algeria). Aquat Ecol 56, 1245–1269 (2022). https://doi.org/10.1007/s10452-022-09980-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-022-09980-8