Abstract
This work aims to evaluate the effects of different environmental factors (i.e., geographical, chemical, and hydrological) on benthic diatoms at 34 sites located in 13 watercourses of northern Italy, and to highlight possible misclassifications of the ecological status of watercourses, sensu Water Framework Directive, related to the normative index currently adopted in Italy (ICMi). The analysis of both the taxonomical and functional composition of diatom communities confirmed the presence of differences in terms of taxonomical richness, diversity, and taxa assemblages, associated to the altitude and the geological characteristics of the investigated watercourses. Moreover, the data analysis revealed differences due to chemical and hydrological alterations. Specifically, our results showed a clear link among these environmental perturbations and the communities’ functional composition expressed through the use of ecological guilds. High abundance and richness of motile diatoms were detected in sites characterized by nutrient enrichment, while high abundance of low-profile diatoms was linked to hydrological alteration. In contrast, these anthropogenic perturbations were not detected by the ICMi, which ranked more than 90% of the analyzed samples in the highest quality class. This study stresses the need for a different approach in diatom data interpretation in order to achieve reliable information about the ecological status of watercourses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benthic diatoms are an important component of phytobenthos in riverine ecosystems. Due to their ubiquity and sensitivity to environmental alteration, diatom communities have been used as bioindicators in many countries long since (e.g., Stevenson and Pan 1999; Jüttner et al. 2003; Lobo et al. 2004; Tan et al. 2015; Stancheva and Sheath 2016; Nhiwatiwa et al. 2017; Pardo et al. 2018). At the European scale, after the transposition of the Water Framework Directive (WFD—EC 2000) by the member states of the European Union (EU), diatom monitoring was included in the routine protocols for the assessment of the ecological status of watercourses (Poikane et al. 2016). To date, the diatom indices applied or even specifically developed for this purpose mainly account for nutrients and organic matter enrichment. Indeed, the index more widely used within this framework is the Indice de Polluosensibilité Specifique (IPS, CEMAGREF 1982), specifically detecting organic pollution (Kelly 2013). As a consequence, in the EU, the assessment of the ecological status of watercourses through benthic diatom communities is currently biased towards the detection of chemical alteration in terms of nutrients and organic pollution, neglecting other environmental pressures. However, as already highlighted by many authors, diatom community composition is also influenced by further environmental factors that could be altered by human activity. With reference to chemical alteration, many studies have stressed the importance of ion concentration and trophic status as major environmental drivers in diatoms distribution (as reviewed by Soininen 2007). Marcel et al. (2017) indicated changes in diatom communities in response to electrical conductivity and pH, the latter influencing in turn nutrient availability, metal solubility, and enzymatic activity (Battarbee et al. 2001; o’Driscoll et al. 2014). Vasiljević et al. (2017) found community changes mainly in response to silicon (Si) and arsenic (As) concentrations. Moreover, in recent years, the role of hydrology and channel morphology in shaping diatom distribution is receiving increasing attention. For instance, some authors reported that morphologically altered sites show lower species diversity (Bona et al. 2008), and that increasing flood frequency causes a decrease in biomass and species richness (e.g., Clausen and Biggs 1997; Heath et al. 2015), according to peaking of benthic diatom biomass at flow velocities within optimal ranges (Wang et al. 2018). Furthermore, Hoyle et al. (2017) found that the abrasion by suspended material during floods was the dominant control factor on periphyton abundance, prevailing on nutrient concentration.
Changes in community composition mirror adaptations of the community to environmental factors, through changes in the prevailing survival strategies. Thus, the link between community structure and function is crucial for the ecological interpretation of biological datasets and for the consequent identification of the best management practices to be implemented in case of environmental quality deterioration. Although in the WFD the good ecological status (GES, i.e. the benchmark for waterbodies protection) was clearly defined taking into account both structure and functioning of aquatic ecosystems (see Article 2, EC 2000), the phytobenthos indices currently used in many EU member states lack the capacity to assess the ecological functions of diatom communities, thus overlooking the value of these communities as indicators (Kelly 2013).
In this study, we used ecological guilds as defined by Rimet and Bouchez (2012), along with the analysis of the community taxonomical composition, to evaluate the effects of different environmental factors (i.e., geographical, chemical and hydrological) on diatoms in 13 watercourses of northern Italy.
Ecological guilds are a crucial approach proposed by Root (1967) and repeatedly discussed later (e.g., MacMahon et al. 1981; Simberloff and Dayan 1991; Wilson 1999; Kornan and Kropil 2014), to interpret community structure, taking into account its linkage with the characteristics of the surrounding ecosystem. Originally, ecological guilds were defined as groups of species that exploit the same class of environmental resources in a similar way, where resources include both food and physical features, such as shelters. By applying this definition to a single taxonomic assemblage (diatoms), ecological guilds, as defined by Passy (2007) and Carrère and Bloor (2009), and then applied by Rimet and Bouchez (2012), are groups of taxa combined on the basis of their potential to exploit nutrients and to withstand physical disturbance, which is linked to their morphological characteristics. Despite the fact that ecological guilds are a user-friendly classification, their use to disentangle the interactions of multiple stressors and define their specific effects on community functions has been limited to a small number of studies (e.g., B-Béres et al. 2014; Lange et al. 2016; Marcel et al. 2017). In our study, we compare the ecological guilds approach to the one of the current Italian normative diatom index (the Intercalibration Common Metric index, ICMi, Mancini and Sollazzo 2009).
Specifically, this study aims to test the following hypotheses: (i) differences in terms of ecological guilds composition can be related to different environmental abiotic features and (ii) the use of the Italian normative diatom index can lead to misclassifications of the ecological status of watercourses. Overall, the results of our study highlight the potential of ecological guilds in supporting the interpretation of the ecological status of waterbodies.
Materials and methods
Study area
The presented dataset concerns seven areas in northern Italy, including five upland river basins (i.e., Mera River, Upper Adda River, Oglio River, Caffaro River, and Tagliamento River basins) in the central and eastern Alps, and the lowland parts of the Adda and Ticino river basins (i.e., below Lake Como and Lake Maggiore, respectively). Except for the Tagliamento River basin, all the study catchments belong to the Po River basin.
All the considered river basins are characterized by temperate climate, with local differences mostly related to altitude and land morphology.
The upland river basins are located in the Alpine region, with altitude ranging between 200 and 2000 m asl and maximum precipitation mostly recorded in spring and autumn. The hydrological regime shifts from nival (i.e., high flow season in late spring and low flows mainly during winter) to pluvio-nival (i.e., two periods of high flows, in spring and autumn, and two periods of low flows, in summer and winter) as the altitude decreases. The most eastern basins are characterized by more abundant precipitations compared to the western ones. However, their karst geology leads to a marked connection between superficial and groundwater and, as a consequence, despite the higher precipitation, the seepage of water into the streambed can locally largely reduce superficial runoff.
Lowland river basins are located in the Po Plain at low altitudes (below 200 m asl) and are characterized by maximum rainfall during spring and autumn, giving pluvio-nival hydrological regime. In these areas, watersheds are intensely modified by human activity.
According to the geographical classification following the WFD principles (MATTM 2010), the watercourses within the study area belong to three different river macrotypes (Table 1): Alpine watercourses in calcareous areas (A1), Alpine watercourses in siliceous areas (A2), and lowland watercourses (C).
Sampling sites
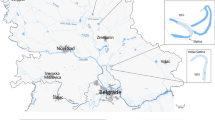
A total of 34 sampling sites within 13 watercourses was monitored, thus investigating river reaches with different geographical characteristics subjected to different anthropogenic pressures (Fig. 1 and Table 1). Data collection took place within research projects for the definition of site-specific minimum flows (MFs).
All the sampling sites are located in river reaches significantly exploited for hydropower and/or irrigation. Hydropower development in northern Italy dates back to the end of the nineteenth century and represents an important electricity source at the National scale (Quadroni et al. 2017). Water diversion for irrigation dates back to at least the thirteenth century in the Po Plain (i.e., the largest Italian area for intensive agriculture). In the study area, since 2009, MFs are released downstream of water intakes as an environmental protection measure (Quadroni et al. 2017; Salmaso et al. 2017, 2018).
The selected sample reaches are mainly characterized by riffle morphology (Montgomery and Buffington 1997) and by coarse substrates composed of boulders and cobbles in varying percentages, with the exception of the most downstream sites of the Lowland Adda (i.e., Ad5–Ad8), where small cobbles and gravel prevail. Some upland sites (i.e., Ad1, Ad2, R1, L2, and Lm1) were affected by controlled sediment flushing operations from hydropower reservoirs, which temporarily altered the substratum increasing the content of fine sediment (Espa et al. 2013, 2015, 2016; Quadroni et al. 2016; Brignoli et al. 2017).
Moreover, many of the sampling sites display some degree of morphological alteration (e.g., embankments, grade control structures).
Chemical alteration mainly characterizes lowland areas, due to diffuse nutrient pollution from agriculture and point source pollution from urban wastewaters (Salmaso et al. 2014, 2018).
Data collection
Data about benthic diatoms were collected along with chemical and hydrological data during a 3-year period (i.e., 2013–2015 for the Tagliamento River basin sites, and 2010–2012 for all the other sampling sites).
Data on benthic diatoms
Diatom samples (n = 6 per site) were collected twice a year, in late spring and in late summer, as required by the Italian protocol (ISPRA 2014) in order to assess the community status after a period of high flow (i.e., late spring, mainly May–June) and one of low flow (i.e., late summer, mainly August–September). Moreover, the sampling dates reflected local climatic and meteorological conditions.
Samples were collected and processed in accordance with the Italian standard method (ISPRA 2014), based on the European standards UNI EN 13946:2005 (CEN 2005) and UNI EN 14407:2004 (CEN 2004), and briefly summarized hereafter. Diatoms were sampled by scrubbing the upper layer of five stones with minimum surface area of 10 cm2 each, collected along a transect. The samples were fixed in 90% ethanol and transported to the laboratory. Diatoms valves were cleaned from organic matter with hydrogen peroxide and from carbonates with hydrogen chloride. Samples were then concentrated through centrifugation and mounted in resin on microscope permanent slides. For each slide, a minimum of 400 valves was counted and identified at species and subspecies level, using a × 1000 magnification optical microscope Nikon Alphaphot 2 YS2. Specific references about Italian diatoms taxonomy (Falasco et al. 2013) were consulted in addition to Krammer and Lange-Bertalot floras (Krammer and Lange-Bertalot 1986, 1988, 1991a, b, 2000; Lange-Bertalot 2001; Krammer 2002) and further international bibliography (Bey and Ector 2013; Fourtanier and Kociolek 2011; Guiry and Guiry 2015; Hofmann et al. 2011). The correspondence between diatom codes and taxa names for the taxa commented in the text and shown in figures is provided in Table 2.
Chemical data
Chemical parameters were analyzed seasonally at sites within the Upper Adda, Mera, and Tagliamento basins, and monthly at the other sites.
Water samples were collected, moved to the laboratory within 24 h, and then analyzed to detect concentration of ammonia-nitrogen (NH4+-N), nitric-nitrogen (NO3−-N), total phosphorous (TP), 5-days biochemical oxygen demand (BOD5), and total suspended solids (TSS) according to standard methods (APAT-IRSA/CNR 2003). pH was measured on-site using a portable probe.
Hydrological data
The average daily streamflows at each monitoring site during the 3-year study period were either gauged by stage measurement or calculated on the basis of gauge measurements in nearby sections by performing basic hydrologic balances (Quadroni et al. 2017; Salmaso et al. 2018).
Mean annual reference flows (Qmr), i.e., the theoretical flows characterizing the sites in absence of upstream diversion, were available from water resource management programs.
Based on these data, the following hydrological parameters were calculated to characterize the streamflow pattern at each monitoring site: the ratio between mean annual flow (Qm) and corresponding Qmr (Qm/Qmr); the coefficient of variation of the streamflow (QCV); the flood frequency (FRE3) as defined by Clausen and Biggs (1997); and the duration of low flows (daysLF), expressed as the number of days per year with streamflow equal or minor than 10% of the Qmr.
Data analysis
For each diatom sample, the ecological status sensu WFD was determined using the ICMi. The ICMi is based on two indices, the IPS and the TI (i.e., the Trophic Index—TI, Rott et al. 1999), which are combined by calculating the arithmetic mean of their ecological quality ratios (i.e., EQRs, the ratios between the observed and reference values). The ICMi is ranked into five quality classes (bad, poor, moderate, good, and high). In order to make ICMi values comparable between different geographical areas, both the reference values used for EQRs calculation and the boundaries among ICMi quality classes are different among river macrotypes (MATTM 2010). For the ICMi calculation, the software Omnidia 5.5 (Lecointe et al. 1993) was used to get the specific sensibility and indicator values for IPS and TI calculation. We avoided using the official Italian taxa list provided by Mancini and Sollazzo (2009) because of some limitation, such as the absence of some common species (e.g., Salmaso et al. 2014; Falasco et al. 2012), and the presence of some wrong values for IPS and TI calculation.
The total number of taxonomical units (species or subspecies, i.e., the community taxonomical richness) and the Shannon-Wiener index (S-W index, i.e., community diversity) were calculated for each sample; the average values of the same metrics for the three river macrotypes were calculated as well. The differences among macrotypes were tested for significance (p < 0.05) using the Kruskal-Wallis test and the Dunn post hoc test.
Each taxon was assigned to one of the four ecological guilds described by Rimet and Bouchez (2012): low-profile (L, i.e., small, fast-growing diatoms), high profile (H, i.e., large or colonial diatoms), motile (M, i.e., fast-moving diatoms), and planktic (P, i.e., diatoms adapted to lentic environment).
A discriminant analysis (DA) was performed using the relative abundance of taxa collected at each sampling site, to detect if taxonomical composition differed among the three river macrotypes. Another DA was performed using data about the relative abundance of diatoms (hereafter called abu_L, abu_H, abu_M, and abu_P, respectively) and the number of diatom taxa (hereafter called n_taxa_L, n_taxa_H, n_taxa_M, and n_taxa_P, respectively) belonging to the four ecological guilds, to detect if these guilds could be predictors of the diatom communities of the three river macrotypes. For both the DAs, log-transformed data about single samples were used.
In order to avoid misinterpretation of DA results, we preliminarily checked seasonal variability in community composition through one-way ANOSIM, which allowed excluding significant (p < 0.05) differences in taxonomical composition and ecological guilds composition among sampling seasons (spring vs summer).
Moreover, one-way ANOSIM was used to test significant (p < 0.05) differences in taxonomical composition and ecological guilds composition among river macrotypes.
In order to investigate possible environmental determinants of diatom communities in terms of ecological guilds composition, three redundancy analyses (RDAs) on the guilds composition of the three different river macrotypes were performed. The values of the environmental variables used for the RDAs are shown in Supplementary Material—Table SM1 and include geographical, chemical, and hydrological parameters. The geographical variables were altitude (Alt, m asl), upstream catchment area (catch, km2), and the degree of shading of the sampling site (shade, defined as follows: sites without shading = 0, sites temporarily shaded by trees canopy = 1, and sites permanently shaded by mountains = 2). The chemical and physical variables were NO3−-N (mg L−1), NH4+-N (mg L−1), TP (mg L−1), BOD5 (mg L−1), and pH, all expressed as median values for the 3-year study period. TSS was not included in the analyses because it resulted lower than detection limit for the majority of samplings. The hydrological parameters were Qmr (m3 s−1), Qm/Qmr, QCV, FRE3 (y−1), daysLF (d y−1). The response variables included abu_L, abu_H, abu_M, abu_P, n_taxa_L, n_taxa_H, n_taxa_M, and n_taxa_P. For the response variables, as well as for chemical and physical explanatory variables, median values for each sampling site were used in order to get the same number of observations (i.e., one observation per site). Both explanatory and response variables were standardized (mean = 0 and standard deviation = 1) to avoid data with different measurement units in the same analysis.
In the ordination analyses, for each sample, only taxa with relative abundance ≥ 5% were used.
Variations among river macrotypes in ICMi, IPS, TI, and the ecological quality ratios of the IPS (EQR_IPS) and TI (EQR_TI) were tested for significant (p < 0.05) differences using the Kruskal-Wallis test and the Dunn post hoc test.
All the numerical analyses were performed using XLSTAT2011 software (Addinsoft 2011).
Results
A total of 201 diatom taxa (at species or subspecies level) were detected in the study area (107 in macrotype A2, 114 in A1 and 152 in C).
On average, the diatom communities showed significantly (p < 0.05) higher richness (i.e., n. species) and diversity (i.e., S-W index) at the lowland sites (i.e., macrotype C) than at the Alpine sites (i.e., macrotypes A1 and A2) (Fig. 2). However, in all the three river macrotypes, Achnanthidium minutissimum (Kützing) Czarnecki (ADMI) was the most abundant taxon (Fig. 2). At A2 sites, this species was dominant (i.e., 30.2% of the total abundance), while it showed a relative abundance similar to Achnanthidium pyrenaicum (Hustedt) Kobayasi 1997 (ADPY) (i.e., approximately 21%) at A1 sites; at C sites, it showed a weaker dominance (16.5% of the total abundance), as a consequence of higher diversity.
Average (+ standard deviation) relative abundance of diatom taxa in river macrotypes A1 (a), A2 (b) and C (c). For brevity, only the 15 most abundant taxa are shown per each macrotype. Number of species (n. species) and values of Shannon-Wiener diversity index (S-W index) are also reported, as average ± standard deviation
Benthic diatom communities differed among the three river macrotypes also in terms of ecological guilds composition, but the most abundant ecological guild was the same for the three macrotypes, i.e., the low-profile guild (70% of total abundance as median value, Table 3).
Differences among river macrotypes, tested through one-way ANOSIM, were highly significant (p < 0.001) for both taxonomical composition and ecological guilds composition.
The a priori grouping according to the river macrotype was confirmed by the DA on taxonomical composition (Fig. 3a) for 92% of the observations. The relative abundances of Cocconeis placentula Ehrenberg (CPLA) and ADPY were the most important parameters differentiating the diatom assemblages of the macrotype C from those of the two macrotypes A, followed by the relative abundances of Nitzschia fonticola Grunow (NFON), Navicula cryptotenella Lange-Bertalot (NCTE), and Fragilaria capucina var. vaucheriae (Kutzing) Lange-Bertalot (FCVA). The highest abundances of CPLA, NFON, NCTE, and FCVA were found at the lowland sites, while the one of ADPY was recorded at the Alpine sites. The two mountainous macrotypes differed each other mainly for the relative abundances of Cocconeis placentula var. lineata (Ehr) Van Heurck (CPLI) and Fragilaria arcus (Ehrenberg) Cleve (FARC), which were higher at A2 sites, and for the relative abundances of Cocconeis pediculus Ehrenberg (CPED), Diatoma moniliformis Kützing (DMON), Gomphonema tergestinum (Grunow) Schmidt (GTER), and Cocconeis euglypta Ehrenberg emend Romero & Jahn (CEUG), which were higher at A1 sites (Fig. 3a).
Ordination plots of the discriminant analyses calculated using samplings as observations (n = 6 per site) and river macrotypes as groupings (left) and distribution on the same plots of the variables used in the ordination (right). a Relative abundances of taxa were used as variables. Taxa with relative abundance lower than 5% were excluded. F1 explained variation = 80.41%, F2 explained variation = 19.59%. b Values of abundance and taxa richness per each ecological guild were used as variables. F1 explained variation = 83.07%, F2 explained variation = 16.93%
The DA on ecological guilds (Fig. 3b) generally confirmed these results. However, river macrotypes appeared less clearly distinguished than in the previous analysis, and the a priori grouping was confirmed for 79% of the observations. The most important ecological parameters differentiating the diatom assemblages were abu_L, higher for Alpine macrotypes than for the lowland one, n_taxa_P, n_taxa_M and abu_M, higher at C sites, and n_taxa_L, n_taxa_H, and abu_H, characterizing A1 sites (Fig. 3b).
The RDAs on the guilds composition of the three different river macrotypes (Fig. 4) allowed to explore environmental parameters influencing diatom communities, excluding major geographical variability.
Biplots of first and second RDA axes for the monitoring sites of river macrotypes A1 (plot a; PC1 explained variation = 46.17%, PC2 = 25.64%), A2 (plot b; PC1 = 40.11%, PC2 = 30.16%), and C (plot c; PC1 = 49.06%, PC2 = 27.06%). For variables description see the “Materials and methods” section
Within river macrotype A1 (Fig. 4a), the first RDA axis (PC1, explained variation 46%) was positively correlated with pH and degree of shading, clearly differentiating site Lm1 (characterized by higher values of taxonomic richness and abundance of high-profile and planktic diatoms) from all the other sites. On the second axis (PC2, 26%), sites at higher altitude, with small drainage area and low Qmr, but more impacted chemically (mainly for TP, NH4+-N and BOD5) and hydrologically (low Qm/Qmr and high daysLF), were characterized by motile diatoms.
Within river macrotype A2 (Fig. 4b), the first RDA axis (PC1, 40%) separated valley sites characterized by higher nutrients concentration (NO3−-N and NH4+-N), Qmr and catchment area, from the high-altitude sites. Motile taxa prevailed at valley reaches, and low-profile diatoms dominated the communities at high-altitude sites. Along the second RDA axis (PC2, 30%), the sites were ordered on the basis of hydrological alteration, with more altered sites (i.e., higher daysLF) characterized by communities mainly dominated by high-profile diatoms and characterized by high taxonomic richness of low-profile diatoms.
Within river macrotype C (Fig. 4c), the first RDA axis (PC1, 49%) separated the more chemically impacted sites (i.e., with higher nutrients concentration), dominated by motile diatoms, from the more hydrologically impacted sites (i.e., with higher QCV and daysLF), dominated by low-profile diatoms and characterized by high taxonomic richness of high-profile diatoms. The second axis (PC2, 27%) was positively correlated with altitude and clearly differentiated the site Ad3 from all the other sites. This site was characterized by high abundance of planktic and high-profile diatoms and by a high number of low-profile taxa.
Based on the ICMi, 94% of the sampling sites resulted in high ecological status, and 6% in good ecological status (as average on the six samples). Regarding the application of the three diatom indices (i.e., IPS, TI, and ICMi; Fig. 5 and Table 3), both IPS and TI indicated a significantly lower quality (Kruskal-Wallis, p < 0.05) at the lowland sites than at the Alpine sites (bear in mind that for IPS, the higher the value, the better the quality, while the opposite is valid for TI). After normalizing by reference values, EQR_IPS resulted significantly different (p < 0.05) only between A1 and A2 macrotypes (i.e., lower in A2 than in A1, Table 3). The EQR_TI, as well as the ICMi, was significantly different (p < 0.05) among the three macrotypes (i.e., ordered from the highest to the lowest as follows: C, A1, A2, Table 3).
Discussion
Diatom assemblages of the study sites
As expected, the diatom assemblages detected at the 34 study sites differed according to the river macrotype sensu WFD. The communities of lowland watercourses (i.e., macrotype C) differed from those of Alpine watercourses (i.e., macrotypes A1 and A2) for the lower relative abundance of pioneer taxa such as ADPY, and for the higher richness and diversity. CPLA, NFON, NCTE, and FCVA can be considered representative taxa of lowland rivers. Among these, CPLA and FCVA are known to be indicators of polluted waters (Szczepocka et al. 2015), while NFON and NCTE, such as other species of Nitzchia and Navicula genera, are silt-tolerant diatoms, able to withstand high current velocity and sediment transport (Bona et al. 2012).
The diatom assemblages of Alpine watercourses in calcareous areas (i.e., macrotype A1) had higher richness and diversity than those of Alpine siliceous watercourses (i.e., macrotype A2). CPED, DMON, and GTER, which are good indicators of unpolluted waters, can be considered representative species for macrotype A1. Differently, CPLI and FARC characterized macrotype A2. They are typical pioneer diatoms of headwaters, with preference for oligotrophic environments (Bona et al. 2012).
The presence of ADMI as dominant species was a common feature of the three macrotypes, probably as an effect of the streamflow alteration (Biggs et al. 1998) characterizing all the study sites. However, as already reported by Kelly (2013), the autecology of this species is still debated, also because of its troubled taxonomy.
Difference between macrotypes in terms of community guilds composition was less accentuated than that regarding taxonomical composition, suggesting that taxonomically different assemblages do not necessarily imply differences in community functionality and that the use of ecological guilds could help the ecological interpretation of complex diatom datasets by discriminating between community structural variability and functional variability. Typical characteristics of the guilds composition in the three macrotypes can be identified as follows. Alpine watercourses were quantitatively dominated by low-profile diatoms. In calcareous areas, this guild was represented by a high number of species, differently from siliceous watercourses that, as already mentioned, resulted species-poor environments. Moreover, at calcareous Alpine sites, high-profile diatoms were also abundant and represented by numerous taxa. Lowland watercourses and valley reaches of Alpine watercourses were instead characterized by high abundance and richness of motile diatoms. In lowland areas, some planktic species were present as well, even if with low abundance.
Environmental variables structuring diatom assemblages
The differences among communities described above mirror the different environmental characteristics and perturbations of the study sites. First, the noticeable distinction among diatom communities from different river macrotypes confirms the importance of natural environmental variables linked to geography, as geology and altitude, as already highlighted by many authors (Potapova and Charles 2002; Rimet 2009; Beltrami et al. 2012; Bona et al. 2012). For instance, Rimet (2009) found that geology was the main factor structuring diatom assemblages in France, clearly separating communities from limestone and siliceous catchments. Moreover, Mao et al. (2018) analyzed the structure of diatom communities in a long river system and stressed the importance of longitudinal variation in physical habitat conditions, overwhelming changes due to water quality. Accordingly, in our study sites, major geographical characteristics played a stronger role as drivers of the community composition, if compared to local environmental factors, including those affected by anthropic alteration. Similar relationships were found among riparian plant communities and geographical features (e.g., Angiolini et al. 2011; Engelhardt et al. 2011; Nucci et al. 2012).
In the three analyzed river macrotypes, the environmental factors defining the functional composition of the diatom communities were different.
In Alpine siliceous watercourses (A2), altitude resulted the key determinant: at lower altitude, assemblages are characterized by higher abundance and richness of motile diatoms, while at higher altitude, low-profile diatoms prevail. However, altitude seems to act indirectly on assemblages, through other parameters which are typically negatively correlated with altitude, as catchment area, water discharge, and nutrients (NO3−-N and NH4+-N) concentration. In fact, the increase of nutrients concentration from upland to lowland is expected as a result of (i) leaching and increase of catchment area and (ii) increase of human population density and of agricultural and zootechnical activities. Thus, the guild composition of the communities in this macrotype was defined by a complex mix of natural and anthropogenic causes.
Since the altitude range concerning the investigated Alpine calcareous watercourses (A1) is narrower than that concerning the siliceous ones (370–850 m asl in the former, 238–1740 m asl in the latter), altitude and related parameters have a minor importance than anthropogenic impairment in shaping the diatom communities. In particular, a high number of species of motile diatoms was detected as a consequence of chemical alteration. Differently from siliceous valley sites, in this case, chemical alteration seems to be due to point source pollution rather than to a diffuse input of nutrients. Indeed, in A1 sites, chemical alteration does not concern valley sites, and nutrients which correlate with biological patterns were TP and NH4+-N. At A1 sites, noticeable high abundance and number of taxa of high-profile and planktic diatoms were detected at Lm1, a reach characterized by high pH values and shaded streambed. We can hypothesize that in permanently shaded sites, high-profile diatoms could be favored by their morphological adaptations (i.e., large surface, erect position, growth in upper layers of microbial mats, Marcel et al. 2017) which maximize the contact with water matrix and thus the exposition to light. Regarding planktic diatoms, it should be underlined that Lm1 is located at a short distance downstream from a large reservoir, which could be the source of these taxa.
In lowland watercourses (C), all the study sites are in low-altitude areas with strong anthropogenic pressure. Therefore, environmental factors linked to human activities resulted the major determinants of the guild composition of diatom communities, and different biological features were linked to different environmental stressors. In particular, high abundance and number of taxa of motile diatoms were detected in response to chemical alteration (high NO3−-N and TP concentrations), while sites affected by heavy hydrological alteration (high QCV, daysLF and FRE3) were numerically dominated by low-profile diatoms and characterized by a high number of taxa of high-profile diatoms. Moreover, at Ad3, as well as for Lm1, the position (i.e., short distance below Lake Como) and the massive upstream withdrawals can be linked to the high abundance of planktic and high-profile diatoms.
Overall, motile diatoms were the most variable component of the studied communities: their abundance and richness increased in response to nutrient enrichment, linked to both point source and diffuse pollution and to the natural increase of drainage area. This guild was already associated to high nutrient concentrations (Passy 2007) because motile diatoms are capable of rapid displacement and migration within the biofilm from resource-depleted to richer areas (Johnson et al. 1997).
Low-profile diatoms were the most abundant guild in the study area, probably due to the streamflow alteration characterizing all the study sites. In particular, at lowland sites, the relative abundance of low-profile diatoms increased with hydrological alteration (in terms of discharge variability and flood frequency). Indeed, as observed by Rimet and Bouchez (2012), diatoms belonging to this guild are resistant to physical disturbance linked to water turbulence and current velocity, which are often modified in magnitude and predictability at hydrologically altered sites. At uppermost sites, low-profile diatoms strongly dominated the community, probably because pioneer species can withstand the extreme conditions characterizing these sites.
Planktic diatoms were rare in the study area and mainly observed downstream of lakes, which seem to represent their source.
Finally, it was more difficult to find out clear relationships between high-profile diatoms and the considered environmental variables. Indeed, due to their morphology, high-profile diatoms are influenced by many factors, including predation (Marcel et al. 2017), a pressure not considered in this study. In fact, invertebrate grazers are more successful at ingesting large, high-profile diatom taxa than small, adnate taxa (e.g., Peterson 1987; Feminella and Hawkins 1995; Steinman 1996). As a consequence, at sites where physical disturbance is considerable, this environmental factor both directly favors low-profile diatoms and indirectly controls grazers, thus influencing the trophic web.
Diatoms as bioindicators sensu WFD
As previously discussed, a link between anthropogenic pressure and the composition of diatom communities emerges for all the three river macrotypes. Thus, in agreement with other authors (see Tapolczai et al. 2016), our study confirms that an alteration of diatom communities structure and function occurs in response to environmental alterations.
As expected, the indices IPS and TI highlighted a significantly lower quality in lowland watercourses (macrotype C), characterized by higher chemical impairment compared to the Alpine ones. However, the ecological status of diatom communities detected through the ICMi (i.e., sensu WFD) resulted significantly higher in lowland watercourses (C) than in the Alpine ones (and, similarly, it resulted significantly higher in A1 than in A2). This difference is clearly connected to the normalization of the IPS and TI values before the ICMi calculation. As mentioned, normalization has the key function to allow for comparisons among different geographical areas, where different chemical characteristics of freshwaters are expected, even in pristine conditions. However, our results showed that in Alpine siliceous watercourses (A2), the abiotic factors mainly influencing the diatom communities are predominantly natural, while in macrotypes A1 and C, they are mostly linked to anthropogenic pressures. Therefore, the reference values currently available for the calculation of EQRs appear unreliable to support a sound application of the ICMi. We believe that a single reference value for each river macrotype is inadequate to account for the variability inside the macrotype, as already proposed by Rimet (2009) in France. For instance, the macrotype C includes numerous and very different watercourse typologies (MATTM 2010) and, in our case, its reference values resulted too low for the application in lowland sites. The status of diatom communities based on the ICMi was classified as good or even high for all our study sites despite evident differences in the severity of anthropogenic alterations. Consequently, the use of the ICMi in the form currently provided by the Italian law does not allow to correctly assess the differences among sites, even in terms of water quality.
Moreover, the complex and frequently changing taxonomy of diatoms makes the identification process time-consuming and requiring high expertise (see also Zampella et al. 2007).
In summary, the low reliability of the ICMi combined to the difficulty in the interpretation of taxonomic datasets, limits the use of diatoms in Italian biomonitoring programs. Actually, their application in streamflow management programs was recently officially reduced (Regione Lombardia 2014; MATTM 2017). This tendency could be reversed by revising current approach following recent international experiences (i.e., Charles et al. (2019) and Pardo et al. (2018)). In this framework, as confirmed by our results, ecological guilds may provide a robust (since not relying on high resolution taxonomy—i.e., species or subspecies level, with problems linked to unstable taxonomy) and user-friendly tool supporting reliable characterization of the ecological condition of watercourses, also in the WFD context.
References
Addinsoft. (2011). Data analysis and statistical solution for Microsoft excel. Paris: Addinsoft.
Angiolini, C., Nucci, A., Frignani, F., & Landi, M. (2011). Using multivariate analyses to assess effects of fluvial type on plant species distribution in a Mediterranean river. Wetlands, 31, 167–177. https://doi.org/10.1007/s13157-010-0118-7.
APAT-IRSA/CNR. (2003). Metodi analitici per le acque. APAT Manuali e linee guida 29/2003.
Battarbee, R., Jones, V., Flower, R., et al. (2001). Diatoms. In J. Smol, H. J. Birks, W. Last, R. Bradley, & K. Alverson (Eds.), Tracking environmental change using lake sediments (pp. 155–202). Netherlands: Springer.
B-Béres, V., Török, P., Kókai, Z., Krasznai, E. T., Tóthmérész, B., & Bácsi, I. (2014). Ecological diatom guilds are useful but not sensitive enough as indicators of extremely changing water regimes. Hydrobiologia, 738, 191–204. https://doi.org/10.1007/s10750-014-1929-y.
Beltrami, M. E., Ciutti, F., Cappelletti, C., Lösch, B., Alber, R., & Ector, L. (2012). Diatoms from alto Adige/Südtirol (northern Italy): characterization of assemblages and their application for biological quality assessment in the context of the water framework directive. Hydrobiologia, 695, 153–170. https://doi.org/10.1007/s10750-012-1194-x.
Bey, M. Y., & Ector, L. (2013). Atlas des diatomées des cours d'eau de la région Rhone-Alpes. Lippmann: Centre de Recherche Public Gabriel.
Biggs, B. J. F., Stevenson, R. J., & Lowe, R. L. (1998). A habitat matrix conceptual model for stream periphyton. Archiv für Hydrobiologie, 143, 21–56. https://doi.org/10.1127/archiv-hydrobiol/143/1998/21.
Bona, F., Falasco, E., Fenoglio, S., Iorio, L., & Badino, G. (2008). Response of macroinvertebrate and diatom communities to human-induced physical alteration in mountain streams. River Research and Applications, 24, 1068–1081. https://doi.org/10.1002/rra.1110.
Bona, F., La Morgia, V., & Falasco, E. (2012). Predicting river diatom removal after shear stress induced by ice melting. River Research and Applications, 28, 1289–1298. https://doi.org/10.1002/rra.1517.
Brignoli, M. L., Espa, P., & Batalla, R. J. (2017). Sediment transport below a small alpine reservoir desilted by controlled flushing: field assessment and one-dimensional numerical simulation. Journal of Soils and Sediments, 17, 2187–2201. https://doi.org/10.1007/s11368-017-1661-0.
Carrère, P., & Bloor, J. M. G. (2009). Lexique thématique à l’usage des techniciens en écologie. Clermont-Ferrand: INRA, EFPA, Unité de Recherche sur l’Ecosystème Prairial 7 p.
CEMAGREF. (1982). Etude de méthodes biologiques quantitatives d’appréciation de la qualité des eaux. Rapport Q.E. Lyon-A.F.B. Rhône-Mediterranée-Corse.
CEN. (2004). UNI EN 14407:2004Water Quality - Guidance Standard For The Identification, Enumeration And Interpretation Of Benthic Diatom Samples From Running Waters. Available at https://infostore.saiglobal.com.
CEN. (2005). UNI EN 13946:2005Water Quality - Guidance standard for the routine sampling and pretreatment of benthic diatoms from rivers. Available at https://infostore.saiglobal.com.
Charles, D. F., Tuccillo, A. P., & Belton, T. J. (2019). Use of diatoms for developing nutrient criteria for rivers and streams: a biological condition gradient approach. Ecological Indicators, 96, 258–269. https://doi.org/10.1016/j.ecolind.2018.08.048.
Clausen, B., & Biggs, B. (1997). Relationships between benthic biota and hydrological indices in New Zealand streams. Freshwater Biology, 38, 327–342. https://doi.org/10.1046/j.1365-2427.1997.00230.x.
EC. (2000). Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities, L327/1. Brussels, European Commission.
Engelhardt, B. M., Weisberg, P. J., & Chambers, J. C. (2011). Influences of watershed geomorphology on extent and composition of riparian vegetation. Journal of Vegetation Science, 23, 127–139. https://doi.org/10.1111/j.1654-1103.2011.01328.x.
Espa, P., Castelli, E., Crosa, G., & Gentili, G. (2013). Environmental effects of storage preservation practices: controlled flushing of fine sediment from a small hydro-power reservoir. Environmental Management, 52, 261–276. https://doi.org/10.1007/s00027-009-0117-z.
Espa, P., Crosa, G., Gentili, G., Quadroni, S., & Petts, G. (2015). Downstream ecological impacts of controlled sediment flushing in an alpine valley river: a case study. River Research and Applications, 31, 931–942. https://doi.org/10.1002/rra.2788.
Espa, P., Brignoli, M. L., Crosa, G., Gentili, G., & Quadroni, S. (2016). Controlled sediment flushing at the Cancano reservoir (Italian Alps): management of the operation and downstream environmental impact. Journal of Environmental Management, 182, 1–12. https://doi.org/10.1016/j.jenvman.2016.07.021.
Falasco, E., Mobili, L., Risso, A. M., & Bona, F. (2012). First considerations on the ICMi diatom index application in north-west Italy. Biologia Ambientale, 26, 21–28.
Falasco, E., Piano, E., & Bona, F. (2013). Guida al riconoscimento e all’ecologia delle principali diatomee fluviali dell’Italia nord occidentale. Biologia Ambientale, 27, 1–288.
Feminella, J. W., & Hawkins, C. P. (1995). Interactions between stream herbivores and periphyton: a quantitative analysis of past experiments. Journal of the North American Benthological Society, 14, 465–509. https://doi.org/10.2307/1467536.
Fourtanier, E., & Kociolek, J. P. (2011). Catalogue of Diatom Names, California Academy of Sciences, on-line version updated 19 sep 2011 available online at: http://research.calacademy.org/research/diatoms/names/index.asp.
Guiry, M. D., & Guiry, G. M. (2015). AlgaeBase, World-wide electronic publication. Galway: National University of Ireland http://www.algaebase.org.
Heath, M. W., Wood, S. A., Brasell, K. A., Young, R. G., & Ryan, K. G. (2015). Development of habitat suitability criteria and in-stream habitat assessment for the benthic cyanobacteria Phormidium. River Research and Applications, 31, 98–108. https://doi.org/10.1002/rra.2722.
Hofmann, G., Werum, M., & Lange-Bertalot, H. (2011). Diatomeen im Sϋβwasser-Benthos von Mitteleuropa. A.R.G. Gantner Verlag K.G., 908 pp.
Hoyle, J. T., Kilroy, C., Hicks, D. M., & Brown, L. (2017). The influence of sediment mobility and channel geomorphology on periphyton abundance. Freshwater Biology, 62, 258–273. https://doi.org/10.1111/fwb.12865.
ISPRA. (2014). Protocollo di campionamento e analisi delle diatomee bentoniche dei corsi d’acqua. In: Metodi biologici per le acque superficiali interne. Roma: ISPRA, Manuali e linee guida, 111/2014.
Johnson, R. E., Tuchman, N. C., & Peterson, C. G. (1997). Changes in the vertical microdistribution of diatoms within a developing periphyton mat. Journal of the North American Benthological Society, 16, 503–519. https://doi.org/10.2307/1468140.
Jüttner, I., Sharma, S., Dahal, B. M., Ormerod, S. J., Chimonides, P. J., & Cox, E. J. (2003). Diatoms as indicators of stream quality in the Kathmandu Valley and Middle Hills of Nepal and India. Freshwater Biology, 48, 2065–2084. https://doi.org/10.1046/j.1365-2427.2003.01138.x.
Kelly, M. (2013). Data rich, information poor? Phytobenthos assessment and the Water Framework Directive. European Journal of Phycology, 48, 437–450. https://doi.org/10.1080/09670262.2013.852694.
Kornan, M., & Kropil, R. (2014). What are ecological guilds? Dilemma of guild concepts. Russian Journal of Ecology, 45, 445–447. https://doi.org/10.1134/S1067413614050178.
Krammer, K. (2002). Cymbella—diatoms of Europe volume 3. Diatoms of the European inland waters and comparable habitats. A. R. G. GantnerVerlag, Rugel.
Krammer, K., & Lange-Bertalot, H. (1986). Bacillariophyceae. 1. Teil: Naviculaceae, Sϋβwasserflora von Mitteleuropa, 2/1. Stuttgart: Gustav Fischer Verlag Neuauflage 1997. REPRINT 2007.
Krammer, K., & Lange-Bertalot, H. (1988). Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. Sϋβwasserflora von Mitteleuropa, 2/2. Stuttgart: Gustav Fischer Verlag Neuauflage 1997. REPRINT 2007.
Krammer, K., & Lange-Bertalot, H. (1991a). Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. Sϋβwasserflora von Mitteleuropa, 2/3. Stuttgart: Gustav Fischer Verlag CORRECTED REPRINT 2004.
Krammer, K., & Lange-Bertalot, H. (1991b). Bacillariophyceae. 4 Teil: Achnanthaceae, kritische Erganzungen zu Achnanthes s.l., Navicula s.str. und Gomphonema. Sϋβwasserflora von Mitteleuropa, 2/4. Stuttgart: Gustav Fischer Verlag REV. ED. 2004.
Krammer, K., & Lange-Bertalot, H. (2000). Bacillariophyceae. Part 5: English and French translation of the keys. Sϋβwasserflora von Mitteleuropa, 2/5. Stuttgart: Gustav Fischer Verlag.
Lange, K., Townsend, C. R., & Matthaei, C. D. (2016). A trait-based framework for stream algal communities. Ecology and Evolution, 6, 23–36. https://doi.org/10.1002/ece3.1822.
Lange-Bertalot, H. (2001) Navicula sensu stricto, 10 genera separated from Navicula sensu lato, Frustulia - diatoms of Europe, volume 2. Diatoms of the European inland waters and comparable habitats. A. R. G. GantnerVerlag, Rugell.
Lecointe, C., Coste, M., & Prygiel, J. (1993). “Omnidia”: software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia, 269(270), 509–513. https://doi.org/10.1007/BF00028048.
Lobo, E. A., Callegaro, V. L. M., Hermany, G., Bes, D., Wetzel, C. A., & Oliveira, M. A. (2004). Use of epilithic diatoms as bioindicators from lotic systems in southern Brazil, with special emphasis on eutrophication. Acta Limnologica Brasilensia, 16, 25–40.
MacMahon, J. A., Schimpf, D. J., Andersen, D. C., Smith, K. G., & Bayn, R. L. (1981). An organism-centered approach to some community and ecosystem concepts. Journal of Theoretical Biology, 29, 287–307. https://doi.org/10.1016/0022-5193(81)90077-1.
Mancini, L., & Sollazzo, C. (Eds.). (2009). Metodo per la valutazione dello stato ecologico delle acque correnti: comunità diatomiche. Roma: Istituto Superiore di Sanità (Rapporti ISTISAN 09/19).
Mao, S., Guo, S., Deng, H., Xie, Z., & Tang, T. (2018). Recognition of patterns of benthic diatom assemblages within a river system to aid bioassessment. Water, 10, 1559. https://doi.org/10.3390/w10111559.
Marcel, R., Berthon, V., Castets, V., Rimet, F., Thiers, A., Labat, F., & Fontan, B. (2017). Modelling diatom life forms and ecological guilds for river biomonitoring. Knowledge & Management of Aquatic Ecosystems, 418, 1–15. https://doi.org/10.1051/kmae/2016033.
MATTM. (2010). D.M. Ambiente 8 novembre 2010 n. 260. Regolamento recante i criteri tecnici per la classificazione dello stato dei corpi idrici superficiali, per la modifica delle norme tecniche del decreto legislativo 3 aprile 2006, n.152, recante norme in materia ambientale, predisposto ai sensi dell’articolo 75, comma 3, del medesimo decreto legislativo.
MATTM. (2017). Decreto n. 30/STA del 13/02/2017, di approvazione delle Linee Guida per l’aggiornamento dei metodi di determinazione del deflusso minimo vitale al fine di garantire il mantenimento nei corsi d’acqua del deflusso ecologico a sostegno del raggiungimento degli obiettivi di qualità ambientale dei corpi idrici definiti ai sensi della Direttiva 2000/60/CE.
Montgomery, D. R., & Buffington, J. M. (1997). Channel-reach morphology in mountain drainage basins. Geological Society of America Bulletin, 109, 596–611. https://doi.org/10.1130/0016-7606(1997)109b0596:CRMIMDN2.3.CO;2.
Nhiwatiwa, T., Dalu, T., & Sithole, T. (2017). Assessment of river quality in a subtropical austral river system: a combined approach using benthic diatoms and macroinvertebrates. Applied Water Science, 7, 4785–4792. https://doi.org/10.1007/s13201-017-0599-0.
Nucci, A., Angiolini, C., Landi, M., & Bacchetta, G. (2012). Influence of bedrock-alluvial transition on plant species distribution along a Mediterranean river corridor. Plant Biosystems, 146, 564–575. https://doi.org/10.1080/11263504.2012.670669.
o’Driscoll, C., de Eyto, E., Rodgers, M., O’Connor, M., Kelly, M., & Xiao, L. (2014). Spatial and seasonal variation of peatland-fed riverine macroinvertebrate and benthic diatom assemblages and implications for assessment: a case study from Ireland. Hydrobiologia, 728, 67–87. https://doi.org/10.1007/s10750-014-1807-7.
Pardo, I., Delgado, C., Abraín, R., Gómez-Rodríguez, C., García-Roselló, E., García, L., & Reynoldson, T. B. (2018). A predictive diatom-based model to assess the ecological status of streams and rivers of northern Spain. Ecological Indicators, 90, 519–528. https://doi.org/10.1016/j.ecolind.2018.03.042.
Passy, S. I. (2007). Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquatic Botany, 86, 171–178. https://doi.org/10.1016/j.aquabot.2006.09.018.
Peterson, C. G. (1987). Gut passage and insect grazer selectivity of lotic diatoms. Freshwater Biology, 18, 455–460. https://doi.org/10.1111/j.1365-2427.1987.tb01330.x.
Poikane, S., Kelly, M., & Cantonati, M. (2016). Benthic algal assessment of ecological status in European lakes and rivers: challenges and opportunities. Science of the Total Environment, 568, 603–613. https://doi.org/10.1016/j.scitotenv.2016.02.027.
Potapova, M., & Charles, D. F. (2002). Benthic diatoms in USA rivers: distribution along spatial and environmental gradients. Journal of Biogeography, 29, 167–187. https://doi.org/10.1046/j.1365-2699.2002.00668.x.
Quadroni, S., Brignoli, M. L., Crosa, G., Gentili, G., Salmaso, F., Zaccara, S., & Espa, P. (2016). Effects of sediment flushing from a small Alpine reservoir on downstream aquatic fauna. Ecohydrology, 9, 1276–1288. https://doi.org/10.1002/eco.1725.
Quadroni, S., Crosa, G., Gentili, G., & Espa, P. (2017). Response of stream benthic macroinvertebrates to current water management in Alpine catchments massively developed for hydro-power. Science of the Total Environment, 609, 484–496. https://doi.org/10.1016/j.scitotenv.2017.07.099.
Regione Lombardia. (2014). D.d.g. 8 maggio 2014 - n. 3816 Integrazione del d.d.g. n. 9001 dell’8 agosto 2008. Approvazione delle linee guida per l’avvio di sperimentazioni sul deflusso minimo vitale in tratti del reticolo idrico naturale regionale. Bollettino Ufficiale regione Lombardia, Serie Ordinaria n. 20 - Lunedì 12 maggio 2014.
Rimet, F. (2009). Benthic diatom assemblages and their correspondence with ecoregional classifications: case study of rivers in north-eastern France. Hydrobiologia, 636, 137–151. https://doi.org/10.1007/s10750-009-9943-1.
Rimet, F., & Bouchez, A. (2012). Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowledge and Management of Aquatic Ecosystems, 406, 1–12. https://doi.org/10.1051/kmae/2012018.
Root, R. B. (1967). The niche exploitation pattern of the Blue-Gray Gnatcatcher. Ecological Monographs, 37, 317–350. https://doi.org/10.2307/1942327
Rott, E., Pipp, E., Pfister, P., Van Dam, H., Ortler, K., Binder, N., & Pall, K. (1999). Indikationslisten für Aufwuchsalgen in Österreichischen Fliessgewassern. Teil 2: Trophieindikation. Vienna: Bundesministerium für Land- und Forstwirtschaft.
Salmaso, F., Quadroni, S., Romanò, A., Compare, S., Gentili, G., & Crosa, G. (2014). Ecological status definition according to D.M. 260/2010 in two lowland rivers (Adda and Ticino) characterized by minimum flow. Biologia Ambientale, 28, 25–37.
Salmaso, F., Quadroni, S., Gentili, G., & Crosa, G. (2017). Thermal regime of a highly regulated Italian river (Ticino River) and implications for aquatic communities. Journal of Limnology, 76, 23–33. https://doi.org/10.4081/jlimnol.2016.1437.
Salmaso, F., Crosa, G., Espa, P., Gentili, G., Quadroni, S., & Zaccara, S. (2018). Benthic macroinvertebrates response to water management in a lowland river: effects of hydro-power vs irrigation off-stream diversions. Environmental Monitoring and Assessment, 190, 33. https://doi.org/10.1007/s10661-017-6390-8.
Simberloff, D., & Dayan, T. (1991). The guild concept and the structure of ecological communities. Annual Review of Ecology and Systematics, 22, 115–143. https://doi.org/10.1146/annurev.es.22.110191.000555.
Soininen, J. (2007). Environmental and spatial control of freshwater diatoms—a review. Diatom Research, 22, 473–490. https://doi.org/10.1080/0269249X.2007.9705724.
Stancheva, R., & Sheath, R. G. (2016). Benthic soft-bodied algae as bioindicators of stream water quality. Knowledge and Management of Aquatic Ecosystems, 417, 15. https://doi.org/10.1051/kmae/2016002.
Steinman, A. D. (1996). Effects of grazers on freshwater benthic algae. In R. J. Stevenson, M. L. Bothwell, R. J. Lowe, & J. H. Thorp (Eds.), Algal ecology (pp. 341–373). San Diego: Academic Press.
Stevenson, R. J., & Pan, Y. (1999). Assessing environmental conditions in rivers and streams with diatoms. In F. Stoermer & J. P. Smol (Eds.), The diatoms: Applications for the environmental and earth sciences (pp. 11–40). Cambridge: Cambridge University Press.
Szczepocka, E., Kruk, A., & Rakowska, B. (2015). Can tolerant diatom taxa be used for effective assessments of human pressure? River Research and Applications, 31, 368–378. https://doi.org/10.1002/rra.2744.
Tan, X., Ma, P., Bunn, S. E., & Zhang, Q. (2015). Development of a benthic diatom index of biotic integrity (BD-IBI) for ecosystem health assessment of human dominant subtropical rivers, China. Journal of Environmental Management, 151, 286–294. https://doi.org/10.1016/j.jenvman.2014.12.048.
Tapolczai, K., Bouchez, A., Stenger-Kovács, C., Padisák, J., & Rimet, F. (2016). Trait-based ecological classifications for benthic algae: review and perspectives. Hydrobiologia, 776, 1–17. https://doi.org/10.1007/s10750-016-2736-4.
Vasiljević, B., Simić, S. B., Paunović, M., Zuliani, T., Krizmanić, J., Marković, V., & Tomović, J. (2017). Contribution to the improvement of diatom-based assessments of the ecological status of large rivers—the Sava River case study. Science of the Total Environment, 605, 874–883. https://doi.org/10.1016/j.scitotenv.2017.06.206.
Wang, H., Li, Y., Li, J., An, R., Zhang, L., & Chen, M. (2018). Influences of hydrodynamic conditions on the biomass of benthic diatoms in a natural stream. Ecological Indicators, 92, 51–60. https://doi.org/10.1016/j.ecolind.2017.05.061.
Wilson, J. B. (1999). Guilds, functional types, and ecological groups. Oikos, 86, 507–522. https://doi.org/10.2307/3546655.
Zampella, R. A., Laidig, K. J., & Lowe, R. L. (2007). Distribution of diatoms in relation to land use and pH in Blackwater coastal plain streams. Environmental Management, 39, 369–384. https://doi.org/10.1007/s00267-006-0041-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Salmaso, F., Quadroni, S., Compare, S. et al. Benthic diatoms as bioindicators of environmental alterations in different watercourses of northern Italy. Environ Monit Assess 191, 158 (2019). https://doi.org/10.1007/s10661-019-7290-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7290-x