Abstract
Hypertrophic cardiomyopathy (HCM) represents one of the primary cardiomyopathies and may lead to heart failure and sudden cardiac death. Among various histologic features of the disease examined, assessment of myocardial fibrosis may offer valuable information, since it may be considered the common nominator for all HCM connected complications. Late gadolinium-enhanced cardiac magnetic resonance (LGE-CMR) has emerged as the reference noninvasive method for visualizing and quantifying myocardial fibrosis in patients with HCM. T1 mapping, a promising new CMR technique, may provide an advantage over conventional LGE-CMR, by permitting a more valid quantification of diffuse fibrosis. On the other hand, echocardiography offers a significantly more portable, affordable, and easily accessible solution for the study of fibrosis. Various echocardiographic techniques ranging from integrated backscatter and contrast-enhanced ultrasound to two- (2D) or three-dimensional (3D) deformation and shear wave imaging may offer new insights into substrate characterization in HCM. The aim of this review is to describe thoroughly all different modalities that may be used in everyday clinical practice for HCM fibrosis evaluation (with special focus on echocardiographic techniques), to concisely present available evidence and to argue in favor of multi-modality imaging application. It is essential to understand that the role of various imaging modalities is not competitive but complementary, since the information provided by each one is necessary to illuminate the complex pathophysiologic pathways of HCM, offering a personalized approach and treatment in every patient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Ιntroduction
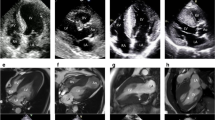
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant genetic disorder with a prevalence of 1:500 in the general population [1]. It is caused by mutations in cardiac sarcomeric proteins [2, 3], two thirds of which are associated with the genes encoding cardiac β-myosin heavy chain, cardiac troponin T, and myosin-binding protein C [1]. HCM is macroscopically defined by eccentric left ventricular (LV) hypertrophy [3], without dilatation of the cavity [4], accompanied by preserved or increased ejection fraction (Fig. 1) [5]. Most commonly, cardiac hypertrophy is located in the basal interventricular septum adjacent to the aortic valve [5]. This septal hypertrophy may favor the hemodynamic conditions for left ventricular outflow tract obstruction (LVOT), which at rest is present in about one third of HCM patients, and it can be found in another one third after provocation (Fig. 1) [5]. In microscopic level, HCM is characterized pathologically by myocyte hypertrophy, disarray, fibrosis, and small vessel disease [6,7,8,9]. Concerning myocardial disarray and fibrosis, these two pathologic processes may co-exist but may not be entirely inter-related in HCM, a point that should be taken into consideration when these two characteristics are assessed through cardiovascular imaging. Based on the main macroscopic characteristics of the disease and the existence or not of fibrosis, two distinct HCM phenotypes have been recently suggested: one group, positive for sarcomere mutations, is more likely to have reverse septal curvature morphology, fibrosis, and no or less resting obstruction while another phenotype, negative for sarcomere mutations, frequently has isolated basal septal hypertrophy with obstruction, but less fibrosis [10].
Clinical diagnosis of HCM is based on the presence of a wall thickness ≥ 15 mm in one or more LV myocardial segments as measured by any imaging technique (echocardiography, cardiac magnetic resonance imaging (CMR), or computed tomography (CT)), not explained solely by loading conditions [11]. However, in first-degree relatives of patients with HCM, the diagnosis of HCM can be confirmed by the presence of otherwise unexplained increased LV wall thickness ≥ 13 mm [11].
The majority of patients with HCM have an excellent prognosis [5, 12], with a mortality rate of 0.5% of a year, similar to that found in the general population [6, 13], while most of the affected individuals remain asymptomatic or minimally symptomatic through their lives [13, 14]. Despite this uneventful course for the majority of HCM patients, a still significant percent will experience adverse events, such as atrial fibrillation, evolution towards a dilated “burnt-out” phenotype leading to concomitant heart failure and in a few cases (ranging from 0.5 up to 2% per year) sudden cardiac death (SCD) [12].
Even though different risk factors and clinical scores have been adopted to successfully predict HCM individuals who might experience unfavorable course and especially SCD [5, 11, 14, 15], all of the existing clinical models present high negative predictive value with a low positive one. This literally means that existing prediction algorithms, used mainly to guide implantation of cardioverter defibrillators (ICDs), are still far by correctly classifying the risk in HCM. This shortcoming makes the recognition of new indices and their inclusion in risk stratification process necessary. Among different parameters tested, assessment of myocardial fibrosis may offer additional information, being possibly the common nominator for all HCM connected complications [1, 11, 16,17,18].
Types and significance of myocardial fibrosis in HCM
Fibrosis is a scarring process indicated by cardiac fibroblast activation and differentiation into myofibroblasts, loss of extracellular matrix homeostasis, and excess deposition of collagen [19, 20]. This pathophysiologic waterfall contributes to increased mechanical stiffness leading to diastolic dysfunction, evolution towards heart failure, as well as development of cardiac arrhythmias [8, 20, 21]. Histologically, 3 different forms of fibrosis can be recognized: (a) replacement/scarring fibrosis, (b) reactive interstitial fibrosis, and (c) perivascular fibrosis with the two first forms being the most common in HCM and discussed in depth later (Fig. 2) [21]. Interstitial fibrosis is characterized by an increase in extra cellular matrix and collagen deposits between the cells without loss of cardiomyocytes [22]. This type of fibrosis is diffusely present throughout the myocardium and is mostly caused by chronic triggers, such as pressure overload (hypertension, obstructive HCM, etc.), inflammation, and aging [22, 23]. Perivascular fibrosis is characterized by accumulation of collagen fibers in the area surrounding the coronary arteries and is mainly observed in the setting of hypertension [23]. Finally, replacement fibrosis presents following cardiomyocyte death, occurring for instance after acute ischemic injury, such as myocardial infarction (MI) [23]. In HCM, patches of replacement fibrosis are more prominently observed in interventricular septum and especially in the insertion points of LV and RV, while it is also mostly recognized in the mid-myocardial layer [22].
Pathophysiologically, all HCM complications may be connected with the existence and evolution of fibrosis. Concerning arrhythmias, the contribution of fibrosis is towards a creation of a vulnerable substrate, while simultaneously facilitating the emergence of triggers, such as premature ventricular complexes (PVCs) [24]. This trigger-substrate combination promotes the initiation of functional or anatomic reentry [24], leading to ventricular tachyarrhythmias/fibrillation (VT/VF) [24] which seems to be the principal mechanism of SCD in HCM patients [25,26,27]. However, the different types of fibrosis may lead to arrhythmias employing different paths/mechanisms [24]. For example, replacement fibrosis provides an inexcitable obstacle which favors arrhythmias by anchoring reentrant waves [20]. On the other hand, diffuse interstitial fibrosis is characterized by the presence of nonconducting collagen septa between myocytes leading to conduction slowing, unidirectional block due to source-sink mismatch and trigger escape, favoring the onset of reentrant activity [20, 24].
Except its role in arrhythmiogenesis, interstitial fibrosis gives rise to increased LV stiffness and higher LV filling pressure [28]. As a result, LV diastolic function is compromised reducing exercise tolerance in HCM patients [5, 28]. Moreover, the increased amount of collagen in the perivascular compartment may provide a possible structural explanation for the impaired coronary vasodilator reserve [29]. In other words, the small intramural coronary arteries, which are embedded in the interstitial fibrous tissue, exhibit wall thickening and narrow lumina, contributing to myocardial ischemia [5]. This ischemia might further play a role in expanding replacement fibrosis, initiating thus a vicious circle, which leads to the dilated “burn out” phase of HCM characterized by wall thinning, cavity dilatation, and systolic dysfunction (heart failure with reduced ejection fraction) [1, 5, 6].
These pathophysiologic sequelae make evident that assessment of fibrosis in HCM plays a significant role in prognostication and risk stratification for the disease. Therefore, aim of this mini-review is to describe which current imaging modalities and techniques may be applied to visualize fibrosis along with their pros and cons. These modalities can be roughly classified into those permitting direct fibrosis visualization and those allowing an indirect assessment of fibrosis burden.
CMR: the gold standard for detecting fibrosis
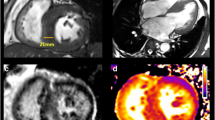
Cardiovascular magnetic resonance (CMR) is able to provide extensive information on cardiac morphology, ventricular function, and myocardial tissue characteristics [11]. Specifically, late gadolinium-enhanced cardiac magnetic resonance (LGE-CMR) has emerged as the reference noninvasive method for visualizing and quantifying myocardial fibrosis in patients with HCM (Fig. 3) [26, 30].
Short-axis late gadolinium-enhanced (LGE) cardiovascular magnetic resonance images from different hypertrophic cardiomyopathy patients (each panel comes from a different patient). White arrows indicate different types of LGE (midmyocardial, subepicardial, etc.) in various levels (basal, mid, apical)
Gadolinium chelates are contrast agents which diffuse quickly and passively leave the vascular space in order to concentrate in the extracellular compartment [31, 32]. They are inert and cannot move to the intracellular space, characteristics which gave rise to the often-used term “extracellular, extravascular contrast agents” [32, 33]. The expanded extracellular compartment in the myocardium of HCM patients, which is caused by fibrosis and additionally by myocardial disarray, leads to increased volume of gadolinium distribution and slower washout kinetics [33]. As a result, the relative accumulation of gadolinium compared to normal myocardium can be detected in the late washout phase [33].
Since the first report in 2002 [34], LGE-CMR plays a pivotal role in fibrosis assessment in HCM [35]. Especially, late gadolinium enhancement (LGE) is a strong independent predictor of adverse effects, such as SCD and progression to heart failure symptoms [36]. In studies which examine the relationship between LGE and prognosis of HCM, LGE was associated with risk of malignant arrhythmias, sustained ventricular tachycardia, and ventricular fibrillation [37, 38]. As a result, LGE can help in risk stratification of HCM patients for SCD, determining the implantation of implantable cardioverter defibrillator (ICD) or not [31, 37].
Despite the fact that LGE-CMR is crucial for depicting replacement fibrosis, it is not suitable to delineate diffuse interstitial fibrosis [31, 36]. As referred by Vöhringer et al., there are two major reasons why LGE applying an all-or-nothing approach is very sensitive to regional Gd accumulation, and therefore able to visualize focal regions of (replacement) fibrosis but not diffuse interstitial fibrosis [31]. The major disadvantage of LGE is its limited voxel resolution, being approximately 1.8 mm × 1.2 mm × 6 mm, so only scar tissue will be visible on CMR [31]. In addition to that, the lack of a common standardized protocol for acquisition and evaluation of LGE imaging combined with the difficulty in setting the reference area make the quantification of fibrosis even more challenging [39,40,41].
A promising new CMR technique is T1 mapping which may provide an advantage over conventional LGE-CMR, by permitting a more valid quantification of diffuse fibrosis [32, 36]. T1 mapping consists of the generation of a pixelated map based on the longitudinal or spin–lattice relaxation of protons that recover towards thermodynamic equilibrium following excitation with the radiofrequency beam [22, 42]. The value of T1 relaxation time varies according to the state of the molecular environment. The native T1 value is a tissue-specific time constant in the absence of an exogenous contrast agent. Tissues generally contain water but pathological processes, including fibrosis, alter the water composition and thereby alter the T1 values [22, 42]. In case of myocardial fibrosis, an increase in native T1 relaxation time will be observed. T1 mapping can also be combined with administration of Gd, which increases proton relaxation, and thus decreases T1 relaxation time [22, 42]. Therefore, in contrast to the native T1 relaxation time, in case of myocardial fibrosis, the post-contrast T1 values will be lower. Native and post-contrast T1 mapping can determine an increase in ECM volume that is not detectable by LGE, reflecting thus the burden of interstitial fibrosis in HCM [22, 42]. Current studies have applied T1 mapping in HCM patients showing promising data about the significance of interstitial fibrosis in disease evolution and potentially prognosis [43, 44].
Even though CMR may be considered the “gold standard” for fibrosis detection and evaluation in HCM, there are still some major limitations that should be taken into consideration. Firstly, gadolinium has been associated with the development of contrast-induced nephropathy (nephrogenic systemic fibrosis (NSF)) in patients with impaired renal function (eGFR < 30 mL/min/1.73 m2) [32, 37, 45]. Secondly, attention should be paid on patients with cardiac implantable electronic devices (CIEDs) as some changes in the pacing rate or the function of the device during the CMR acquisition have been documented [39]. Last but not least, facts such as claustrophobia, financial cost, and access to medical centers with expertise in CMR should be considered [11, 32].
All the aforementioned “flaws” of CMR make the development and application of new imaging techniques, which assess myocardial fibrosis, highly desirable [30].
Echocardiography for direct fibrosis visualization
As opposed to CMR, echocardiography offers a significantly more portable, affordable and easily accessible solution for the study of cardiomyopathies, especially HCM. Despite this fact, direct visualization of fibrotic regions in HCM as a part of a tissue characterization process is still not completely feasible.
During early stages of tissue characterization struggle, integrated backscatter, which measures the ultrasonic reflectivity of the region of interest, was a major focus of tissue characterization research [46, 47]. Studies in small series of 20 patients showed that backscatter was correlated with fibrosis, disarray, and myocyte diameter in HCM (albeit correlated with RV endomyocardial biopsy findings) [48]. Another echocardiographic measure, based on backscatter methodology, is the signal intensity coefficient (SIC) [49]. SIC is based on the fact that interactions between the ultrasound signal and myocardial tissue (e.g., absorption and refraction) influence the grayscale signal intensity values produced at the myocardial-pericardial interface, which can yield measurable differences between diseased versus healthy myocardium [49]. In a pilot study, Hiremath et al. applied this method to quantify subclinical alterations in LVM of HCM sarcomere mutation carriers (28 patients) [49]. They concluded that among people who carry genetic variants associated with HCM, the SIC values differ between those with LV overt hypertrophy and those without [49]. Moreover, the SIC differentiated between phenotypically silent mutation carriers and healthy controls [49]. Despite these promising signs, integrated backscatter technique has still major limitations such as the need for an intrinsic reference frame, the actual position of the sample volume, the limited validated views, the effect of image setting, and the presence of artifacts and other reflectors which significantly harass the accuracy and reproducibility of the method [46, 50].
Accordingly, contrast echocardiography (either as two-dimensional (2D) or three-dimensional (3D) imaging) has been applied to detect fibrosis in HCM. More specifically, an increased capture of ultrasound contrast agent in regions of replacement fibrosis similar to the ones depicted by CMR-LGE have been described in the literature [51], whereas a pulse cancellation ultrasound technique (scar imaging echocardiography with ultrasound multipulse scheme (eSCAR)) was found promising to differentiate normal from scarred myocardium among coronary artery disease patients [52]. The application of the last method in HCM patients, however, may be very challenging since replacement fibrosis patterns are heterogenous, spotty, and small as opposed to clearly delineated scar corresponding to a coronary territory. Additionally, existence of replacement fibrosis at the basal RV insertion sites may pose further difficulties in visualization and in discrimination of fibrotic areas from the membranous septum and surrounding nonmuscular structures, making thus this method nonproposed for HCM fibrosis assessment unless a dedicated study provides opposite clues.
Although tissue characterization is already (partially) performed using echocardiography, the greatest contribution of echo towards the study of underlying HCM histology is through assessment of myocardial function [53]. Nowadays, more advanced echocardiography methods provide detailed information on the function of the underlying tissue, which could be used in both translational and clinical research, and ultimately translated to clinical practice. Advanced deformation-strain imaging is the technique presenting the most data on this field, evaluating the impact of underlying pathology on tissue function [46].
Basic principles of strain imaging
Strain is the fractional change in length of a myocardial segment relative to its baseline length, and it is expressed as a percentage [2, 54]. Strain rate is the temporal derivative of strain providing data on the time at which the deformation occurs [2, 54]. In order to provide a detailed description of the deformation of a myocardial segment, three normal and six shear strain components are required [54]. The normal strains, which are normally used in clinical practice, are oriented along the three axes-directions (x, y, z) of the heart (longitudinal strain (LS), circumferential strain (CS), radial strain (RS)) (Fig. 4) [54]. Lengthening or thinning of the myocardium are represented by positive strain values, whereas shortening or thickening by negative ones [54].
Basic deformation parameters used in everyday clinical practice. EDV end-diastolic volume, ESV end-systolic volume, MVC mitral valve closure indicating beginning of cardiac cycle, AVC aortic valve closure indicating peak systolic strain, GLS global longitudinal strain, GCS global circumferential strain, GRS global radial strain. Right panels present a typical example of longitudinal strain assessment resulting in bull’s eye (bottom) including all regional longitudinal strain values
Tissue Doppler echocardiography was the first echocardiographic approach to myocardial deformation, offering high temporal resolution, being however highly dependent on angle of acquisition [2, 54, 55]. The introduction of speckle tracking deformation imaging (STE) overcame inherent limitations of tissue Doppler and allowed evaluation of whole LV systolic function through “global strain” [54]. In the longitudinal direction, global longitudinal strain (GLS), as an average of the three apical views, reflects the deformation of the whole LV (Fig. 4) [54] and it can be used as an alternative parameter to ejection fraction (EF) [54]. GLS offers additional information compared to EF, while in hypertrophic hearts, GLS may be earlier impaired compared to EF, providing an early sign of LV systolic dysfunction [54, 56]. Combination of the diagnostic information offered both by EF and GLS in a single index (EFSR ratio) can be used for discrimination of hypertrophic pathology [56]; however, a detailed approach of this ratio is beyond the scope of this review.
As mentioned before, STE allows the objective quantification of global and regional myocardial function [57]. The major advantages of this technique are the multidirectional tracking ability with angle independency, large availability, high feasibility, fast and user-friendly (semi-) automated post-processing [54, 57]. It is important to mention that this method has been validated against sonomicrometry and CMR and among all deformation parameters GLS is qualified as a parameter of LV systolic function with the best clinical reliability and reproducibility [54].
However, there are still some facts regarding STE which should not be overlooked. In contrast to GLS, the quantification of segmental LV strains has a higher degree of measurement variability among different vendors [57, 58]. Except the inter-vendor bias, the fidelity of different software varies considerably [58]. This implies that the comparison of quantitative data obtained with different post-processing software should be made with caution in clinical practice. Segmental strain pattern analysis may potentially be a more objective alternative [54, 57].
Despite the fact that there are some pitfalls as mentioned above, further standardization and pre-release testing, potentially including hardware or software phantoms, will provide a more solid basis for wide spread use of this technique [54, 57].
2D-STE: role in myocardial fibrosis assessment
Nowadays, echocardiographic deformation imaging plays an important role in clinical assessment of myocardial fibrosis in HCM, contributing to tissue characterization of this disease [53]. Previous studies applying STE strain analysis for fibrosis detection validated their results against CMR-LGE or histologic specimens. In the majority of the studies performed, longitudinal deformation (either GLS or SLS) has been almost exclusively used to be correlated with CMR or histologic findings, showing great reliability and reproducibility (Table 1) [28, 59,60,61,62,63,64,65,66,67].
Popovic et al., analyzed the impact of myocardial fibrosis on segmental LV function in patients with HCM, assessed by strain parameters derived from 2D speckle tracking echocardiography (2D-STE) (myocardial fibrosis was previously detected by LGE-CMR) [62]. Authors report that the amount of fibrosis significantly correlated with global end-systolic longitudinal strain, while a correlation was also established in the regional level (r = 0.24, P < 0.0001) [62]. Additionally, the presence of fibrotic lesions and end-diastole wall thickness were both independent predictors for presence of lower regional LS in a multivariate regression analysis [62].
Another study indicated that in HCM patients, regional peak–LS was notably lower in fibrotic than nonfibrotic LV myocardial segments (myocardial fibrosis was previously detected by computed tomography) [2]. Consequently, Yajima et al. reported that regional peak–LS assessed by 2D-STE may be a useful noninvasive tool to distinguish fibrotic from nonfibrotic lesions in HCM [2].
In the same direction is also the paper by Chang et al. [62]. SLS was significantly decreased in the segments with LGE compared to those without LGE (− 12.4 ± 5.6% vs − 18.3 ± 6.53%, P < 0.001) [63]. Regarding GLS (global longitudinal strain), the study revealed that total LGE volume and LV mass index were independent determinants of GLS on multivariate analysis [63]. To conclude, authors reported that increase in segmental fibrosis and thickness was related to decreased segmental longitudinal function, whereas increase in global fibrosis burden was associated with decrease in global longitudinal function independently of myocardial mass [63].
Wiedemann et al. demonstrated that a distinct pattern (called “double peak sign”) of the strain rate curve allows for reproducible and accurate qualitative assessment of the presence of myocardial fibrosis [64]. “Double peak sign” is characterized by a first peak in early systole followed by a fall in strain rate close to zero and a second peak during isovolumic relaxation [64]. Second strain rate peak should be at least 50% in magnitude compared with the first peak in order to be differentiated from the physiological induced post-systolic deformation after aortic valve closure [64].
Another pilot study which is worth mentioning was conducted by Galli E et al. [65]. Specifically, they estimated the myocardial work by pressure strain loops (PSLs) [65]. The PSLs analysis showed that global constructive work (GCW) is reduced in HCM despite normal LVEF and is associated with LV fibrosis as assessed by LGE [65].
Despite the fact that late gadolinium-enhanced cardiac magnetic resonance (LGE-CMR) has emerged as the reference noninvasive method for visualizing and quantifying myocardial fibrosis in patients with HCM [27, 30], Almaas et al. demonstrated some really interesting results, although conclusions reached by this study may be limited due to study’s small size and nonadjusted analysis [66]. Authors report that reduced longitudinal septal strain correlated better with interstitial and total fibrosis in myectomy specimens compared to LGE, whereas a reduction in longitudinal septal strain was indicated to play a role in arrythmiogenesis being related also to myocardial disarray [66].
Lastly, Haland et al. came to the conclusion that mechanical dispersion (heterogeneous myocardial contraction) was an independent predictor of arrhythmic events (ventricular arrhythmias) and was correlated with the amount of fibrosis, even though GLS was only borderline related to VAs and the presence of fibrosis [67]. It is critical to underline that reduced LS and increased mechanical dispersion may not be explained by the presence of fibrosis alone but may reflect the whole pathophysiological status of HCM (hypertrophy, myocardial disarray, macro- and microvascular ischemia, intraventricular obstruction) [67]. The fact that an impaired SLS reflects not only fibrosis but also hypertrophy, and possibly the underlying microvascular dysfunction, has also been evidenced by another study previously conducted by authors [53].
Taking into consideration all previous studies [28, 59,60,61,62,63,64,65,66,67], strain by STE has the ability to detect reduced myocardial function in HCM patients despite normal LVEF [54, 57, 66], and LS assessment may be useful to distinguish fibrotic from non-fibrotic lesions [2, 63, 64]. Moreover, reduced LS in HCM patients may not be solely explained by the presence of myocardial fibrosis, but hypertrophy, disarray, and macro-/microvascular ischemia seem to play a role [53, 67]. Reduced LS may also be a marker of arrhythmic events and occurrence of SCD or appropriate ICD shock [16, 67]; however, further evidence is needed before LS is used to improve risk stratification of HCM patients for malignant VAs and SCD [16, 67].
From 2D to 3D speckle tracking echocardiography
The recently developed 3D speckle tracking echocardiography (3D-STE) seems to be a promising method to overcome the limitations of 2D-STE [68, 69]. It is important to mention that the left ventricular myofibers have a complex arrangement and contract at the same time in different directions [70]. The spatial orientation of muscle fibers changes from a left-handed helix in the subepicardium to a right-handed helix in the subendocardium [71]. This shows that LV mechanics and contraction are a 3D phenomenon and a 3D imaging method is required for its accurate assessment [70].
3D strain overcomes inherent limitations of 2D imaging including foreshortening of apical views and time-consuming calculations of strain parameters based on spatially and temporally different views and out-of-plane motion [70, 72,73,74]. Another interesting fact about 3D-STE is that analysis of data derived from this method may provide characteristic echocardiographic patterns for various conditions, playing an important role in differential diagnosis of phenocopies (discriminating for example athlete’s heart from HCM or HCM vs amyloidosis) [70, 75]. These profound advantages, however, come at a cost of a lower temporal and spatial resolution which might significantly affect the quality of 3D deformation estimates [76, 77]. Indeed, according to a previous study performed by our group, 3D longitudinal and circumferential absolute strain values are significantly lower compared to 2D ones, while there is a very low correlation and consistency between values obtained by the two modalities [78]. This inconsistency may be attributed both to strain-thickness interactions in HCM as well as to smoothing and calculating assumptions of the 3D software applied [78].
Regarding, the significance of 3D-STE in detecting underlying fibrosis in HCM limited data exist. In a study by Spartera et al., 3DGLS showed a modest correlation (r = 0.465) with fibrosis burden as this was approached by LGE CMR (> 10% myocardial mass) in a mixed patient population including also 8 HCM patients [79]. According to the authors, 3DGLS presented a low positive but a very high negative predictive value for fibrosis detection; however, when it came to segmental correlations between SLS and fibrosis, accuracy decreased [79]. Previous studies have also suggested that 3D segmental area strain (SAS) may be a potentially valuable parameter for the detection of fibrosis [3]. In a previous study by our group, 3D SLS, SAS, and radial strain showed a similar diagnostic ability which, besides that, was significantly lower compared to 2D SLS in a cohort of 40 HCM patients [78]. The observed superiority of 2D strain parameters may not necessarily reflect the true potential of 3D deformation parameters as the current data quality and available algorithms are limiting factors [78].
In the future, 3D-STE may contribute more to global and regional myocardial function evaluation, as well as to tissue characterization in various pathologic substrates and HCM (Fig. 5) [77]. Software improvements, including spatial and temporal resolution optimization, along with decrease of intervendor bias are crucial steps towards this direction [78, 80].
Late gadolinium-enhanced (LGE) cardiovascular magnetic resonance images (upper panels) and longitudinal strain bull’s eyes (lower panels) calculated by means of two-dimensional (2D) and three-dimensional (3D) deformation imaging. Evident is the correlation between fibrosis position indicated by LGE (arrows) and the functional impairment as highlighted by 2D and 3D longitudinal strain (blue areas show greater impairment)
Promising techniques and future directions
Myocardial stiffness (MS) is known to have an important diagnostic and prognostic value in diastolic left ventricular function [81]. MS seems to have a physiological variation with age but is also affected by pathological alterations of cellular tissue and extracellular compartment [81]. So far, the clinical assessment of MS and the diastolic function is challenging [81]. Villemain et al. investigated the potential role of shear wave imaging (SWI), in their attempt to quantify noninvasively the diastolic MS. SWI is an ultrasound-based technique which has been already used in the field of breast lesions and liver imaging and offers quantitative, local, and noninvasive characterization of soft tissue’s stiffness [81]. Firstly, the authors aimed to measure MS with SWI in a population of healthy adult volunteers and to clarify the dependence of MS values with age, and then compared it to significantly altered MS in 20 HCM patients with HFpEF (heart failure with preserved ejection fraction). As a result, MS was higher in HCM patients with HFpEF than in healthy volunteers and positive correlation was found between MS and fibrosis markers in LGE-CMR [81]. In another study, naturally occurring myocardial shear waves (produced after mechanical excitation of the myocardium, for example after mitral valve closure) have also been applied to assess MS both in normal volunteers and amyloidosis patients, showing promising results [82]. In the future, myocardial SWI might have a key role in clinical assessment of diastolic stiffness and fibrosis burden [81, 82].
Finally, molecular imaging has a lot of potentials and it can be combined with different imaging modalities to detect specific histologic findings including fibrosis [83]. Even though molecular echocardiography, based on fibrosis-specific binding molecules detected by the application of contrast echo techniques, is still in a bench level [83], nuclear medicine has significantly evolved toward fibrosis detection. Positron emission tomography imaging performed by using 15O-labelled water (H2 15O) and carbon monoxide (C15O) allowed the noninvasive quantification of both myocardial perfusion and fibrosis [83, 84]. Myocardial fibrosis can be indirectly assessed through calculation of the perfusable tissue index (PTI), separating perfusable and nonperfusable tissues. A reduction in PTI serves as an estimate of fibrosis in a chronic MI model and in human dilated cardiomyopathy [83, 85]. In addition, combining PET and MRI has the potential for sensitive and quantitative imaging of cardiovascular anatomy and function with detection of molecular events at the same time [83, 86]. A fused PET-MRI image allows the simultaneous detection of myocardial global and regional function, extracellular volume, and tissue perfusion and metabolism [87].
Conclusions
Fibrosis is a significant histologic characteristic of HCM pathology linked to diagnosis and prognosis of the disease. Both evolution towards burnt-out phenotype and SCD are triggered by the severity and extent of underlying fibrosis, either replacement or interstitial. Apart from LGE-CMR and newer sequences applied to detect fibrosis in the hypertrophic myocardium, several other echocardiography-based techniques are available today for substrate characterization. Among them, deformation imaging, especially longitudinal strain assessment, may offer functional information beyond the impact of fibrosis burden. It is essential to understand that the role of various imaging modalities is not competitive but complementary, since the information provided by each one is necessary to illuminate the complex pathophysiologic pathways of HCM, offering a personalized approach and treatment in every patient. Hybrid imaging [88], combining information from different modalities, should allow for the maximization of the performance and advantages of imaging tests, and its application in everyday medical practice is a one-way solution to the assessment of cardiomyopathies and HCM in the future.
References
Hughes SE (2009) The pathology of hypertrophic cardiomyopathy. Histopathology 44:412–427
Yajima R, Kataoka A, Takahashi A, Uehara M, Saito M, Yamaguchi C, Lee K, Komuro I, Funabashi N (2012) Distinguishing focal fibrotic lesions and non-fibrotic lesions in hypertrophic cardiomyopathy by assessment of regional myocardial strain using two-dimensional speckle tracking echocardiography: comparison with multislice CT. Int J of Cardiol 158:423–432
Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG (2014) Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 7:11–19
Arteaga E, Araújo AQ, Bernstein M, Ramires FJA, Ianni BM, Fernandes F, Mady C (2009) Prognostic value of the collagen volume fraction in hypertrophic cardiomyopathy. Arq Bras Cardiol 92(3):210–214
Marian AJ, Braunwald E (2017) Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 121(7):749–770
Chowdhry SS, Jacoby D, Moon JC, McKenna WJ (2016) Update on hypertrophic cardiomyopathy and a guide to the guidelines. Nat Rev 13:651–675
Hurtado-de-Mendoza D, Corona-Villalobos CP, Pozios I, Gonzales J, Soleimanifard Y, Sivalokanathan S, Montoya-Cerrillo D, Vakrou S, Kamel I, Mormontoy-Laurel W, Dolores-Cerna K, Suarez J, Perez-Melo S, Bluemke DA, Abraham TP, Zimmerman SL, Abraham MR (2017) Diffuse interstitial fibrosis assessed by cardiac magnetic resonance is associated with dispersion of ventricular repolarization in patients with hypertrophic cardiomyopathy. J Arrhythm 33:201–207
Fujita T, Konno T, Yokawa J, Masuta E, Nagata Y, Fujino N, Funada A, Hodatsu A, Kawashiri M, Yamagishi M, Hayashi K (2015) Increased extent of myocardial fibrosis in genotyped hypertrophic cardiomyopathy with ventricular tachyarrhythmias. J Cardiol 66:63–68
Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ (2006) Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circ 18:2216–2225
Neubauer S, Kolm P, Ho CY, Kwong RY, Desai MY, Dolman DF, Appelbaum E, Desvigne-Nickens P, DiMarco JP, Friedrich MG, Geller N, Harper AR, Jarolim P, Jerosch-Herold M, Kim DY, Maron MS, Schulz-Menger J, Piechnik SK, Thomson K, Zhang C, Watkins H, Weintraub WS, Kramer CK, on behalf of the HCMR Investigators (2019) Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM Registry. J Am Coll Cardiol 74:2333–2345
Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H (2014) 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J 35:2733–2779
Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y (2016) Prognostic Value of LGE-CMR in HCM. Meta-Anal Cardiovasc Imaging 9:1392–1402
Leonardo S (2019) Myocardial fibrosis in hypertrophic cardiomyopathy: what remains to be proven? Arq Bras Cardiol 112(3):290–291
Zhang L, Mmagu O, Liu L, Li D, Fan Y, Baranchuk A, Kowey PR (2014) Hypertrophic cardiomyopathy: can the noninvasive diagnostic testing identify high risk patients? World J Cardiol 6(8):764–770
Klopotowski M, Kukula K, Malek LA, Spiewak M, Polanska-Skrzypczyk M, Jamiolkowski J, Dabrowski M, Baranowski R, Klisiewicz A, Kusmierczyk M, Jasinska A, Jarmus E, Kruk M, Ruzyllo W, Witkowski A, Chojnowska L (2016) The value of cardiac magnetic resonance and distribution of late gadolinium enhancement for risk stratification of sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiol 68:49–56
Vergé MP, Cochet H, Reynaud A, Morlon L, Peyrou J, Vincent C, Rooryck C, Ritter F, Lafitte S, Réant P (2018) Characterization of hypertrophic cardiomyopathy according to global, regional, and multi-layer longitudinal strain analysis, and prediction of sudden cardiac death. Int J Cardiovasc Imaging 34:1091–1098
Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D’Andrea A, Bruder O, Cosyns B, Davin L, Donal E, Freitas A, Habib G, Kitsiou A, Petersen SE, Schroeder S, Lancellotti P (2015) Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J – Cardiovasc Imaging 16:280
Mavrogeni S, Petrou E, Kolovou G, Theodorakis G, Iliodromitis E (2013) Prediction of ventricular arrhythmias using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 14:518–525
Almaas VM, Haugaa KH, Strøm EH, Scott H, Dahl CP, Leren TP, Geiran OR, Endresen K, Edvardsen T, AakhusT AJP (2013) Increased amount of interstitial fibrosis predicts ventricular arrhythmias, and is associated with reduced myocardial septal function in patients with obstructive hypertrophic cardiomyopathy. Europace 15:1319–1327
Disertori M, Masè M, Ravelli F (2017) Myocardial fibrosis predicts ventricular tachyarrhythmias. Trends Cardiovasc Med 27:363–372
O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK (2010) Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. JACC 56:867–874
Eijgenraam TR, Silljé HHW, de Boer RA. Current understanding of fibrosis in genetic cardiomyopathies (2019) Trends Cardiovasc Med. pii: S1050–1738(19)30132-X
de Boer RA , De Keulenaer G , Bauersachs J , BrutsaertD , Cleland JG , Diez J, et al (2019) Towards better definition, quantification and treatment of fibrosis in heart failure. a scientific roadmap by the committee of translational research of the heart failure association (HFA) of the European Society of Cardiology. Eur J Heart Fail 21(3):272–85
Nguyen TP, Qu Z, Weiss JN (2014) Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol 70:83–91
Morita N, Mandel WJ, Kobayashi Y, Karagueuzian HS (2014) Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrhythm 30:389–394
Shiozaki AA, Senra T, Arteaga E, Filho MM, Pita CG, A’vila LFR, Filho JRP, Mady C, Kalil-Filho R, Bluemke DA, Rochitte CE, (2013) Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr 7:173–181
Fluechter S, Kuschyk J, Wolpert C, Doesch C, Veltmann C, Haghi D, Schoenberg SO, Sueselbeck T, Germans T, Streitner F, Borggrefe M, Papavassiliu T (2010) Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 12:30
Maragiannis D, Alvarez PA, Ghos MG, Chin K, Hinojosa JJ, Buergler JM, Shah DJ, Nagueh SF (2018) Left ventricular function in patients with hypertrophic cardiomyopathy and its relation to myocardial fibrosis and exercise tolerance. Int J Cardiovasc Imaging 34:121–129
Shirani J, Pick R, Roberts WC, Maron BJ (2000) Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. JACC 35:36–44
Prinz C, Schwarz M, Ilic I, Laser KT, Lehmann R, Prinz EM, Bitter T, Vogt J, Buuren F, Bogunovic N, Horstkotte D, Faber L (2013) Myocardial fibrosis severity on cardiac magnetic resonance imaging predicts sustained arrhythmic events in hypertrophic cardiomyopathy. Can J Cardiol 29:358–363
Vöhringer M, Mahrholdt H, Yilmaz A, Sechtem U (2007) Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR). Herz 32:129–137
Scully PR, Bastarrika G, Moon JC, Treibel TA (2018) Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep 20:15
Moon JCC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ (2004) The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. JACC 43(12):2260–2264
Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ (2002) Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. JACC 40(12):2156–2164
Cheng S, Fang M, Cuil C, Chen X, Yin G, Prasad SK, Dong D, Tian J, Zhao S (2018) LGE-CMR-derived texture features reflect poor prognosis in hypertrophic cardiomyopathy patients with systolic dysfunction: preliminary results. Eur Radiol 28:4615–4624
Cooper RM, Raphael CE, Liebregts M, Anavekar NS, Veselka J (2017) New developments in hypertrophic cardiomyopathy. Can J Cardiol 33:1254–1265
Patel N, Kolakalapudi P, Arora G (2018) Contrast applications in CMR. Echocardiography 35(3):401–409
JCS Joint Working Group (2016) Guidelines for diagnosis and treatment of patients with hypertrophic cardiomyopathy (JCS 2012). Circ J 80:753–774
Horwood L, Attili A, Luba F, Ibrahim ELS, Parmar H, Stojanovska J, Gadoth-Goodman S, Fette C, Oral H, Bogun F (2017) Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace 19:812–817
Freitas P, Ferreira AM, Arteaga-Fernández E, de Oliveira AM, Mesquita J, Abecasis J, Marques H, Saraiva C, Matos DN, Rodrigues R, Cardim N, Mady C, Rochitte CE (2019) The amount of late gadolinium enhancement outperforms current guideline-recommended criteria in the identification of patients with hypertrophic cardiomyopathy at risk of sudden cardiac death. J Cardiovasc Magn Reson 21(1):50
Teramoto R, Fujino N, Konno T, Nomura A, Nagata Y, Tsuda T, Tada H, Sakata K, Yamagishi M, Hayashi K, Kawashiri M (2018) Late Gadolinium enhancement for prediction of mutation-positive hypertrophic cardiomyopathy on the basis of panel-wide sequencing. Circ J 82:1139–1148
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M (2016) T1 mapping basic techniques and clinical applications. JACC Cardiovasc Imaging 9(1):67–81
Ando K, Nagao M, Watanabe E, Sakai A, Suzuki A, Nakao R, Ishizaki U, Sakai S, Hagiwara N (2020) Association between myocardial hypoxia and fibrosis in hypertrophic cardiomyopathy: analysis by T2* BOLD and T1 mapping MRI. Eur Radiol. https://doi.org/10.1007/s00330-020-06779-9
Brouwer WP, Baars EN, Germans T, de Boer K, Beek AM, van der Velden J, van Rossum AC, Hofman MB (2014) In-vivo T1 cardiovascular magnetic resonance study of diffuse myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 16:28
Wang C, Zheng J, Sun J, Wang Y, Xia R, Yin Q, Chen W, Xu Z, Liao J, Zhang B, Gao F (2015) Endogenous contrast T1rho cardiac magnetic resonance for myocardial fibrosis in hypertrophic cardiomyopathy patients. J Cardiol 66:520–526
Moharram MA, Lamberts RR, Whalley G, Williams MJA, Coffey S (2019) Myocardial tissue characterisation using echocardiographic deformation imaging. Cardiovasc Ultrasound 17(1):27
Milunski MR, MohrJE GA, Pérez JE, Vered Z, Wear KA, Gessler CJ, Sobel BE, Miller JG, Wickline SA (1989) Ultrasonic tissue characterization with integrated backscatter Acute myocardial ischemia reperfusion and stunned myocardium in patients Circulation 80(3):491
Mizuno R, Fujimoto S, Yamaji K, Yutani C, Hashimoto T, Nakamura S (2001) Myocardial ultrasonic tissue characterization for estimating histological abnormalities in hypertrophic cardiomyopathy: comparison with endomyocardial biopsy findings. Cardiol 96(1):16–23
Hiremath P, Lawler PR, Ho JE, Correia AW, Abbasi SA, Kwong RY, Jerosch-Herold M, Ho CY, Cheng S (2016) Ultrasonic assessment of myocardial microstructure in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Heart Fail 9(9)
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP et al (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12(3):167–205
Chaudeurge A, Garçon P, Cador R (2011) Potential value of myocardial contrast echocardiography for the detection of myocardial fibrosis in hypertrophic cardiomyopathy. Arch Cardiovasc Dis 104(12):682–683
Gaibazzi N, Bianconcini M, Marziliano N, Parrini I, Conte MR, Siniscalchi C, Faden G, Faggiano P, Pigazzani F, Grassi F, Albertini L (2016) Scar detection by pulse-cancellation echocardiography: validation by CMR in patients with recent STEMI. JACC Cardiovasc Imaging 9(11):1239–1251
Pagourelias ED, Mirea O, Vovas G, Duchenne J, Michalski B, Van Cleemput J, Bogaert J, Vassilikos VP, Voigt JU (2019) Relation of regional myocardial structure and function in hypertrophic cardiomyopathy and amyloidois: a combined two-dimensional speckle tracking and cardiovascular magnetic resonance analysis. Eur Heart J Cardiovasc Imaging 20(4):426–437
Mirea O, Duchenne J, Voigt JU (2016) Recent advances in echocardiography: strain and strain rate imaging. F1000Res 5. pii: F1000 Faculty Rev-787 https://doi.org/10.12688/f1000research.7228.1
Ghio S, Revera M, Mori F, Klersy C, Raisaro A, Raineri C, Serio A, Pasotti M, Visconti LO (2009) Regional abnormalities of myocardial deformation in patients with hypertrophic cardiomyopathy: correlations with delayed enhancement in cardiac magnetic resonance. Eur J Heart Fail 11(10):952–957
Pagourelias ED, Mirea O, Duchenne J, Van Cleemput J, Delforge M, Bogaert J, Kuznetsova T, Voigt JU (2017) Echo parameters for differential diagnosis in cardiac amyloidosis: a head-to-head comparison of deformation and nondeformation parameters. Circ Cardiovasc Imaging 10(3):e005588
Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, Voigt JU, EACVI-ASE-Industry Standardization Task Force, (2018a) Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging 11(1):25–34
Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, Voigt JU, EACVI-ASE-Industry Standardization Task Force, (2018b) Variability and reproducibility of segmental longitudinal strain measurement: a report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging 11(1):15–24
Funabashi N, Takaoka H, Ozawa K, Kamata T, Uehara M, Komuro I, Kobayashi Y (2018) Quantitative differentiation of LV myocardium with and without layer-specific fibrosis using MRI in hypertrophic cardiomyopathy and layer-specific strain TTE analysis. Int Heart J 59(3):523–530
Funabashi N, Takaoka H, Ozawa K, Uehara M, Komuro I, Kobayashi Y (2018) 2D speckle-tracking TTE-based quantitative classification of left ventricular myocardium in patients with hypertrophic cardiomyopathy by the presence or the absence of fibrosis and/or hypertrophy. Heart Vessels 33(9):1046–1051
Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, Sumimoto T, Inaba S, Nishimura K, Inoue K, Ogimoto A, Shigematsu Y, Hamada M, Higaki J (2012) Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 13(7):617–623
Popovic´ ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, Flamm SD, Thomas JD, Lever HM, Desai MY (2008) Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr 21(12):1299–1305
Chang SA, Lee SC, Choe YH, Hahn HJ, Jang SY, Park SJ, Choi JO, Park SW, Oh JK (2012) Effects of hypertrophy and fibrosis on regional and global functional heterogeneity in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 28:133–140
Weidemann F, Niemann M, Herrmann S, Kung M, Störk S, Waller C, Beer M, Breunig F, Wanner C, Voelker W, Ertl G, Bijnens B, Strotmann JM (2007) A new echocardiographic approach for the detection of non-ischaemic fibrosis in hypertrophic myocardium. Eur Heart J 28(24):3020–3026
Galli E, Vitel E, Schnell F, Le Rolle V, Hubert A, Lederlin M, Donal E (2019) Myocardial constructive work is impaired in hypertrophic cardiomyopathy and predicts left ventricular fibrosis. Echocardiography 36:74–82
Almaas VM, Haugaa KH, Strøm EH, Scott H, Smith HJ, Dahl CP, Geiran OR, Endresen K, Aakhus S, Amlie JP, Edvardsen T (2014) Noninvasive assessment of myocardial fibrosis in patients with obstructive hypertrophic cardiomyopathy. Heart 100(8):631–638
Haland TF, Almaas VM, Hasselberg NE, Saberniak J, Leren IS, Hopp E, Edvardsen T, Haugaa KH (2016) Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 17:613–621
Hayat D, Kloeckner M, Nahum J, Ecochard-Dugelay E, Dubois-Randé JL, Jean-François D, Guéret P, Lim P (2012) Comparison of real-time three-dimensional speckle tracking to magnetic resonance imaging in patients with coronary heart disease. The American Journal of Cardiology 109:180–186
Badran HM, Faheem N, Soliman M, Hamdy M (2019) Yacoub M (2019) Comparison of vector velocity imaging and three-dimensional speckle tracking echocardiography for assessment of left ventricular longitudinal strain in hypertrophic cardiomyopathy. Glob Cardiol Sci Pract 1:6
Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L (2018) Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn and Ther. 8(1):101–117
Saito K, Okura H, Watanabe N, Hayashida A, Obase K, Imai K, Maehama T, Kawamoto T, Neishi Y, Yoshida K (2009) Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr 22:1025–1030
Trache T, Stöbe S, Tarr A, Pfeiffer D, Hagendorff A (2014) The agreement between 3D, standard 2D and triplane 2D speckle tracking: effects of image quality and 3D volume rate. Echo Res Pract 1:71–83
Badano LP, Cucchini U, Muraru D, Nono OA, Sarais C, Iliceto S (2013) Use of three-dimensional speckle tracking to assess left ventricular myocardial mechanics: inter-vendor consistency and reproducibility of strain measurements. Eur Heart J Cardiovasc Imaging 14:285–293
Maffessanti F, Nesser H-J, Weinert L, Steringer-Mascherbauer R, Niel J, Gorissen W et al (2009) Quantitative evaluation of regional left ventricular function using three-dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol 104:1755–1762
Baccouche H, Maunz M, Beck T, Gaa E, Banzhaf M, Knayer U, Fogarassy P, Beyer M (2012) Differentiating cardiac amyloidosis and hypertrophic cardiomyopathy by use of three-dimensional speckle tracking echocardiography. Echocardiography 29(6):668–677
Hjertaas JJ, Fosså H, Dybdahl GL, Grüner R, Lunde P, Matre K (2013) Accuracy of real-time single- and multi-beat 3-d speckle tracking echocardiography in vitro. Ultrasound Med Biol 39:1006–1014
Seo Y, Ishizu T, Atsumi A, Kawamura R, Aonuma K (2014) Tree-dimensional speckle tracking echocardiography – a promising tool for cardiac functional analysis –. Circ J 78:1290–1301
Pagourelias ED, Mirea O, Duchenne J, Unlu S, Van Cleemput J, Papadopoulos CE, Bogaert J, Vassilikos VP, Voigt JU (2020) Speckle tracking deformation imaging to detect regional fibrosis in hypertrophic cardiomyopathy: a comparison between 2D and 3D echo modalities. Eur Heart J Cardiovasc Imaging pii: jeaa057. https://doi.org/10.1093/ehjci/jeaa057
Spartera M, Damascelli A, Mozes F, De Cobelli F, La Canna G (2017) Three-dimensional speckle tracking longitudinal strain is related to myocardial fibrosis determined by late-gadolinium enhancement. Int J Cardiovasc Imaging 33:1351–1360
Jasaityte R, Heyde B, D’hooge J, (2013) Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr 26(1):15–28
Villemain O, Correia M, Mousseaux E, Baranger J, Zarka S, Podetti I, Soulat G, Damy T, Hagège A, Tanter M, Pernot M, Messas E (2019) Myocardial stiffness evaluation using noninvasive shear wave imaging in healthy and hypertrophic cardiomyopathic adults. JACC Cardiovasc Imaging 12(7):1135–1145
Petrescu A, Santos P, Orlowska M, Pedrosa J, Bézy S, Chakraborty B, Cvijic M, Dobrovie M, Delforge M, D’hooge J, Voigt JU, (2019) Velocities of naturally occurring myocardial shear waves increase with age and in cardiac amyloidosis. JACC Cardiovasc Imaging 12(12):2389–2398
Gyöngyösi M, Winkler J, Ramos I, Do QT, Firat H, McDonald K, González A, Thum T, Díez J, Jaisser F, Pizard A, Zannad F (2017) Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail 19(2):177–191
Sado DM, Flett AS, Moon JC (2011) Novel imaging techniques for diffuse myocardial fibrosis. Future Cardiol 7:643–650
Knaapen P, Götte MJ, Paulus WJ, Zwanenburg JJ, Dijkmans PA, Boellaard R, Marcus JT, Twisk JW, Visser CA, van Rossum AC, Lammertsma AA, Visserv FC (2006) Does myocardial fibrosis hinder contractile function and perfusion in idiopathic dilated cardiomyopathy? PET and MR imaging study. Radiology 240:380–388
Rischpler C, Nekolla SG, Dregely I, Schwaiger M (2013) Hybrid PET/MR imaging of the heart: potential, initial experiences, and future prospects. J Nucl Med 54:402–415
Li XG, Roivainen A, Bergman J, Heinonen A, Bengel F, Thum T, Knuuti J (2015) Enabling [18 F]-bicyclo[6.1.0]nonyne for oligonucleotide conjugation for positron emission tomography applications: [18 F]-anti-microRNA-21 as an example. Chem Commun 51:9821–9824
Pagourelias E, Delforge M, Claus P, Gheysens O (2016) Functional and molecular correlative imaging in a patient with amyloidosis. Eur Heart J 37(23):1834
Acknowledgments
Efstathios Pagourelias is a Kakkis-Metaxas fellowship awardee while the fulfillment and completion of this project was possible through a scholarship from the Greek State Scholarships Foundation (IKY).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Efstathios Pagourelias and Georgios Alexandridis. The first draft of the manuscript was written by Georgios Alexandridis, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pagourelias, E.D., Alexandridis, G.M. & Vassilikos, V.P. Fibrosis in hypertrophic cardiomyopathy: role of novel echo techniques and multi-modality imaging assessment. Heart Fail Rev 26, 1297–1310 (2021). https://doi.org/10.1007/s10741-020-10058-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-10058-6