Abstract
To evaluate the severity of hypertrophic cardiomyopathy (HCM) according to global, regional, and multi-layer longitudinal strain (LS) analysis using speckle-tracking echocardiography. From February 2007 to November 2014, we prospectively evaluated 375 consecutive HCM patients referred to our specialized cardiomyopathy center. Demographics, clinical, and rest and exercise echocardiographic parameters were collected according to a completely standardized protocol. Global, regional, and multilayer strain analyses were performed. Correlations between LS and other characteristics were evaluated, and we assessed their prognostic value to predict sudden cardiac death (SCD) or appropriate implantable cardioverter defibrillator (ICD) shocks during follow-up, using Cox proportional hazards analyses. We finally included 217 patients (50.1 ± 15.6 years, 67% male) but only 179 (82%) had LS analysis of sufficient quality. An inverse relation was observed between the mean basal left ventricular (LV) LS and diastolic parameters [E/Ea (r = − 0.30) and left atrium indexed volume (r = − 0.23)], as well as between the resting LV outflow-tract maximal gradient (r = − 0.26) or during peak exercise (r = − 0.20). Mean LS in the LV hypertrophic area was particularly related with maximal wall thickness (r = − 0.47) and transmural global LS with the degree of myocardial fibrosis in cardiac magnetic resonance (r = − 0.32). During a median follow-up of 2.8 ± 1.5 years, mean transmural LS in the hypertrophic area was predictor of SCD and appropriate ICD shock (10 events/179 patients, hazard ratio = 0.83 [95% CI 0.72–0.95], p = 0.01). Basal LS and hypertrophic area LS are valuable parameters to evaluate HCM severity. Mean hypertrophic area LS particularly seems predictive of SCD occurrence and appropriate ICD shocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) has a prevalence of 1/500 in the general population and is believed to cause disabling symptoms particularly during exercise, as well as complications like heart failure, atrial fibrillation, and sudden cardiac death (SCD) owing to ventricular fibrillation [1].
SCD can be avoided by implantable cardioverter defibrillator (ICD) when the risk has been foreseen [2, 3]. However, current evaluations and recommended scores are unsuccessful in fully eliminating the risk of SCD, and other helpful parameters, such as myocardial fibrosis in cardiac magnetic resonance (CMR), are actively researched to help physicians to better identify which patients are at higher risk. Left ventricular (LV) longitudinal strain (LS) analysis by speckle-tracking echocardiography can moreover provide valuable information about HCM patient status [4,5,6,7,8].
A recently-developed speckle-tracking method enables LV myocardial strain to be evaluated by multilayer (endocardial/epicardial strain) analysis [9,10,11]. This is an innovative and newly validated tool, though it has not yet been fully evaluated in HCM [9].
We hypothesized that a regional analysis of LV strains in hypertrophied areas, basal segments, and the endocardial part of the LV wall could provide more details about HCM severity and the prognosis in terms of SCD or appropriate ICD shocks for ventricular tachycardia/fibrillation, in comparison with global longitudinal strain (GLS).
The main objective of our study was to evaluate the relationship between regional and multi-layer LS analyses, as well as with other parameters representative of HCM severity. The second objective of this study was to evaluate the relationship between regional and multi-layer LS analysis and the occurrence of SCD or appropriate ICD shock for ventricular tachycardia/fibrillation.
Methods
This is a single-center, observational, cohort study performed in a tertiary referral center (Bordeaux University Cardiologic Hospital, France) for patients with HCM. From February 2007, we prospectively evaluated a cohort of consecutive HCM patients who were clinically and ultrasonically evaluated according to a standardized acquisition protocol involving two-dimensional analysis of LV LS using speckle-tracking.
The inclusion criteria were: (1) diagnosis of HCM based on LV hypertrophy on echocardiography (≥ 15 mm) unexplained by abnormal loading conditions; (2) age ≥ 16 years; (3) at least two visits to the center during the study period to track events.
The exclusion criteria were: other cardiac or systemic disease that may produce LV hypertrophy (i.e., mitochondrial disease, neuromuscular diseases, malformation syndromes, infiltrative disease); prosthetic valve; known severe untreated coronary artery disease; poor ultrasound window quality; systolic blood pressure ≥ 160 mmHg at the time of the echocardiography; atrial fibrillation or stimulated rhythm by a pace maker; moderate to severe mitral regurgitation; previous history of myectomy or septal alcohol ablation.
Information concerning the study and data collection was provided to all patients, and the institutional review board approved the study protocol. Patients were included after providing informed consent.
Demographic characteristics, age, and circumstances of HCM discovery, common cardiovascular risk factors, therapies, history of atrial fibrillation, unexplained syncope (< 5 years), current cardiovascular symptoms, and family history of HCM or unexplained SCD were collected from the hospital records at the time of the first clinical evaluation, when echocardiography was also performed.
Non-sustained ventricular tachycardia (NSVT) was defined as three or more consecutive ventricular extras-systoles ≥ 120 beat/min, and lasting < 30 s, it was documented by a 24–48 h Holter ECG recording [3]. Previous paroxysmal (> 30 s) or permanent atrial fibrillation episodes were also recorded.
Each patient was evaluated at rest using transthoracic echocardiography according to a standardized acquisition protocol and current guidelines [12], with either Vivid 7 or E9 (General Electric Medical System®, Horten, Norway).
Recordings in standardized views were acquired in two-dimensional, pulsed, continuous, color-flow Doppler, and pulsed-tissue Doppler modalities, then stored for subsequent analysis. Three consecutive cardiac cycles were stored systematically. Off-line analysis was performed by the same operator using Echopac® software version BT 11.3.
Exercise echocardiography was conducted in accordance with European Association of Echocardiography guidelines [13]. The exercise consisted of bicycle exertion in a semi-supine position (50°) increasing by 25 W every 2 min up to the maximum tolerated effort.
Maximal end-diastolic LV wall thickness was measured on two-dimensional imaging. Volumes and LV ejection fraction were calculated from the apical two- and four-chamber views using Simpson’s biplan method. Biplane maximal left atrial volume was calculated using the area-length method and indexed to body surface area [14]. Mitral pulsed Doppler velocities were also measured. LV filling pressures were estimated using the ratio of mitral orifice peak E wave velocity to septal and lateral annular Ea peak velocities (mean E/Ea ratio). Systolic pulmonary artery pressure was calculated as recommended [14]. Mitral regurgitation, if present, was graded (none, mild, moderate, severe).
Particular attention was paid to the left ventricular outflow-tract (LVOT) area to identify and analyze systolic anterior motion of the mitral valve, as well to perform continuous Doppler. LVOT obstruction at rest was defined as a maximal gradient ≥ 30 mmHg [15] using pulsed and continuous wave Doppler from the apical three- and five-chamber views. LVOT obstruction during exercise was defined as a maximal gradient ≥ 50 mmHg [8, 15].
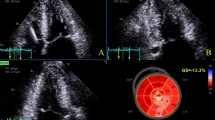
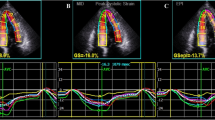
Longitudinal LV strain at rest was quantified in the three apical views using the two-dimensional speckle-tracking echocardiographic method [5, 16] GLS was presented in magnitude throughout the manuscript. LS was assessed in the three apical views using Echopac® software version BT 11.3 (General Electric Medical System®, Horten, Norway), enabling transmural (common LS) and both endocardial and epicardial strain analyses. Adequate tracking could be verified in real time and corrected numerically by adjusting the region of interest or manually by correcting the contour to ensure optimal tracking of LV hypertrophic myocardium. We also calculated the mean LS in hypertrophied segments for each patient and that of the LV basal segments.
Patients underwent CMR studies using a 1.5 T scanner (Magnetom Avanto®, Syngo MR B15 version; Siemens Medical Solutions®, Erlangen, Germany). Late gadolinium enhancement (LGE) sequences were performed, with images acquired 10–20 min following intravenous administration of 0.2 mmol/kg gadolinium-DTPA with breath-hold 2D segmented inversion-recovery sequence or phase-sensitive inversion-recovery sequences in identical planes as in cine images. Regional LGE extent was semi-quantitatively assessed, then calculated as follows: LGE area/each regional segment area × 100 (%) (0, 1–25, 26–50, 51–75, and 76–100%) (0 = no LGE in the segment, 1 = LGE ≤ 25% of the myocardial segment, 2 = 25–75% of LGE in the myocardial segment, 3 = LGE ≥ 75% of the myocardial segment), multiplied by the number of segments according to the 17-segment bull’s-eye model, enabling a score of 0–51 to be established, as suggested by Chan et al. [17].
Long-term follow-up was achieved using regular consultations every 6 months in our hospital or via patient and cardiologist recalls. The follow-up period for each patient was determined using the time from initial consultation to echocardiography and the last data recorded from the patient. In the event of death, the date and cause were both specified.
Endpoints: SCD was defined as sudden and unexpected collapse or nocturnal death with no previous deterioration. Aborted SCD or appropriate ICD shock were also considered events.
To assess LS analysis intraobserver variability, the data from 16 subjects was analyzed twice offline by the same operator, with at least 1 week between the two analyses. A second observer blinded to the results of the previous investigations also performed these same 16 analyses in order to assess interobserver variability. The percentage of variability was calculated as the absolute difference divided by the average of the two measurements of the same dataset.
Statistical analyses
Continuous and qualitative variables were expressed as mean with standard deviation (SD), and discrete variables were expressed as absolute numbers and percentages. Descriptive data was analyzed for normality using visual histograms and the Shapiro–Wilk test.
Linear correlations were evaluated between LV strains and some relevant echocardiographic variables using Pearson test. Specific survival, as defined for the end-point, was measured from inclusion until the first end-point or last follow-up, estimated using Cox proportional univariate hazards regression analyses. Estimated hazard ratios (HR) and their 95% confidence intervals (CI) were calculated. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using Stata software Version 11.0® (StataCorp LP, College Station, TX).
Results
Over 375 consecutive patients evaluated and followed at the HCM Competence Center from February 2007 to November 2014, 217 HCM patients were enrolled and included in this study. The reasons for exclusion have been presented in a flow chart (Fig. 1).
The mean age on initial evaluation was 50.1 ± 15.6 years, and 146 (67%) were male. Demographic and clinical characteristics of the population (n = 217) are presented in Table 1. The median follow-up duration was 2.8 ± 1.5 years.
In our population, the mean LV ejection fraction was 69.5 ± 8.6% (Table 2). In total, 61 (28%) patients had LVOT gradients ≥ 30 mmHg at rest, and the mean LV maximal end-diastolic wall thickness was 20.3 ± 4.6 mm.
During exercise (Table 2), the maximal level of exercise was 6.9 ± 1.9 METS equivalent.
LV LS strain analysis was feasible and obtained for 179/217 (82%) patients. We obtained relatively good intra- and inter-observer reproducibility for global LV LS (variability of 3.4 and 4.2%, respectively), regional transmural LS (variability of 8.2–12.2%, respectively), regional endocardial (variability of 7.8–12.8%, respectively) LS, and epicardial LS analyses (variability of 8.9–13.0%, respectively).
The mean global LS (GLS) was 15.7 ± 4.0%; mean transmural GLS in the hypertrophic area was 14.2 ± 4.1%, and transmural LS in the basal segments was 13.6 ± 3.7%.
Table 3 presents the best relations observed between other imaging variables and strains (GLS, mean LS in hypertrophic area, or mean LS in basal segments). A relative correlation was observed between global transmural LS and LV ejection fraction (r = 0.30) (Table 3).
An inverse correlation was observed between mean basal transmural LS and diastolic parameters [E/Ea (r = − 0.30) and maximal left atrium indexed volume (r = − 0.23)]; as well as with LVOT maximal gradient at rest (r = − 0.26) or at peak bicycle exercise (r = − 0.20) and max exercise level in watts (r = 0.28) or METS (r = 0.26).
The mean of (transmural) LS in hypertrophic area was particularly correlated with maximal LV wall thickness (r = − 0.47), and GLS with myocardial fibrosis score in CMR (r = − 0.32).
During a median follow-up of 2.8 ± 1.5 years, ten events occurred (four SCDs and six appropriate ICD shocks) for the 179 patients. In the Cox univariate analyses (Table 4), only maximal wall thickness, and mean LV LS in hypertrophic area were significantly predictive of SCD and appropriate ICD shock (p < 0.05), particularly transmural LS (HR = 0.83 [95% CI 0.72–0.95], p = 0.01). As multicolinearity between maximal wall thickness and LS in hypertrophic area was detected by a VIF test > 3.0, we did not performed any Cox multivariate analysis. Kaplan–Meier survival curves of (transmural) LS in hypertrophic area predicting SCD or appropriate ICD shock are presented in Fig. 2 (p for log-rank = 0.014). Figure 3 displays an example of a patient.
Kaplan–Meier survival curves for mean transmural longitudinal strain in hypertrophic area in the prediction of SCD or appropriate ICD shocks in HCM: p for log-rank was significant (p = 0.014) between patients with value ≤ 14.5% who presented more SCD or appropriate ICD shock than those with value > 14.5%
Discussion
Our study underlines the value of using LS analysis in the HCM population. It particularly highlights a relationship between mean basal LS and LV diastolic function parameters as well as LVOT obstruction, in addition to correlations between LS in the hypertrophic area and myocardial fibrosis in CMR, as well as the greater efficacy of LS measurement in the hypertrophic area compared to GLS for predicting SCD or appropriate ICD shocks.
Longitudinal deformation-related alteration of the LV occurs earlier than LV ejection fraction alteration in HCM [5]. Previous studies have pointed out the diagnostic role of LS analysis to detect subclinical forms of HCM [18] and this measurement’s role in predicting overall cardiovascular outcomes including heart failure evolution [19] or ICD therapies [20,21,22]. Debonnaire et al. [20] demonstrated that GLS < 14% and a left atrial indexed volume ≥ 34 mL/m2 were independent predictors off ICD therapy during follow-up. However, this study assessed only HCM patients with ICD. Haland et al. recently demonstrated that HCM patients with ventricular arrhythmia had worse GLS than controls (15.7 ± 3.6% vs. 21.1 ± 1.9%, p = 0.001) [21]. In our study, GLS was not significantly predictive of SCD or appropriate ICD shock (p = 0.19).
In our study, an inverse relationship was observed between mean basal transmural LS and both diastolic parameters [E/Ea (r = − 0.30) and left atrium indexed volume (r = − 0.23)]. These results are in line with those observed [23] in amyloidosis patients, where E/Ea was independently associated with basal LS (β = − 0.54, p < 0.0001). This inverse relationship in HCM could also be explained by the presence of relaxation abnormalities secondary to the microcirculation damages caused by LV hypertrophy, as well as by compliance abnormalities in these segments secondary to local myocardial fibrosis and maximal LVOT gradient.
Moreover, the basal segments seem to be altered in early stages of HCM. As recently published by Peyrou et al. [18], basal strain is significantly impaired in the HCM phenotype−/genotype+ subjects compared to controls.
In our study, mean transmural LS in the hypertrophic area was particularly related to maximal LV thickness (r = − 0.47). We also observed a relationship between myocardial fibrosis in CMR and LS in the hypertrophic area, as well as between transmural GLS and the fibrosis score (r = − 0.32). Finally, Cox analyses demonstrated that mean LS in the hypertrophic area was predictive of SCD or appropriate ICD shock during follow-up in HCM patients (p = 0.01), and to a greater extent than GLS (p = 0.19).
These results are also in line with Di Salvo’s work, which demonstrated in multivariate analysis that an LS ≤ 10% in > 3 segments was an independent parameter predictive of NSVT occurrence [24].
Multilayer strain is a very recent and so far poorly-described technique in the literature [9, 10]. To the best of our knowledge, our study is the first to evaluate the interest of multilayer strain analysis in HCM. Unfortunately, endocardial strain failed to perform better than transmural strain in predicting SCD or ICD shock during follow-up. However, multilayer strain can be useful in other situations. As recently described by Zhang et al. [25], it could provide a more sensitive and specific diagnostic index for the differential diagnosis of amyloid light-chain (AL) cardiac amyloidosis from HCM.
This is the largest cohort study to characterize HCM according to global, regional, and multi-layer LS analysis. However, as a tertiary center for HCM management, we cannot exclude the possibility there was a referral bias. We did not collect mortality data from a National registry, either, relying rather on our hospital’s records and direct communication with the patients or their doctors.
LS analysis clearly merits its place in clinical practice in the evaluation of HCM patients, and our present study provides additional data about its prognostic value. Previous studies have already pointed the potential prognostic implication of LS analysis in HCM. Reduced GLS is associated with more severe disease and confers an increased risk for major cardiac events, independently of other clinical and echocardiographic risk factors [26]. Our study particularly highlights the benefits of LS analysis in the prevention of SCD, a major challenge in this disease, demonstrating LS in the hypertrophic area as being more predictive than GLS.
Conclusion
In line with GLS, basal LS, and LS in the hypertrophic area are valuable parameters for evaluating HCM severity. Mean LS in the hypertrophic area in particular appears more predictive of SCD occurrence and appropriate ICD shocks than GLS.
Abbreviations
- CI:
-
Confidence interval
- GLS:
-
Global longitudinal strain
- HCM:
-
Hypertrophic cardiomyopathy
- HR:
-
Hazard ratio
- ICD:
-
Implantable cardioverter defibrillator
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- LVOT:
-
Left ventricular outflow-tract
- NSVT:
-
Non-sustained ventricular tachycardia
- CMR:
-
Cardiac magnetic resonance
- SCD:
-
Sudden cardiac death
References
Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS (2014) Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol 64:83–99
Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H (2014) ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35:2733–2779
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124:e783–e831
Maron MS, Pandian NG (2010) Risk stratification in hypertrophic cardiomyopathy: is two-dimensional echocardiographic strain ready for prime time? J Am Soc Echocardiogr 23:591–594
Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R, Lafitte S (2006) Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol 47:1175–1181
Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, Sumimoto T, Inaba S, Nishimura K, Inoue K, Ogimoto A, Shigematsu Y, Hamada M, Higaki J (2012) Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 13:617–623
Hiemstra YL, Debonnaire P, Bootsma M, Van Zwet EW, Delgado V, Schalij MJ, Atsma DE, Bax JJ, Marsan NA (2017) Global longitudinal strain and left atrial volume index provide incremental prognostic value in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 10:e005706
Reant P, Reynaud A, Pillois X, Dijos M, Arsac F, Touche C, Landelle M, Rooryck C, Roudaut R, Lafitte S (2015) Comparison of resting and exercise echocardiographic parameters as indicators of outcomes in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 28:194–203
Ozawa K, Funabashi N, Takaoka H, Kamata T, Kanaeda A, Saito M, Nomura F, Kobayashi Y (2015) Characteristic myocardial strain identified in hypertrophic cardiomyopathy subjects with preserved left ventricular ejection fraction using a novel multi-layer transthoracic echocardiography technique. Int J Cardiol 184C:237–243
Ozawa K, Funabashi N, Kamata T, Kobayashi Y (2017) Inter- and intraobserver consistency in LV myocardial strain measurement using a novel multi-layer technique in patients with severe aortic stenosis and preserved LV ejection fraction. Int J Cardiol 228:687–693
Sebastian SI, Haugaa KH, Zahid W, Bendz B, Aakhus S, Aaberge L, Edvardsen T (2013) Layer-specific quantification of myocardial deformation by strain echocardiography may reveal significant CAD in patients with non–ST-segment elevation acute coronary syndrome. JACC Cardiovasc Imaging 6:535–544
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL (2008) European Association of Echocardiography. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 9:415–437
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ (2003) Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 348:295–303
Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z (2004) Two-dimensional strain-a novel software for realtime quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 17:1021–1029
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS (2014) Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130:484–495
Peyrou J, Réant P, Reynaud A, Cornolle C, Dijos M, Rooryck-Thambo C, Landelle M, Montaudon M, Laurent F, Roudaut R, Lafitte S (2016) Morphological and functional abnormalities pattern in hypertrophy-free HCM mutation carriers detected with echocardiography. Int J Cardiovasc Imaging 32:1379–1389
Reant P, Mirabel M, Lloyd G, Peyrou J, Lopez Ayala JM, Dickie S, Bulluck H, Captur G, Rosmini S, Guttmann O, Demetrescu C, Pantazis A, Tome-Esteban M, Moon JC, Lafitte S, McKenna WJ (2016) Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 102:741–747
Debonnaire P, Thijssen J, Leong DP, Joyce E, Katsanos S, Hoogslag GE, Schalij MJ, Atsma DE, Bax JJ, Delgado V, Marsan NA (2014) Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int J Cardiovasc Imaging 30:549–558
Haland TF, Almaas VM, Hasselberg NE, Saberniak J, Leren IS, Hopp E, Edvardsen T, Haugaa KH (2016) Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 17:613–621
Candan O, Gecmen C, Bayam E, Guner A, Celik M, Doğan C (2017) Mechanical dispersion and global longitudinal strain by speckle tracking echocardiography: predictors of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy. Echocardiography 34:835–842
Schiano-Lomoriello V, Galderisi M, Mele D, Esposito R, Cerciello G, Buonauro A, Della Pepa R, Picardi M, Catalano L, Trimarco B, Pane F (2016) Longitudinal strain of left ventricular basal segments and E/e′ ratio differentiate primary cardiac amyloidosis at presentation from hypertensive hypertrophy: an automated function imaging study. Echocardiography 33:1335–1343
Di Salvo G, Pacileo G, Limongelli G, Baldini L, Rea A, Verrengia M, D’Andrea A, Russo MG, Calabrò R (2010) Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J Am Soc Echocardiogr 23:581–590
Zhang L, Zhou X, Wang J, Mu Y, Liu B, Lv W, Wang Y, Liu H, Liu H, Zhi G (2017) Differentiation of light-chain cardiac amyloidosis from hypertrophic cardiomyopathy using myocardial mechanical parameters by velocity vector imaging echocardiography. Int J Cardiovasc Imaging 33:499–507
Zegkos T, Parcharidou D, Ntelios D, Efthimiadis G, Karvounis H (2017) The prognostic implications of two-dimensional speckle tracking echocardiography in hypertrophic cardiomyopathy: current and future perspectives. Cardiol Rev. https://doi.org/10.1097/CRD.0000000000000172
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Vergé, MP., Cochet, H., Reynaud, A. et al. Characterization of hypertrophic cardiomyopathy according to global, regional, and multi-layer longitudinal strain analysis, and prediction of sudden cardiac death. Int J Cardiovasc Imaging 34, 1091–1098 (2018). https://doi.org/10.1007/s10554-018-1323-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1323-3