Abstract
We used peak longitudinal strain (PLS) on TTE in HCM patients to differentiate LV myocardium (LVM) into the following 4 groups: group 1—no fibrosis or hypertrophy (≥ 13 mm), group 2—no fibrosis but hypertrophy evident, group 3—fibrosis present but without hypertrophy, and group 4—both fibrosis and hypertrophy. Seventeen HCM patients (13 males, 56 ± 16 years) underwent both 1.5 T CMR and TTE. On TTE, PLS (absolute values) for each LVM segment from 17 AHA-defined lesions was calculated. Of 289 LVM lesions, the numbers in each group, 1–4, were 156, 53, 39, and 41, respectively. PLS for LVM segments in group 1 (13.6 ± 6.4%) were significantly greater than those in group 2 (8.5 ± 4.9%, P < 0.001), group 3 (10.4 ± 5.0%, P = 0.006), and group 4 (7.1 ± 4.4%, P < 0.001). PLS for LVM segments in group 3 was significantly greater than those in group 4 (P = 0.016). However, significant differences in PLS in LVM between groups 2 and 3, and between 2 and 4 were not observed. Using regional PLS, we demonstrate successful differentiation of LVM in HCM patients for group 1 (LVM with zero fibrosis or hypertrophy) from LVM belonging to groups 2–4 and we also demonstrate successful differentiation of LVM with fibrosis present but without hypertrophy from LVM with both fibrosis and hypertrophy. However, it is not possible to differentiate between LVM with no fibrosis but hypertrophy evident and those with fibrosis present but without hypertrophy and also between LVM with no fibrosis but hypertrophy evident and those with both fibrosis and hypertrophy. Our findings have significant implications for the management of HCM patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) patients have several abnormal findings such as coronary artery disease [1], gene mutations [2], and the extent of late gadolinium enhancement (LGE), suggesting the presence of fibrosis in the left ventricular myocardium (LVM) measured by cardiac magnetic resonance (CMR) [3], and these parameters may have prognostic predictive values.
Both fibrosis and hypertrophy in the LVM measured by CMR adversely influence peak longitudinal strain (PLS) values (absolute values) measured by two-dimensional speckle-tracking transthoracic echocardiography (TTE) at corresponding sites [4,5,6,7].
We hypothesized that quantitative values of PLS assessed in HCM patients by TTE could be used to differentiate LVM into the following 4 groups: group 1—zero fibrosis or hypertrophy, group 2—no fibrosis but hypertrophy evident, group 3—fibrosis present but no hypertrophy, and group 4—presence of fibrosis and hypertrophy.
Methods
Patient population
Seventeen HCM patients were enrolled and (13 males, mean age 56 ± 16 years) underwent both 1.5 T CMR (Achieva, Philips) and TTE (Vivid E9, GE Healthcare). Patient background and basic TTE findings are presented in Table 1.
Speckle-tracking TTE
On TTE, PLS (absolute values) for each LVM segment among 17 lesions (as defined by American Heart Association [8]) were calculated using Echo PAC, version 113 (GE Healthcare) (Fig. 1) [9, 10].
Actual images of left ventricular (LV) regional peak longitudinal strain (PLS) strain analysis (upper image) and its strain chart (lower figure) on two-dimensional speckle-tracking transthoracic echocardiogram (TTE). PLS measured by TTE for each LV segment from 17 lesions (as defined by American Heart Association) was calculated using Echo PAC, version 113 (GE Healthcare). In the strain chart, the dotted line indicates global strain (GS), an average of the six solid lines. Each colored line indicates strain change during the cardiac cycle at the corresponding colored site in the LV myocardium shown in the upper TTE image. AVC aortic valve closure
CMR protocol
Both fibrosis and hypertrophy were determined by CMR [11]. On CMR, the patients were placed supine in a clinical 1.5 T magnetic resonance imaging (MRI) with 5-channel cardiac coils around the chest. All CMR were obtained using electrocardiogram gating and during repeated breath-holds, as previously reported [12]. Surface-coil intensity correction was performed for cine CMR and CMR for LGE. Cine CMR images were acquired with a steady-state free precession sequence. After acquiring cine CMR on the 2- and 4-chamber long-axis projections, we obtained short axis cine CMR encompassing the LV from base to apex. CMR for LGE were acquired 10–15 min after intravenous administration of 0.15 mmol/kg of gadopentetate dimeglumine (Magnevist; Schering AG). An inversion recovery prepared, T1-weighted, three dimensional gradient-echo sequence was used to obtain CMR for LGE in the same plane as cine images. The inversion time was adjusted to minimize the signal from the normal myocardium in each patient, using a look-locker sequence to find a null point in normal myocardium. A typical inversion time for LGE CMR ranged from 230 to 300 ms. Imaging data were analyzed on workstations (Ziostation 2, Ziosoft).
Fibrosis was defined as LGE on T1-weighted images in CMR (Fig. 2).
Typical late gadolinium enhancement image observed in T1-weighted cardiac magnetic resonance, suggesting the presence of myocardial fibrosis, in a patient with hypertrophic cardiomyopathy. Myocardial fibrosis was observed in the LV anterior and inferior wall (arrows). LV and RV indicate left and right ventricles, respectively
Hypertrophy was defined as myocardial tissue with ≥ 13 mm wall thickness on CMR [13].
We compared regional PLS in each LVM segment stratified into groups 1–4 as described above.
Statistical analysis
For all analyses, P < 0.05 was considered to be statistically significant. Regional PLS (absolute values) on speckle-tracking TTE between LV lesions with and without fibrosis and LV lesions with and without hypertrophy on CMR were compared using the t test (SPSS software, version 17.0, SPSS, Inc.). Regional PLS (absolute values) in each LVM segment stratified into groups 1–4 as described above were compared using Tukey–Kramer method or Games–Howell method depending upon the results of Levene test (SPSS software, version 17.0, SPSS, Inc.).
This is a retrospective study according to guidelines. TTE and CMR were required clinically in all subjects. We obtained written informed consent from all subjects.
Results
Of 289 LVM lesions, LGE, suggesting fibrosis, was detected in 80 LVM lesions, and not detected in 209 LVM lesions.
PLS was significantly smaller in fibrotic lesions (8.7 ± 5.0%) than in non-fibrotic lesions (12.3 ± 6.4%) (P < 0.001) (Fig. 3).
Comparison of peak longitudinal strain (PLS) (absolute values) between left ventricular lesions, with and without fibrosis, in patients with hypertrophic cardiomyopathy. PLS was significantly smaller in fibrotic lesions (N = 80) (8.7 ± 5.0%) than in non-fibrotic lesions (N = 209) (12.3 ± 6.4%) (P < 0.001)
PLS values in LVM with hypertrophy (N = 94) (7.9 ± 4.7%) were significantly less than that in LVM without hypertrophy (N = 195) (12.9 ± 6.3%) (P < 0.001) (Fig. 4).
Comparison of peak longitudinal strain (PLS; absolute values) between left ventricular (LV) myocardium classified by the presence or absence of hypertrophy (≥ 13 mm wall thickness) measured by MRI in patients with hypertrophic cardiomyopathy. PLS values in LV myocardium with hypertrophy (N = 94) (7.9 ± 4.7%) were significantly less than that in LV myocardium without hypertrophy (N = 195) (12.9 ± 6.3%) (P < 0.001)
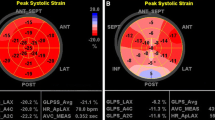
Of 289 LVM lesions, the numbers in each group, 1–4, were 156, 53, 39, and 41, respectively.
PLS for LVM segments in group 1 (13.6 ± 6.4%) were significantly greater than those in group 2 (8.5 ± 4.9%, P < 0.001), group 3 (10.4 ± 5.0%, P = 0.006), and group 4 (7.1 ± 4.4%, P < 0.001) (Fig. 5). PLS for LVM segments in group 3 was significantly greater than those in group 4 (P = 0.016). However, significant differences in PLS in LVM between groups 2 and 3, and between 2 and 4 were not observed.
Comparison of peak longitudinal strain (PLS) (absolute values) among 4 types of left ventricular (LV) myocardium classified by presence or absence of fibrosis and/or hypertrophy in patients with hypertrophic cardiomyopathy. On a segment based analysis, LV myocardium was divided into four groups; group 1—no fibrosis or hypertrophy, group 2—no fibrosis but evident hypertrophy, group 3—fibrosis present but no hypertrophy, and group 4—both fibrosis and hypertrophy. Hypertrophy was defined as myocardial tissue with ≥ 13 mm wall thickness measured by magnetic resonance imaging. PLS for LV segments in group 1 (13.6 ± 6.4%) were significantly greater than those in group 2 (8.5 ± 4.9%, P < 0.001), group 3 (10.4 ± 5.0%, P = 0.006), and group 4 (7.1 ± 4.4%, P < 0.001). PLS for LV segments in group 3 was significantly greater than those in group 4 (P = 0.016). However, significant differences in PLS in LV segments between groups 2 and 3, and between 2 and 4 were not observed
Discussion
The relationship of regional PLS to LGE has been reported previously [14], and our results are consistent with those findings. Furthermore, in this study, we reported the influences of myocardial hypertrophy or the combination of LGE and myocardial hypertrophy on PLS in HCM patients.
Our hypothesis is that PLS is influenced by both the presence of fibrosis and wall hypertrophy, although the mechanism of the influence of hypertrophy on PLS values is unclear. As the low numbers of HCM patients in this study makes it difficult to conclude whether PLS can or cannot (the conclusion of this study) differentiate LVM in the proposed 4 groups. However, our results indicate that PLS in LVM without fibrosis or hypertrophy was significantly greater than that of the other 3 groups. In Fig. 3, PLS values were significantly lower in the LVM with fibrosis group than in the LVM without fibrosis group. In Fig. 4, PLS values were significantly lower in the LVM with hypertrophy group than in the LVM without hypertrophy group. Furthermore, in Fig. 5, PLS values were the lowest in the LVM with both fibrosis and hypertrophy among the proposed 4 groups. From these results, we suggest that hypertrophy may have some influence on PLS values.
Strain is defined as the deformation of an object, normalized to its original shape [15] and is influenced by the presence of fibrotic or hypertrophic lesions [4,5,6,7]. Okada reported that timing and waveform analyses of systolic strain rate may be useful to distinguish between HCM and hypertension [16]. Even in this small study using a limited numbers of HCM patients (N = 17) and using regional PLS, we were still able to successfully differentiate LVM from HCM patients in group 1 (LVM with neither fibrosis nor hypertrophy) from LVM belonging to groups 2–4. However, it was not possible to differentiate LVM with fibrosis from that with hypertrophy. The reason of this result may be (1) the number of patients was small and (2) we defined hypertrophy as myocardial tissue with ≥ 13 mm wall thickness by CMR.
Quantitative absolute values of LV wall thickness measured in a larger numbers of patients may produce better results.
On the other hand, LGE on CMR, suggesting the presence of fibrotic lesions, indicates irreversible myocardial modification. Therefore, there may be disagreement between the presence of LGE seen on CMR and the reduction in PLS indicating functional abnormality.
Significant implications for patient management
If the patients had advanced renal dysfunction, performing an MRI-enhanced examination is contraindicated in such patients. Therefore, in the future, if myocardium with fibrotic change can be detected with two-dimensional speckle-tracking TTE, similar to the detection of LGE on MRI, we can predict future cardiac events and can evaluate the appropriateness of application of an implantable cardioverter defibrillator by TTE rather than LGE on CMR in patients with advanced renal dysfunction.
Furthermore, the numbers of institute, with available CMR, are limited, and if TTE can detect fibrotic lesions, the significance of speckle-tracking TTE would increase.
Previously, we reported that multilayer strain measurement on TTE allows layer-specific PLS measurement (endocardial, epicardial, and whole layer myocardial strain) in HCM [17], ischemic heart disease [10], collagen disease [9], pulmonary hypertension [18], and valvular disease [19,20,21]. This new layer PLS measurement technique may help to better estimate PLS surrogacy to myocardial fibrosis and hypertrophy in the future.
Study limitations
This is a non-randomized study in a single center with small numbers of patients.
In this study, we measured regional PLS in whole layer LV myocardium. However, measurement of regional PLS in asymmetrical and abnormal wall thickened LV myocardium in HCM patients may not be consistent with the measurement of a true whole layer PLS, because the configuration of the thickened LV myocardium is beyond the pre-defined contour. This discrepancy would be influenced by the degree of hypertrophy or spatial relationship with hypertrophic site.
We did not attempt to detect hypertrophy from PLS, because hypertrophy can be detected by TTE. However, we aimed to detect fibrotic change, as confirmed on CMR, by TTE, with consideration of the influence of hypertrophy.
Multi-variable analysis should be performed to evaluate the relationship between the PLS values, myocardial fibrosis, and the influence of wall thickening. However, due to the small numbers of the patients, a limitation of this study, we could not perform such analysis.
In our study, a new finding of “the impaired influence of hypertrophy on PLS” is added to “the impaired influence of the presence of fibrosis on PLS”, the latter of which has been already reported [14].
In the near future, we plan to evaluate our hypothesis in a larger patient population. Furthermore, in future studies, we hope to differentiate LV hypertrophy with LV wall thickening due to infiltrative disease (such as cardiac amyloidosis) or storage disease (such as cardiac Fabry disease) using this technique.
Normal values of PLS vary depending upon the location of the LVM and between locations. In particular, PLS values in the LV apex tend to be greater than that in other locations [22], and this locational effect on PLS values should be considered when we examine a larger population in the future.
Diffuse fibrosis may not be detected as LGE on CMR, but the presence of diffuse fibrosis may influence the PLS values. Therefore, T1 mapping on MRI [23] should also evaluate the presence of diffuse fibrosis in LVM, which may influence PLS values on TTE.
We have previously evaluated inter- and intra-observer consistency in LV myocardial strain measurement in patients with severe aortic stenosis and preserved LV ejection fraction [19]. However, in this present study, we did not evaluate the performance of inter- and intra-observer consistency in the detection of fibrosis and hypertrophy.
This is not a quantitative, but a qualitative study, designed to evaluate detection of LGE and hypertrophy. LV mass index may be more preferable as the indicator of LV wall hypertrophy than the presence of ≥ 13 mm LV wall thickness, we did not quantify the area, volume or the degree of LGE.
Conclusion
Using regional PLS, we have demonstrated successful differentiation of LVM from HCM patients in group 1 (LVM with no fibrosis or hypertrophy) from LVM belonging to groups 2–4. We have also demonstrated successful differentiation of LVM with fibrosis present but without hypertrophy from LVM with both fibrosis and hypertrophy. However, it is not possible to differentiate between group 2 (LVM with no fibrosis but hypertrophy evident) and group 3 (those with fibrosis present but without hypertrophy) and also between group 2 (LVM with no fibrosis but hypertrophy evident) and group 4 (those with both fibrosis and hypertrophy). Our findings could have significant implications for the management HCM patients.
References
Okayama S, Soeda T, Kawakami R, Takami Y, Somekawa S, Ueda T, Sugawara Y, Matsumoto T, Sung JH, Nishida T, Uemura S, Saito Y (2015) Evaluation of coronary artery disease and cardiac morphology and function in patients with hypertrophic cardiomyopathy, using cardiac computed tomography. Heart Vessels 30:28–35
Chida A, Inai K, Sato H, Shimada E, Nishizawa T, Shimada M, Furutani M, Furutani Y, Kawamura Y, Sugimoto M, Ishihara J, Fujiwara M, Soga T, Kawana M, Fuji S, Tateno S, Kuraishi K, Kogaki S, Nishimura M, Ayusawa M, Ichida F, Yamazawa H, Matsuoka R, Nonoyama S, Nakanishi T (2017) Prognostic predictive value of gene mutations in Japanese patients with hypertrophic cardiomyopathy. Heart Vessels 32:700–707
Funada A, Kanzaki H, Noguchi T, Morita Y, Sugano Y, Ohara T, Hasegawa T, Hashimura H, Ishibashi-Ueda H, Kitakaze M, Yasuda S, Ogawa H, Anzai T (2016) Prognostic significance of late gadolinium enhancement quantification in cardiac magnetic resonance imaging of hypertrophic cardiomyopathy with systolic dysfunction. Heart Vessels 31:758–770
Reant P, Metras A, Detaille D, Reynaud A, Diolez P, Jaspard-Vinassa B, Roudaut R, Ouattara A, Barandon L, Dos Santos P, Lafitte S (2016) Impact of afterload increase on left ventricular myocardial deformation indices. J Am Soc Echocardiogr 29:1217–1228
Edwards NC, Moody WE, Yuan M, Hayer MK, Ferro CJ, Townend JN, Steeds RP (2015) Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am J Cardiol 115:1311–1317
Ishizu T, Seo Y, Kameda Y, Kawamura R, Kimura T, Shimojo N, Xu D, Murakoshi N, Aonuma K (2014) ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension 63:500–506
Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG (2014) Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 7:11–19
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Ozawa K, Funabashi N, Sugiura A, Kobayashi Y (2016) Layer specific strain measurement and its relationship to heart failure indicators in systemic autoimmune disorder patients: a multi-layer transthoracic echocardiography study. Int J Cardiol 220:693–699
Nishi T, Funabashi N, Ozawa K, Takahara M, Fujimoto Y, Kamata T, Kobayashi Y (2016) Resting multilayer 2D speckle-tracking transthoracic echocardiography for the detection of clinically stable myocardial ischemic segments confirmed by invasive fractional flow reserve. Part 1: vessel-by-vessel analysis. Int J Cardiol 218:324–332
Sakuma H (2014) Late gadolinium enhancement and prognosis of hypertrophic cardiomyopathy. Circ J 78:832–834
Takaoka H, Funabashi N, Uehara M, Iida Y, Kobayashi Y (2017) Diagnostic accuracy of CT for the detection of left ventricular myocardial fibrosis in various myocardial diseases. Int J Cardiol 228:375–379
Valente AM, Lakdawala NK, Powell AJ, Evans SP, Cirino AL, Orav EJ, MacRae CA, Colan SD, Ho CY (2013) Comparison of echocardiographic and cardiac magnetic resonance imaging in hypertrophic cardiomyopathy sarcomere mutation carriers without left ventricular hypertrophy. Circ Cardiovasc Genet 6:230–237
Betancur J, Simon A, Halbert E, Tavard F, Carré F, Hernández A, Donal E, Schnell F, Garreau M (2016) Registration of dynamic multiview 2D ultrasound and late gadolinium enhanced images of the heart: application to hypertrophic cardiomyopathy characterization. Med Image Anal 28:13–21
Uematsu M (2015) Speckle tracking echocardiography–Quo Vadis? Circ J 79:735–741
Okada K, Kaga S, Mikami T, Masauzi N, Abe A, Nakabachi M, Yokoyama S, Nishino H, Ichikawa A, Nishida M, Murai D, Hayashi T, Shimizu C, Iwano H, Yamada S, Tsutsui H (2017) Characteristic systolic waveform of left ventricular longitudinal strain rate in patients with hypertrophic cardiomyopathy. Heart Vessels 32:591–599
Ozawa K, Funabashi N, Takaoka H, Kamata T, Kanaeda A, Saito M, Nomura F, Kobayashi Y (2015) Characteristic myocardial strain identified in hypertrophic cardiomyopathy subjects with preserved left ventricular ejection fraction using a novel multi-layer transthoracic echocardiography technique. Int J Cardiol 184:237–243
Ozawa K, Funabashi N, Tanabe N, Tatsumi K, Kobayashi Y (2016) Contribution of myocardial layers of right ventricular free wall to right ventricular function in pulmonary hypertension: analysis using multilayer longitudinal strain by two-dimensional speckle-tracking echocardiography. Int J Cardiol 215:457–462
Ozawa K, Funabashi N, Kamata T, Kobayashi Y (2017) Inter- and intraobserver consistency in LV myocardial strain measurement using a novel multi-layer technique in patients with severe aortic stenosis and preserved LV ejection fraction. Int J Cardiol 228:687–693
Ono R, Funabashi N, Ozawa K, Takaoka H, Kamata T, Kobayashi Y (2015) An educational intervention to help medical students achieve accurate and consistent measurement of longitudinal myocardial strain on transthoracic echocardiogram. Int J Cardiol 201:300–301
Ozawa K, Funabashi N, Kobayashi Y (2016) Left ventricular myocardial strain gradient using a novel multi-layer transthoracic echocardiography technique positively correlates with severity of aortic stenosis. Int J Cardiol 221:218–226
Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Nakatani S, JUSTICE Investigators (2012) Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J 76:2623–2632
Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, Rolf A, Zitzmann S, Peker E, D’Angelo T, Pathan F, Elen Valbuena S, Hinojar R, Arendt C, Narula J, Herrmann E, Zeiher AM, Nagel E, International T1 Multicentre CMR Outcome Study (2018) Native T1 and ECV of noninfarcted myocardium and outcome in patients with coronary artery disease. J Am Coll Cardiol 71:766–778
Acknowledgements
This work is partially supported by a Grant from Japan Heart Foundation Research Grant (no Grant numbers). The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the Heart and Vessels.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Funabashi, N., Takaoka, H., Ozawa, K. et al. 2D speckle-tracking TTE-based quantitative classification of left ventricular myocardium in patients with hypertrophic cardiomyopathy by the presence or the absence of fibrosis and/or hypertrophy. Heart Vessels 33, 1046–1051 (2018). https://doi.org/10.1007/s00380-018-1155-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1155-z