Abstract
The volatile organic compounds (VOCs) emitted by plant rhizobacteria play a significant role in the promotion of plant growth. However, it is unclear how VOCs play a role in plant growth and which component participates in this process. In this study, we assessed the effect of the VOCs emitted by Bacillus sp. JC03 on the promotion of plant growth and identified the overall functional mechanism. The results indicated that the VOCs produced by JC03 could significantly promote the biomass accumulation of Arabidopsis and tomato. Furthermore, an analysis of Arabidopsis mutants perturbations in hormone production and signaling, in conjunction with analyses of hormone contents and gene expression levels, indicated that auxin and strigolactone played essential roles in the promotion of plant growth induced by the VOCs produced by JC03. The results showed that the ARF1 and CCD7 genes were significantly upregulated in the Arabidopsis seedlings exposed to the VOCs emitted by JC03 and the results of the endogenous hormone levels detection experiment reached the same conclusion. Furthermore, the VOC-induced phenotype was reduced or, even lost in the ARF1, and CCD7 mutant lines, while the phenotype remained in A. thaliana ecotype Col-0 seedlings and in other mutants, such as etr1, OST1 and gai1. Finally, GC-MS analysis results positively identified the compounds released from JC03, including 3-hydroxy-2-butanone, 1, 3-propanediol, 2-methyl-dipropanoate, tetrahydrofuran-3-ol, 2-heptanone, 2-ethyl-1-hexanol. Only tetrahydrofuran-3-ol, 2-heptanone and 2-ethyl-1-hexanol, at different concentrations, significantly promoted the growth of the Arabidopsis seedlings. In this study, we first demonstrated that the VOCs emitted by JC03 promoted plant growth through the action of auxin and strigolactone, and identified several new compounds, tetrahydrofuran-3-ol, 2-heptanone and 2-ethyl-1-hexanol, that could promote plant growth. The important achievement of our study is the further elucidation of the interacting mechanisms related to plant responses to the VOCs emitted by microbes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are living organisms that play a major rolein the environment and life on the earth; however, there are many factors that affect plant growth and reduce their ability to reach their greatest growth potential (Kopsell and Kopsell 2008). Plant growth factors influence or dictate plant characteristics as well as plant adaptation. Generally, there are two factors, which can affect plant growth and development: genetic factors and environmental factors. Genetic factors are also called internal factors, and environmental factors are considered external; nevertheless, the two factors interact in various ways. Environmental factors determine the growth and development of plants (Kopsell and Kopsell 2008).
There are many environmental factors that affect plant growth, such as climate, temperature, water, light, nutrition, soil characteristics, (Li et al. 2010; Valverdebarrantes et al. 2017). In addition, an important environmental factor that can significantly promote plant growth, rhizobacteria, has been well studied in recent years; rhizobacteria are generally referred to PGPR (plant-growth-promoting rhizobacteria) (Lugtenberg and Kamilova 2009). Plant growth can be affected by PGPR in two different ways, directly or indirectly. The direct promotion of plant growth by PGPR entails providing the plant either with compounds biosynthesized by the bacteria, such as an essential nutrient from the environment, or hormones (Beneduzi et al. 2012). In addition, the growth of plants can be limited by low levels of soluble phosphate. However, some PGPR can solubilize inorganically or organically bound phosphates, thereby promoting plant growth (Vassilev et al. 2006). The indirect promotion of plant growth occurs when PGPR decrease or prevent the harmful effects of one or more plant pathogens (Beneduzi et al. 2012). Additionally, the development and growth of plant organs could also influence crop production by modulating photosynthesis, nutrient remobilization, and index of harvest (Iqbal et al. 2012, 2017). Phytohormones, such as auxin, ethylene, jasmonates, salicylic acid, and strigolactone (SL), have been shown to increase the growth of plants (Iqbal et al. 2017). Jasmonates were demonstrated to be naturally occurring plant growth regulators in the plant kingdom (Per et al. 2018). In addition, ethylene is also regarded as a multifunctional phytohormone that regulates growth (Iqbal et al. 2017). Mostofa and associates found that strigolactones, a class of carotenoid-derived phytohormones, were initially discovered as an “ecological signal” for parasitic seed germination and the establishment of a symbiotic relationship between beneficial microbes and plants (Mostofa et al. 2018).
The significant role of VOCs emitted by plant rhizobacteria in plant growth promotion has recently been well documented (Ryu et al. 2003; Rath et al. 2018). The first study mentioning the effect of the VOCs emitted by bacteria on plant growth was reported by Cook and Stall (1969); however, research on VOCs only began to become popular following the discovery of bacterial VOCs promoting the growth of Arabidopsis in 2003. The results of that study showed that the total leaf area of Arabidopsis exposed to the VOCs released by B. amyloliquefaciens GB03 for 10 days increased by ~ fivefold (Ryu et al. 2003). Additionally, other bacterial species have been evaluated as growth inducers through VOC emission. Zou and associates (2010) demonstrated that B. megaterium XTBG-34 can increase the fresh weight of Arabidopsis by releasing volatiles (Zou et al. 2010). Gutiérrez-Luna found that some VOCs emitted by Bacillus sp. can modify the root architecture and promote nutrient uptake, thus promoting the growth of plants (Gutiérrez-Luna et al. 2010). A study carried out by Orozco-Mosqueda indicated that the chlorophyll concentration and, fresh weight of shoots and roots of Medicago truncatula seedlings treated with the VOCs released from Arthrobacter agilis UMCV2, were increased by 35%, 35%, 40%, respectively (Orozco-Mosqueda et al. 2013). In addition, some fungal species were reported to promote plant growth by releasing VOCs. Minerdi and associates (2011) demonstrated that Fusarium oxysporum MSA35 could promote plant growth, as measured by increasing root and shoot length, fresh weight and chlorophyll concentration, by emitting volatiles (Minerdi et al. 2011). Trichoderma viride, a species of fungus, was reported to promote plant growth through releasing volatiles by Hung et al. in (2013). Additionally, VOCs can stimulate induced systemic resistance (ISR) in several plant species in response to pathogen challenge (Kloepper et al. 2004; Ryu et al. 2005).
Over the past decades, studies on VOCs have targeted individual microbes, mainly focusing on a few bacterial and fungal species, such as Bacillus sp., Arthrobacter sp., Trichoderma sp. (Ryu et al. 2003, 2004; Orozco-Mosqueda et al. 2013; Hung et al. 2013; Tahir et al. 2017). However, the mechanisms associated with the VOCs emitted by microbes were also investigated to varying extents. Previous studies indicated that VOCs can promote plant growth by four primary mechanisms: essential nutrient modulation, sugar accumulation, metabolism and hormonal balance (Hung et al. 2013). It was demonstrated that the VOCs emitted by B. subtilis strain JS had the ability to regulate tobacco gene expression, which was associated with metabolism and cellular processes, thus regulating plant growth and development (Kim et al. 2015). Afterward, Wang and associates (2017) suggested that VOCs could modulate the distribution of essential nutrients in plants (Wang et al. 2017). Moreover, the modulation of plant hormones plays an important role in plant growth and development. Until now, there have been few new developments or findings in this research area. For example, B. amyloliquefaciens GB03 could modulate endogenous photosynthesis and sugar transduction by reducing ABA synthesis-related gene expression (Sánchez-López et al. 2016). Hao and associates (2016) demonstrated that B. amyloliquefaciens FZB42 could regulate plant growth by releasing VOCs and activating the expression of different genes related to plant hormones (Hao et al. 2016). However, this area of research is still new and it is still unknown whether plants react to the VOCs emitted by microbes through conserved regulated mechanisms and which signaling pathways are involved in this process.
In addition, recent research has focused on characterizing the volatile compounds and series of VOCs produced by various PGPR have been identified that can significantly promote plant growth, including 2,3-butanediol, 13-tetradecadien-1-ol, 2-pentylfuran, 2-methyl-n-1-tridecene, 2-butanone and acetoin (3-hydroxy-2-butanone) (Farag et al. 2006; Zou et al. 2010; Park et al. 2015). However, it is unclear whether there are any additional components in the volatile compounds released by microbes that can promote plant growth. Therefore, to investigate these questions, we established the model of plant and Bacillus sp. JC03 interaction that was previously identified to significantly promote characteristics of plant growth. In our study, we demonstrated that the VOCs emitted by JC03 could significantly promote the biomass accumulation of Arabidopsis and tomato seedlings; moreover, the VOCs could promote the elongation of Arabidopsis roots. The results detecting the regulation of gene expression levels showed that auxin and SL played essential roles in the response to the VOCs produced by JC03 promoting plant growth as well. Finally, we determined that tetrahydrofuran-3-ol, 2-heptanone and 2-ethyl-1-hexanol, which were components of the compounds released from JC03, significantly promoted growth and that different concentrations of these chemicals increased fresh weight. In this study, we first demonstrated that the VOCs emitted by JC03 promoted plant growth through auxin and SL action and identified several new compounds, tetrahydrofuran-3-ol, 2-heptanone and 2-ethyl-1-hexanol that promoted plant growth. The significant achievement of our study is the further elucidation of the mechanisms plant responses to the VOCs emitted by microbes.
Materials and methods
Plants, bacteria, and growth conditions
Bacillus sp. JC03, which was isolated from the pear rhizosphere soil by our lab and collected from Zhejiang Province, China and has demonstrated plant growth promotion characteristics, was used in our study. JC03 was cultured on Luria–Bertani (LB) agar medium and incubated at 28 °C for 24 h. In this study, Escherichia coli DH5α was used as a negative control, and DH5α was cultured on LB agar medium and incubated at 37 °C for 24 h. Moreover, Solanum lycopersicum (Micro-Tom), A. thaliana (ecotype Col-0) and phytohormone biosynthesis and metabolism-related gene mutants (ARF1, CCD7, etr1, OST1 and gai1) were studied. All the test plant seeds were surface-sterilized by soaking in 70% ethanol for 30 s followed by soaking in 10% sodium hypochlorite solution for 2–3 min and then soaking in sterilized water 4–5 times until the sodium hypochlorite was completely removed. The surface-sterilized Arabidopsis seeds were laid on a plate divided into two sections containing 0.5 × Murashige and Skoog Basal Medium (MS medium) (PhytoTech™, M519), while the seeds of S. lycopersicum were placed in a tissue culture bottle containing 0.5 × MS medium. All the seedlings were cultured in growth chambers with 14 h light (150 µmol photons m−2 /s)/10 h dark photoperiod at 22 °C.
Growth-promotion evaluation of the VOCs emitted by Bacillus sp. JC03
To assess the plant growth promotion activity of the VOCs emitted by JC03, one day before the assessment experiments, the rhizobacteria JC03 were streaked onto LB plates and incubated at 28 °C in darkness for 24 h. Bacterial cells were harvested from LB plates with sterile water, and the concentration was adjusted to 1 × 109 CFU/mL. JC03 suspension culture (20 µL) was applied dropwise to the non-plant side of the Petri dishes divided into two sections that contained the Arabidopsis seedlings [refer to the method reported by Zhang et al. (2007)]. By positioning the plants and bacteria on separate sides of the petri dish, the plants are exposed to bacterial VOCs without physical contact. In these experiments, E. coli DH5α was introduced as a negative control, and sterile water was used as a mock control. The plant structure and biomass measurement indexes, such as taproot length, lateral root number, fresh weight, plant height, and stem diameter, were determined 30 days post-treatment. The effect of VOCs on the promotion of tomato growth was determined according to the same experimental design principle. However, because the tomato plants grow faster and larger than Arabidopsis seedlings, we cultured the tomato plants in tissue culture bottles and placed a small beaker containing bacteria on one side of the tissue culture bottle to prevent direct contact between microbes and plants. To verify the growth-promoting activity of each identified VOC component, plant growth and compound inoculations were established in the same manner as described above. All experiments were performed three times.
Plant RNA extraction and Q-RT-PCR analysis
The total RNA of Arabidopsis was extracted by using the TRIZOL reagent (Life Invitrogen™, Cat. No. 15596-026). Total RNA (1 mg) was used for reverse transcription and treated with DNase I (gDNA wiper from Vazyme™, Cat. No. R133-01). Then, reverse transcription was conducted by using HiScript™ Q Select RT SuperMix (Vazyme™, Cat. No. R133-01). Q-RT-PCR was performed by using the ABI 7500 system (ABI) with the SYBR premix Ex-Taq mixture (Takara). The reaction was performed under the following conditions: 94 °C for 5 min, followed by 45 cycles of 94 °C for 10 s, 55 °C for 20 s, and 72 °C for 30 s, and ending with 72 °C for 5 min. The ß-Tub 4 gene was employed as the internal standard.
Effect of the VOCs emitted by JC03 on the transcription of genes involved in phytohormone biosynthesis
Total RNA was extracted from 1.0 g fresh weight Arabidopsis leaves that were collected 30 days after exposure to the VOCs emitted by JC03 using TRIZOL reagent (Life Invitrogen™, Cat. No. 15596-026). E. coli DH5α was introduced as a negative control, and sterile water was used as a mock control. The expression levels of ARF1, ABF4, ERF2, CCD7 and GA2ox1 were analyzed. All the primers used in this study are listed in the Supporting Information (Table S1).
Quantification of endogenous phytohormones in Arabidopsis seedlings
For endogenous phytohormone quantification, 1.0 g fresh weight Arabidopsis leaves were harvested and frozen in liquid nitrogen and pulverized with a mortar and pestle. Indole acetic acid (IAA), abscisic acid (ABA), gibberellins (GA) and ethylene (ETH) were measured by using the following kits: IAA ELISA kit (Biomatik™, Cat. No. EKU04915); ABA ELISA kit (Agrisera™, Cat. No. AS111748); GA ELISA kit (Biomatik™, Cat No. EKU04370); and ETH ELISA Kit (Biotsz™, Cat. No. JM-E100015053). The extraction, purification and quantification of each phytohormone (IAA, ABA, SL, GA, and ETH) were performed according to the manufacturer’s instructions. The data describing the detected endogenous phytohormones, which were calculated according to each corresponding conversion formula and the detection value from the enzyme labeling instrument, were statistically analyzed.
For SL analysis, 1.0 g of fresh weight Arabidopsis leaves was ground in a mortar with liquid nitrogen. The samples were extracted and purified according to the protocol described by López-Ráez et al. (2010). Strigolactone was detected and quantified by LC–MS/MS. Data acquisition and analysis were performed by using the software MassLynx version 4.1 (WATERs™). The total area of all the corresponding MRM transitions was used for statistical analysis.
Signaling pathway dependence analysis
To analyze the regulatory pathways involved in the plant growth promoted by the VOCs emitted by JC03, mutant lines of plant hormone synthesis coding genes (ARF1, CCD7, etr1, OST1 and gai1) were used in this study. The of VOC to promote the growth of the different Arabidopsis mutants was assessed, and the fresh weight of the mutants was determined 45 days after exposure to the VOCs emitted by JC03. E. coli DH5α was introduced as a negative control, and sterile water was used as a mock control.
Extraction and analysis of the VOCs emitted by JC03 (SPME–GC/MS analysis)
Divinylbenzene/carboxen on polydimethylsiloxane (DCP/PDMS, 50/30 µm) solid phase microextraction (SPME) fiber (Supelco, Bellefonte, PA, USA, Cat. No. 57299-U) was used for VOC extraction. A 2 cm SPME fiber was recommended. Twenty microliters of JC03 suspension culture was applied dropwise to a 200 ml vial containing MS agar medium, and the mixture was incubated at 28 °C. E. coli DH5α incubated with MS agar medium and MS agar medium alone were used as the negative control and the mock control. To collect the VOCs, the SPME fiber was inserted into the headspace of the vial 7 days after incubation and exposed to the emitted VOCs at room temperature for 15 h, which was previously determined to be the optimal extraction time based on the combination of the number and abundance of compounds recorded (Curran et al. 2005).
GC–MS analysis was performed by using a Bruker 450-GC gas chromatograph in combination with a Bruker 320-MS mass spectrometer as described by Farag et al. (2006). The column was an HP5-MS of 30 m, 0.25 µm, and 0.25 mm with helium as the carrier gas (flow rate: 1.0 ml/min). The SPME fibers were desorbed at 220 °C for 5 min, and the GC method was initiated with an initial oven temperature of 40 °C for 5 min. The temperature was then increased by 10 °C/min until it reached 240 °C, and then the temperature was held at 240 °C for 2 min. The mass spectra for the VOCs were analyzed by referring to the data in the NIST/EPA/NIH Mass Spectrum Library. All of the VOCs from Bacillus sp. JCO3 that were characterized were purchased from Sigma-Aldrich and evaluated individually for their ability to promote plant growth in our assessment system.
Statistical analysis
All bioassays were conducted three times with 24 seedlings per treatment. The data were subjected to analyses of variance (ANOVA) by using SPSS 24.0 (SPSS Inc., USA). Standard errors and standard deviations were calculated. For percentage data, an arcsine transformation was applied prior to ANOVA. Means were assessed by Fisher’s Protected least significant difference (LSD) test at the level of P = 0.05.
Results
VOCs emitted by JC03 promote plant growth in Arabidopsis and tomato
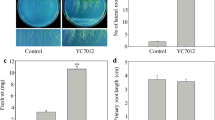
To assess the effect of the VOCs emitted by JC03 on Arabidopsis seedling growth, divided petri dishes (I-plates) supplied with MS-agar medium were used (Fig. 1). The results showed that the VOCs emitted by JC03 were able to stimulate the production of plant biomass; the fresh weight of Arabidopsis plants exposed to VOCs was increased by threefold compared to that of mock-treated plants (Fig. 1a, b; Table 1). Moreover, the VOCs emitted by JC03 were able to promote the extension of the root and increase in the lateral root in Arabidopsis seedlings (Fig. 1d, e; Table 1). However, no significant increase in the production of biomass and the formation of lateral roots were observed in plants inoculated with E. coli DH5α compared to those of mock-treated plants (Fig. 1a, c, d, f; Table 1).
Effect of volatile organic compounds emitted by Bacillus sp. JC03 on the growth of Arabidopsis. The Arabidopsis seedlings were cultured in growth chambers following 30 days JC03 volatile exposure by using two division plate assay system. The growth of seedling expose to JC03 30 for days were photographed (b, e). A non-growth-promoting E. coli strain DH5α (c, f) and water treatment alone (a, d) were carried out in this study for control
To verify the growth-promoting effect of the VOCs emitted by JC03, the tomato model system was used to repeat the assessment experiment. As shown in Supplemental Fig. 1, the VOCs emitted by JC03 were able to stimulate the production of plant biomass in tomato; in tomato seedlings inoculated with JC03, the fresh weight increased by almost tenfold and the plant height, stem diameter and number of leaves increased significantly compared to mock-treated plants (Fig. S1; Table S2). However, no significant increase in biomass production was observed in the tomato seedlings inoculated with E. coli DH5α (Fig. S1; Table S2).
VOCs emitted by JC03 promote plant growth by affecting the transcription of genes involved in phytohormone biosynthesis and metabolism
The promotion of plant growth is often accompanied by the regulation of the transcription of genes involved in phytohormone biosynthesis and metabolism. In this study, the expression levels of ARF1, ABF4, ERF2, CCD7 and GA2ox1, which are involved in phytohormone biosynthesis in Arabidopsis, were analyzed by quantitative reverse-transcription PCR (Q-RT-PCR). As demonstrated in Fig. 2a, the VOCs emitted by JC03 could modify the transcription of ARF1 and CCD7 in Arabidopsis; the results revealed that VOCs emitted by JC03 could increase the expression of the ARF1 gene and could significantly decrease the expression of the CCD7 gene. However, there was no difference in the transcription levels of these two genes in the Arabidopsis seedlings treated with DH5α (Fig. 2a). In addition, we also quantified the transcription of genes involved in other phytohormone biosynthetic and metabolic processes, such as ABF4, ERF2, and GA2ox1, which are involved in abscisic acid, ethylene and gibberellin biosynthetic processes, respectively. Q-RT-PCR results revealed that there was no significant difference among the treatments (Fig. 2a). Therefore, the results indicate that the VOCs emitted by JC03 promote plant growth probably via the auxin and strigolactone signaling pathways.
Effects of VOC emitted by JC03 on encoding genes expression level and content changes of endogenous hormone in Arabidopsis. a Transcriptional expression profiles of genes involved in plant hormone synthesis in Arabidopsis. Q-RT-PCR was performed to determine the relative expression levels of genes involved in plant hormone synthesis such as ARF4, ABF4, ERF2, CCD7 and GA2ox1 in each treatment. The expression values of the individual genes were normalized using β-Tub4 gene as an internal standard. b Detection of endogenous phytohormones in Arabidopsis treated with VOC components produced by Bacillus sp. JC03. Leaves of Arabidopsis in different treatments were harvested at the indicated time points for the quatity detection of phytohormones. The content of IAA, ABA, ETH, strigolactone and GA were determined in each treatment in this study. All data are presented as means of three replicates ± SD, and error bars represent the standard errors of three independent treatment samples. All experiments were performed three times, and similar results were obtained
The level of phytohormones in Arabidopsis was altered by the VOCs released from JC03
Many studies have shown that plant growth is regulated by a variety of endogenous hormones. Therefore, in this study, we also investigated the effect of the VOCs released from JC03 on phytohormone accumulation levels. We quantitatively determined IAA, ABA, GA, ETH and SL accumulation in Arabidopsis leaves with different treatments. As expected, the VOCs released from JC03 led to a significant increase in the endogenous levels of IAA and a significant decrease in the level of SLs in Arabidopsis plants; however, there were no significant differences in the levels of ABA, GA and ETH (Fig. 2b). As shown in Fig. 2b, the accumulation of endogenous IAA in the leaves of Arabidopsis treated with the VOCs released from JC03 gradually increased with treatment time and reached a maximum at 35 dpt; however, in the leaves of Arabidopsis, neither E. coli DH5α nor the mock treatment resulted in changes in the endogenous phytohormone content at the same time points (Fig. 2b). Furthermore, we also found that the level of SLs in the leaves of Arabidopsis treated with VOCs released from JC03 gradually decreased and reached a minimum at 25 dpt (Fig. 2b). Additionally, the results of the other phytohormone tests (such as ABA, GA and ETH) showed that there was no significant difference in the hormone levels among the treatments (Fig. 2b). The above results indicated that the VOCs emitted by JC03 promote plant growth, mainly by regulating the levels of auxin and strigolactone in plants.
Auxin and strigolactone were involved in VOCs emitted by JC03 promoting plant growth
To verify the conclusions described above, we compared the ability of the VOCs emitted by JC03 to promote plant growth in the phytohormone biosynthesis- and metabolism-related gene mutants, etr1, ARF1, CCD7, OST1 and gai1. The fresh weight of the Arabidopsis mutants in each treatment was determined. As shown in Fig. 3, consistent with the results in the wild type Col-0, the VOCs emitted by JC03 led to a significant (P < 0.05) increase in plant growth in all tested Arabidopsis plants, except the ARF1 and CCD7 mutants, compared with the respective mock controls (Fig. 3). The results showed that the fresh weight of the Arabidopsis seedlings exposed to the VOCs emitted by JC03 was significantly increased in etr1, OST1 and gai1 mutants, while there were no significant (P < 0.05) differences in ARF1 and CCD7 mutants (Fig. 3). The above results indicated that ARF1 and CCD7 mutations could affect the growth promoting effect of the VOCs emitted by JC03 on plants. Thus, we can conclude that the growth promotion of the VOCs emitted by JC03 depends on the auxin and strigolactone signaling pathways. Furthermore, we also determined the fresh weight of the Arabidopsis seedlings exposed to the VOCs released from E. coli DH5α and there were no significant (P < 0.05) differences compared with the respective mock controls. The above results were in accordance with the previous results shown in the wild type plants.
Auxin and strigolactone actin were involved in VOCs emitted by JC03 promoting plant growth. The Arabidopsis hormone synthesize coding gene mutant lines (ARF1, CCD7, etr1, OST1 and gai1) were carried out to analyze the regulatory pathways which take part in VOCs emitted by JC03 promoting plant growth. The growth promoting ability of VOCs to different A. thaliana mutants was assessed and the fresh weight of different A. thaliana mutants were detected at 45 days after exposure to VOCs emitted by JC03. The E. coli DH5α was introduced as negative control, and the sterile water as a mock control. Data are presented as means of three replicates ± SD, and error bars represent SD for three replicates. Means with asterisk have significant differences (*P < 0.05; **P < 0.01; LSD test). All experiments were performed three times, and similar results were obtained
Tetrahydrofuran-3-ol, 2-heptanone and 2-ethyl-1-hexanol were the main components of the VOCs that promoted plant growth
To determine which components in the VOCs functioned in plant growth promotion, solid phase microextraction (SPME) coupled with GC–MS was carried out to analyze the volatile compounds in this study. As shown in Fig. 4a, five volatile compounds from Bacillus sp. JC03 that had relatively high peak areas, e.g., ≥ 1%, and were not similar to the E. coli DH5α and mock control treatment were identified (Fig. 4a). The identified VOCs included two alcohols (2-ethyl-1-hexanol and tetrahydrofuran-3-ol), two ketones (3-hydroxy-2-butanone and 2-heptanone) and one acid (1,3-propanediol-2-methyl-dipropanoate). Furthermore, the peak areas of tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol were much higher than the other two compounds (Fig. 4a).
Identification of each component of microbial VOC and evaluation of its promoting function. a Chromatographic profiles of volatiles releasing from Bacillus sp. JC03. The asterisk on the peak means the specific compounds released from JC03. The compounds released from strains JC03 positively identified include 3-hydroxy-2-butanone, 1, 3-propanediol, 2-methyl-dipropanoate, tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol. B-C Growth promotion response of A. thaliana to different concentration of VOC components produced by Bacillus sp. JC03. The Arabidopsis seedlings were cultured in growth chambers following 30 days JC03 volatiles exposure, with different concentrations by using two division plate assay system. b The growth of seedling expose to different volatiles (tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol) for 30 days were photographed. c The growth promoting ability of identified bacterial volatiles to A. thaliana ecotype Col-0 was assessed and the fresh weight of Arabidopsis seedlings in different treatment (tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol) were determined at 45 days after exposure to the bacterial volatiles. The Data are presented as means of three replicates ± SD, and error bars represent SD for three replicates. Means with asterisk have significant differences with each control treatment (*P < 0.05; **P < 0.01; LSD test). All experiments were performed three times, and similar results were obtained
To verify that the growth promotion of Arabidopsis induced by the VOCs emitted by JC03 was due to the above identified components, the divided petri dishes (I-plates) supplied with MS–agar medium were used and the growth promoting capacity of each compound was evaluated with at different concentrations (Fig. 4b). As shown in Fig. 4b, tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol were able to promote plant growth. We found that the seedlings treated with tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol grew more robustly than mock control. Moreover, we also noticed that, for each of these three compounds, the concentration that promoted the best plant growth was different (Fig. 4b). The results in Fig. 4b shows that the best concentrations of tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol to promote plant growth were 1 µg/µl, 10 ng/µl, and 1 µg/µl, respectively.
To quantitatively demonstrate the growth-promoting effects of various substances on Arabidopsis seedlings, the fresh weight of plants in each treatment was determined. As shown in Fig. 4c, compared with the blank control, the fresh weight of plants treated with compounds such as tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol increased significantly (Fig. 4c). Tetrahydrofuran-3-ol and 2-ethyl-1-hexanol exhibited the strongest increase in the fresh weight of the seedlings at a concentration of 1 µg/µl, while 2-heptanone exhibited the strongest increase in the fresh weight of seedlings at a concentration of 10 ng/µl (Fig. 4c). The above results indicate that the promotion of plant growth by the VOCs released by JC03 may not be the result of a single component but may be a result of the interaction of multiple components, for instance, tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol.
Discussion
Plant growth can be affected by plant growth rhizobacterium (PGPR) in two different ways, directly and indirectly. The plant growth-promoting VOCs emitted by PGPR are defined to act directly on plants and have been studied previously (Ryu et al. 2003). It has been demonstrated that some PGPR, such as B. amyloliquefaciens IN937a, B. amyloliquefaciens GB03, P. fluorescens SS101 and Paenibacillus polymyxa E681, emit volatile compounds that stimulate the growth of Arabidopsis (Ryu et al. 2003; Lee et al. 2012; Asari et al. 2016). Zhang et al. (2008) reported that volatile emissions from B. amyloliquefaciens GB03 augment photosynthetic capacity by increasing chlorophyll content and photosynthetic efficiency in Arabidopsis (Zhang et al. 2008). Additionally, Park et al. (2015) recently found that the VOCs emitted by P. fluorescens SS101 could stimulate plant growth and development. In our study, we demonstrated that B. subtilis JC03 could promote various plant growth indexes by releasing VOCs through establishing a noncontact co-culture system of plants and bacteria. We observed a clear and significant increase in the root length, leaf area and fresh weight of Arabidopsis and tomato plants following exposure to the VOCs emitted by JC03 (Figs. 1 and S1). It is well known that the lateral root architecture, is an important component of the plant root system, directly affects plant root morphogenesis and plays a critical role in the absorption of nutrients by plants (Bensmihen 2015). A study carried out by Gutiérrez-Luna et al. (2010) concluded that Bacillus species modified root architecture, eliciting increases in total fresh weight, primary root length, lateral root number, and lateral root length in Arabidopsis. In this study, we also found that the volatile compounds released by JC03 could modify the development of plant roots, thereby affecting the uptake of nutrients by plants. A clear and significant increase was observed in the root length and lateral root numbers of Arabidopsis treated with the VOCs emitted by JC03 (Fig. 1; Table 1). Thus, we hypothesized that the VOCs emitted by JC03 promoted plant growth mainly by affecting plant root morphogenesis.
It is generally known that phytohormones regulate numerous important biological processes in plant development. PGPR have been determined to modulate plant growth by regulating the process of plant hormone synthesis (Tahir et al. 2017). Zhang et al. (2007) reported that microbial VOCs induced numerous physiological changes related to growth hormones and photosynthesis. Arabidopsis mutants of cre1 and ein2 have been reported to be insensitive to the volatiles produced by B. amyloliquefaciens GB03, indicating a role for cytokinins in the detection of PGPR signals (Ryu et al. 2003). Our results showed that the VOCs emitted by JC03 could modify the transcription of ARF1 and CCD7 in Arabidopsis. As a result, the expression of the ARF1 gene was increased, and the expression of the CCD7 gene was significantly decreased in Arabidopsis exposed to the VOCs emitted by JC03; however, there was no significant difference in the expression levels of the ABF4, ERF2, and GA2ox1 genes (Fig. 2a). An analysis of Arabidopsis mutants perturbations in hormone production and signaling, in conjunction with analyses of hormone contents, has indicated that auxin and strigolactone (but not ETH, GA and ABA) may participate in the growth-promoting effect of the VOCs emitted by JC03. The quantification of plant endogenous hormones indicated that the VOCs released from JC03 led to a significant increase in the endogenous levels of IAA and a significant decrease in the level of SLs in Arabidopsis plants; however, there were no significant differences in the levels of ABA, GA and ETH (Fig. 2b). Moreover, the fresh weight of the Arabidopsis seedlings exposed to the VOCs emitted by JC03 was significantly increased in etr1, OST1 and gai1 mutants, while there were no significant (P < 0.05) differences in ARF1 and CCD7 mutants (Fig. 2b). The above results indicated that the VOCs emitted by JC03 promoted plant growth mainly by regulating the levels of auxin and strigolactone in plants. Our results were not quite the same as those reported by Zhang et al. (2007, 2008), in which the VOCs emitted by B. amyloliquefaciens GB03 promoted plant growth in a manner dependent on ABA, auxin and cytokinin pathways. Our results also demonstrated that these plant signaling pathways were not the same as those involved in the regulation of plant growth promoted by the VOCs released from Bacillus species.
Microbial VOCs are signaling molecules with low molecular weight (< 300 g/mol), high vapor pressure (0.01 kPa at 20 °C), and low boiling point, which are ideal characteristics for traveling through the air, soil, and water and for modulating physiological processes (Kanchiswamy et al. 2015). Over the past few decades, diverse chemical compounds emitted by the metabolism of rhizobacteria have been identified by GC–MS (Korpi et al. 2009). These compounds are produced during primary and secondary metabolism, including mainly the metabolism of ketones, alcohols, furans, sulfur compounds and terpenes. The first identified compound, 2, 3-butanediol, was reported by Ryu et al. (2003). Ann et al. (2013) determined that 3-hydroxy-2-butanone acted as an elicitor that could increase the fresh weight of tobacco at 1 to 10 ppm. Subsequently, several new compounds that could act as growth elicitors were identified, such as 2-methyl-η-1-tridecene, 13-tetradecadien-1-ol and 2-butanone, which can increase the fresh weight of tobacco (Park et al. 2015). Additionally, in this study, we demonstrated that tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol were able to promote plant growth. It was found that the seedlings treated with tetrahydrofuran-3-ol, 2-heptanone, or 2-ethyl-1-hexanol grew more robustly (Fig. 4b, c). Furthermore, we noticed that the concentration that promoted the best plant growth was different for each of the three compounds. The above results indicated that the plant growth induced by the VOCs released by JC03 might not be the result of a single component, but a result of the interaction of multiple components, for instance tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol. In this study, we determined that the VOCs emitted by JC03 promote plant growth, mainly by regulating the levels of auxin and strigolactone in plants. Thus, the question of how the VOCs activate the auxin and strigolactone signaling pathways is raised. Do the three substances we identified (tetrahydrofuran-3-ol, 2-heptanone, and 2-ethyl-1-hexanol) take part in this process, or is there another mechanism? All these questions need to be discussed in future studies.
Abbreviations
- VOCs:
-
Volatile organic compounds
- PGPR:
-
Plant-growth-promoting rhizobacteria
- IAA:
-
Indole acetic acid
- ABA:
-
Abscisic acid
- GA:
-
Gibberellins
- ETH:
-
Ethylene
- SLs:
-
Strigolactone
- MRM:
-
Multiple reaction monitoring mode
- SPME:
-
Solid phase microextraction
- LSD:
-
Least significant difference
References
Ann M, Cho Y, Ryu H, Kim H, Park K (2013) Growth promotion of tobacco plant by 3-hydroxy-2-butanone from Bacillus vallismortis EXTN-1. Korean J Pestic Sci 17:388–393
Asari S, Matzén S, Petersen MA, Bejai S, Meijer J (2016) Multiple effects of Bacillus amyloliquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol Ecol 92(6):fiw070
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35(4):1044–1051
Bensmihen S (2015) Hormonal control of lateral root and nodule development in legumes. Plants 4(3):523–547
Cook A, Stall R (1969) Necrosis in leaves induced by volatile materials produced in vitro by bacteria. Phytopathology 59:259–260
Curran AM, Rabin SI, Prada PA, Furton KG (2005) Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J Chem Ecol 31(7):1607–1619
Farag MA, Ryu CM, Sumner LW, Paré PW (2006) Gc-ms spme profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67(20):2262–2268
Gutiérrez-Luna FM, López-Bucio J, Altamirano-Hernández J, Valencia-Cantero E, Cruz HRDL, Macías-Rodríguez L (2010) Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 51(1):75–83
Hao HT, Zhao X, Shang QH, Wang Y, Guo ZH, Zhang YB, Zhong KX, Wang RY (2016) Comparative digital gene expression analysis of the Arabidopsis response to volatiles emitted by Bacillus amyloliquefaciens. PLoS One 11:e0158621
Hung R, Lee S, Bennett JW (2013) Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecol 6:19–26
Iqbal N, Khan NA, Nazar R, Teixeira da Silva JA (2012) Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur. Environ Exp Bot 78:84–90
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Mir K (2017) Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci 8(9):1–19
Jiang CH, Fan ZH, Ping X, Guo JH (2016) Bacillus cereus AR156 extracellular polysaccharides served as a novel micro-associated molecular pattern to induced systemic immunity to Pst DC3000 in Arabidopsis. Frontiers in Microbiology 7(9):1–16
Kanchiswamy C, Malnoy M, Maffei M (2015) Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci 20:206–211
Kim JS, Lee J, Seo SG, Lee C, Woo SY, Kim SH (2015) Gene expression profile affected by volatiles of new plant growth promoting rhizobacteria, Bacillus subtilis strain JS, in tobacco. Genes Genom 37:387–397
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94(11):1259–1266
Kopsell DA, Kopsell DE (2008) Genetic and environmental factors affecting plant lutein/zeaxanthin. Agro Food Industry Hi Tech 19(2):44–46
Korpi A, Järnberg J, Pasanen AL (2009) Microbial volatile organic compounds. Crit Rev Toxicol 39:139–193
Lee B, Farag MA, Park HB, Kloepper JW, Lee SH, Ryu CM (2012) Induced resistance by a long-chain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS ONE 7:e48744
Li ZG, Liu AQ, Wu HS, Tan L, Long YZ, Gou YF (2010) Influence of temperature, light and plant growth regulators on germination of black pepper (Piper nigrum l.) seeds. Afr J Biotech 9(9):1354–1358
López-Ráez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TD, Thompson AJ, Ruyter-Spira C, Bouwmeester H (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187(2):343–354
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 2009(1):541–556
Minerdi D, Bossi S, Maffei M, Gullino M, Garibaldi A (2011) Fusarium oxysporum and its bacterial consortium promote lettuce growth and expansin A5 gene expression through microbial volatile organic compounds (MVOC) emission. FEMS Microbiol Ecol 76:342–351
Mostofa MG, Li W, Nguyen KH, Fujita M, Lam-Son PT (2018) Strigolactones in plant adaptation to abiotic stresses: an emerging avenue of plant research. Plant Cell Environ 41:2227–2243
Orozco-Mosqueda M, Velázquez-Becerra C, Macías-Rodríguez LI, Santoyo G, Flores-Cortez I, Alfaro-Cuevas R, Valencia-Cantero E (2013) Arthrobacter agilis UMCV2 induces iron acquisition in Medicago truncatula (strategy I plant) in vitro via dimethylhexadecylamine emission. Plant Soil 362(1–2):51–66
Park YS, Dutta S, Ann M, Raaijmakers JM, Park K (2015) Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem Biophys Res Commun 461(2):361–365
Per TS, Khan MIR, Anjum NA, Masood A, Hussain SJ, Khan NA (2018) Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environ Exp Bot 145:104–120
Rath M, Mitchell TR, Gold SE (2018) Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture dependent. Microbiol Res 208:76–84
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. P Natl Acad Sci USA 100:4927–4932
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Ryu CM, Hu CH, Locy RD, Kloepper JW (2005) Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil 268(1):285–292
Sánchez-López AM, Baslam M, De Diego N, Muñoz FJ, Bahaji A, Almagro G, Ricarte-Bermejo A, García-Gómez P, Li J, Humplík JF, Novák O, Spíchal L, Doležal K, Baroja-Fernández E, Pozueta-Romero J (2016) Volatile compounds emitted by diverse phytopathogenic microorganisms promote plant growth and flowering through cytokinin action. Plant Cell Environ 39(12):2592–2608
Tahir HA, Gu Q, Wu H, Raza W, Hanif A, Wu L, Colman MV, Gao XW (2017) Plant growth promotion by volatile organic compounds produced by Bacillus subtilis syst2. Front Microbiol 8(e48744):171
Valverdebarrantes OJ, Freschet GT, Roumet C, Blackwood CB (2017) A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytol 215(4):1562–1573
Vassilev N, Vassileva M, Nicolaeva I (2006) Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol 71:137–144
Wang J, Zhou C, Xiao X, Xie Y, Zhu L, Ma Z (2017) Enhanced iron and selenium uptake in plants by volatile emissions of Bacillus amyloliquefaciens (bf06). Appl Sci 7(1):85
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Paré PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW (2008) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56:264–273
Zou C, Li Z, Yu D (2010) Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J Microbiol 48(4):460–466
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Young Scholars Program) (31701829), National Natural Science Foundation of China (31471812, 31672075), Natural Science Foundation of Jiangsu Province (Young Scholars Program) (BK20170709), National Postdoctoral Program for Innovative Talents (BX201600074), China Postdoctoral Science Foundation (2017M611839).
Author information
Authors and Affiliations
Contributions
CJ and JG designed research. CJ, YX, KZ, ZL and NW performed experimental work. CJ wrote the paper and GY, JG revised the paper.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, CH., Xie, YS., Zhu, K. et al. Volatile organic compounds emitted by Bacillus sp. JC03 promote plant growth through the action of auxin and strigolactone. Plant Growth Regul 87, 317–328 (2019). https://doi.org/10.1007/s10725-018-00473-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-018-00473-z