Abstract
Background and aims
Iron is an essential nutrient for plant growth. Although abundant in soil, iron is poorly available. Therefore, plants have evolved mechanisms for iron mobilization and uptake from the rhizospheric environment. In this study, we examined the physiological responses to iron deficiency in Medicago truncatula plants exposed to volatile organic compounds (VOCs) produced by Arthrobacter agilis UMCV2.
Methods
The VOC profiles of the plant and bacterium were determined separately and during interaction assays using gas chromatography. M. truncatula plants exposed to A. agilis VOCs and pure dimethylhexadecylamine were transferred to conditions of iron deficiency, and parameters associated with iron nutritional status were measured.
Results
The relative abundance of the bacterial VOC dimethylhexadecylamine increased 12-fold when in co-cultures of A. agilis and M. truncatula, compared to axenic cultures. Plants exposed to bacterial VOCs or dimethylhexadecylamine exhibited a higher rhizosphere acidification capacity, enhanced ferric reductase activity, higher biomass generation, and elevated chlorophyll and iron content relative to controls.
Conclusions
The VOCs emitted by A. agilis UMCV2 induce iron acquisition mechanisms in vitro in the Strategy I plant M. truncatula. Dimethylhexadecylamine is the signal molecule responsible for producing the beneficial effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) is an essential nutrient for plants as it allows them to maintain cellular homeostasis (Hell and Stephan 2003). Although iron is abundant in the soil, its low solubility restricts its availability in aerobic soils to levels low enough to limit plant growth. This problem of solubility is due to the chemical nature of iron. As a transition metal, iron possesses the ability to gain or lose electrons, which gives it important properties in redox reactions (Vert et al. 2002). Fe2+ is the form available to plants because it is relatively soluble, but is rapidly oxidized to Fe3+ by atmospheric oxygen. The solubility of Fe(III) dramatically decreases with increasing pH due to hydrosilation, polymerization, and precipitation with inorganic anions (Hell and Stephan 2003). The scarcity of iron reduces agricultural production and causes a decline in the nutritional value of crops (Schmidt 1999).
Plants require effective mechanisms to acquire iron from the soil to fulfill growth and developmental demands, while avoiding toxicity due to excess. Efficient acquisition of iron in plants occurs via 2 mechanisms: Strategy I and Strategy II (Römheld and Marschner 1986). Strategy II plants include grasses whose roots secrete compounds known as phytosiderophores (PS) that chelate Fe3+ in the rhizosphere. Subsequently, the Fe3+-PS complex is introduced into the cell via a carrier protein in the plasmalemma known as YS1 (Hell and Stephan 2003). Strategy I plants are all higher plants (except grasses), and iron acquisition occurs via 3 mechanisms: i) excretion of protons across the plasma membrane through an ATPase to acidify the rhizosphere and enhance Fe3+ solubilization; ii) reduction of Fe3+ to the ferrous form (Fe2+) by the expression of the ferric chelate reductase protein, encoded in Arabidopsis by the FRO2 gene (Robinson et al. 1999), in roots; and iii) transfer of Fe2+ into the root cells through the plasma membrane by the transporter IRT (Eide et al. 1996). Besides the 3 main mechanisms described above, iron deficiency also promotes the excretion of phenolic compounds, organic acids, and flavins, which facilitate the reduction and solubility of external iron (Susín et al. 1994; Welkie and Miller 1988). In Strategy I plants, the 3 main inducing conditions are: iron deficiency (Hell and Stephan 2003), presence of humic acids (Aguirre et al. 2009), and presence of growth-promoting microorganisms (Zhang et al. 2009). Regarding the latter, the molecules responsible for the induction of iron acquisition in Strategy I plants are unknown.
Plant growth-promoting rhizobacteria (PGPR) stimulate plant growth via different mechanisms, including the synthesis of plant growth regulators, such as auxins, cytokinins, and even cyclopeptides (Ortíz-Castro et al. 2008; 2011; Spaepen et al. 2009). Recently, we have shown that volatile organic compounds (VOCs) produced by PGPR alter plant development, particularly the root system (Gutiérrez-Luna et al. 2010). Further, a previous study by our group showed that Arthrobacter agilis UMCV2 can improve the nutritional status of leguminous plants by promoting iron acquisition mechanisms involving the reduction and dissolution of Fe3+ present in the soil (Valencia-Cantero et al. 2007), and that emission of the VOC N, N-dimethylhexadecylamine (dimethylhexadecylamine or DMHDA) acts as a signal to promote the growth of the legume Medicago sativa, besides drastically modifying the roots (Velázquez-Becerra et al. 2011).
To dissect the mechanisms and determine the compounds involved in the modulation of iron acquisition responses in Strategy I plants, we examined the overall effect of the VOCs emitted by the PGPR A. agilis UMCV2 on the development of the model legume M. truncatula. In this study we used the legume model M. truncatula instead of M. sativa for 2 reasons: 1) it is easier to compare our results with those of other studies employing M. truncatula, particularly those related to plant-bacteria interactions and plant iron acquisition in vitro; 2) the genome of M. truncatula has been recently sequenced and released, which will facilitate future studies on the components of the strategy I iron uptake at genetic or genomic level.
The results confirm that the growth-promoting effect of the VOCs produced by A. agilis UMCV2 can be extended to other legumes, such as M. truncatula, and also show that, regardless of its effect on root development, the VOCs produced by the strain UMCV2, and dimethylhexadecylamine in particular, induce at least 2 Strategy I components for iron acquisition in plants.
Materials and methods
Biological material and growth conditions
M. truncatula seeds (ecotype Jemalong A17) were subjected to chemical scarification (Boisson-Dernier et al. 2005) and immersed in a vial containing 1 to 2 mL of concentrated anhydrous sulfuric acid with intermittent agitation until the appearance of small black spots on the integument (5–15 min). Excess acid was removed and the seeds were rinsed with 5 washes of sterile deionized water. For sterilization, seeds were soaked in a solution of sodium hypochlorite (12 %) for 3 min and rinsed with 6 washes of sterile deionized water. Seeds were germinated in Petri dishes with Murashiege and Skoog MS medium and transferred to a Percival growth chamber with a photoperiod of 16 h light/8 h dark at a light intensity of 200 mol m2 s−1 at 22°C.
The PGPR strain A. agilis UMCV2 was isolated from lightly acid soil, as previously described (Valencia-Cantero et al. 2007). The bacterium was grown on nutrient agar (NA) at 26°C.
Effect of UMCV2 on the growth of M. truncatula
Newly scarified seeds were placed in plastic Petri dishes containing MS medium at 4°C for 48 h and then placed in a Percival growth chamber with a photoperiod of 16 h light/8 h dark at a light intensity of 200 mol m2 s−1 at 22°C. The germinated sprouts were placed in glass flasks of 170 mL containing 25 mL of MS nutrient medium, 6 g of agar (Phytotechnology, Shawnee Mission KS, US) per liter (L), and a vial with 5 mL of NA (Fig. 1d). After 5 days, half the flasks were incubated with small vials containing an A. agilis UMCV2 inoculum, and the other half were incubated with vials devoid of bacteria (axenic) and were used as controls. The experiment was maintained until the plants were 10 days old. After this time, plants were measured and weighed. Chlorophyll concentration in the plant shoots was determined using a spectrophotometric method described below.
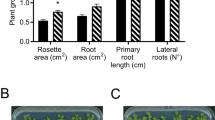
Arthrobacter agilis UMCV2 VOCs promote the growth of Medicago truncatula. Newly germinated M. truncatula seedlings were placed in glass flasks containing MS medium. After 5 days, A. agilis was inoculated in vials containing nutrient agar (NA) medium, and the systems were incubated for 5 days. Values represent mean (standard error [SE]) (n = 9). a Shoot and root length, b plant biomass fresh weight (FW), c chlorophyll content, d general view of the plant-bacteria interaction system, e 10-day-old uninduced plants, and f A. agilis UMCV2 VOC-induced plants. Asterisks indicate statistically significant differences (p < 0.05; Student t test)
Analysis of volatile compounds
For chromatographic analysis, the following treatments were carried out: I) flasks were incubated with vials containing UMCV2 for 5 days; II) 3 plants were axenically grown in flasks for 10 days; III) plants were grown for 5 days in flasks, followed by addition of a vial containing the bacterial inoculum and co-incubation of plant and bacteria for 5 days; and IV) controls. At the end of the incubation, gas chromatographic analysis was performed to detect bacterial and plant VOCs (treatments I and II), VOCs emitted during the plant-bacteria interaction (treatment III), and VOCs from controls. VOCs from axenic media were also analyzed to be discarded in the final table of results.
The gas chromatography was performed as previously described (Velázquez-Becerra et al. 2011). Briefly, the analysis (Gas Chromatographer Agilent 6850 Series II; Agilent, Foster City, CA, U.S.A.) was performed using the solid-phase microextraction technique (SPME), exposing the PDMS/DVB fiber (Supelco, Inc., Bellafonte, PA, USA.) to the sample headspace for 30 min at 30°C, and desorbing at 180°C for 30 s in the injection port of the gas chromatographer coupled to a mass spectrometer (Agilent 5973). A 25 mm × 0.52 mm capillary column with 0.32 μm film thickness (HP-FFAP; Agilent) was used; ultra pure helium (1 mL·min−1) was used as the carrier gas and the detector temperature was 250°C. The column was held for 13 min at 40°C, and then programmed to rise at a rate of 3°C per min to a final temperature of 180°C, which was maintained for 25 min. The source pressure and filament voltage were 7 Pa and 70 eV, respectively, and the scan rate was 1.9 scan·s−1. The compounds were identified by comparing with mass spectra from the library (NIST/EPA/NIH, “Chem Station’Agilent Technologies Rev. D.04.00 2002). The identity of dimethylhexadecylamine was further confirmed by comparing the retention time in the VOC profiles to a sample of the pure standard (Sigma-Aldrich catalog 40460; CAS: 112-69-6). Dimethylhexadecylamine was quantified using an external standard. Five microliters of dimethylhexadecylamine (100 μM) was spotted on a vial with 5 mL of AN inside the flask, as described above. The peak area of the pure standard compound was recorded and compared with the axenic and bacterial compound peak areas, as well as with that from the interaction experiment.
Analysis of rhizosphere acidification
The pH change in the root environment or in the plant rhizosphere was measured by a change in the color of the pH indicator bromocresol purple. Plants were grown in culture flasks containing MS medium with iron sufficiency (100 μM) in a growth chamber for 5 days. The induction was conducted as follows: an A. agilis UMCV2 inoculum (1 × 106 CFU) was placed in a vial, and dimethylhexadecylamine (5 μL to 100 μM) and water (control) were placed separately in glass vials with 5 mL of NA and then placed into culture flasks containing the plants, so that there was no physical contact between M. truncatula and the bacterium. Plants were induced for 2 days, after which a group of plants was transferred to Petri dishes containing MS medium (0.6 % sucrose and 0.8 % agar) with iron sufficiency (100 μM). Another group was transferred to MS medium with iron deficiency (1 μM); the colorant bromocresol purple (0.006 %) (modified from Zhang et al. 2009) was included in both cases. Photographs were taken at 24 and 48 h after initiation of iron stress. The color of bromocresol purple was compared with a colorimetric scale prepared with culture media plates containing bromocresol purple at a pH range of 4 to 7.
Quantification of ferric chelate reductase activity
Ferric chelate reductase activity was analyzed using a spectrophotometric quantification method for the formation of Fe2+-ferrozine complex (Yi and Guerinot 1996). Five-day-old seedlings grown in MS medium were exposed to the volatile compounds of A. agilis or dimethylhexadecylamine for 48 h in the system described above. After the induction, a group of plants was transferred to plates containing MS medium with iron sufficiency or deficiency at different times according to the experiment. The root system was immersed in a solution containing 0.5 mM Fe(III)-EDTA and 4.4 mM ferrozine at pH 6.5. The absorbance was read at 562 nm after 1 h of incubation at room temperature in darkness. The Fe(II)-ferrozine concentration was calculated from a previously formulated standard curve equation. An identical solution but without the root system was used as a blank. The pH of the solution was measured using a potentiometer.
Determination of chlorophyll content
Quantification of chlorophyll was carried out according to the method of Lichtenthaler and Wellburn (1983). The plant tissue was ground in a mortar with a solution of 80 % acetone. The solution containing the pigment was filtered with Whatman No. 1 paper. The samples were gauged to 5 mL with 80 % acetone and read in a spectrophotometer at 663 and 646 nm. The concentration of chlorophyll was calculated using the following formulas: Chlorophyll a = (12.21)·(E663) - (2.81)·(E646); chlorophyll b = (20.13) (E646) - (5.03) (E663); Total chlorophyll = ([mL acetone]·[pigment content])/sample weight
Determination of endogenous Fe content
Endogenous iron in plants of M. truncatula was quantified using the AY-5 analysis of plant tissue wet digestion method (Perkin-Elmer Corp. 1996). For every 1 g of plant tissue powder, 10 mL of HNO3 was added and allowed to stand overnight. The solution was carefully heated in a water bath until the production of nitrous oxide fumes ceased. Liquid solutions were allowed to cool at room temperature and 4 mL of hydrogen peroxide (trace metal grade) was added. The mixture was reheated to evaporate into a small volume of approximately 3 μL and transferred to 50 mL of sterile deionized water. We generated a standard curve for Fe according to the recommended conditions for the equipment (Atomic Absorption Spectrometer AAnalyst 200; Perkin-Elmer Corporation, USA).
Statistical analysis
All experiments were performed 2–3 times. The results were analyzed using the Statistica 6.0 software (Statsoft Inc. 2001). The Student’s t test was used to compare the means of 2 groups, and the ANOVA test and Duncan’s means separation test were used for multiple comparisons (p < 0.05).
Results
A. agilis promotes the growth of M. truncatula seedlings via VOC emission
To determine the effects of VOCs produced by A. agilis UMCV2 on M. truncatula growth we employed a separate compartment system (Fig. 1d), where both organisms were grown in the same gas phase but without physical contact. Under these conditions, bacterial VOCs had a stimulatory effect on the growth of shoots and roots (Fig. 1a, e, and f) and particularly on the plant biomass. In general, plants grown in the presence of bacterial VOCs exhibited 40 % and 35 % increases in shoot and root fresh weights compared to control plants (Fig. 1b).
Plants grown in the presence of UMCV2 also exhibited a 35 % increase in chlorophyll concentration, compared to control plants (Fig. 1c). This is particularly interesting, given that chlorophyll concentration is considered an indicator of iron nutritional status in plants (Masalha et al. 2000; Terry and Abadia 1986). Thus, A. agilis is able to increase the chlorophyll concentration and promote the overall growth of M. truncatula via mechanisms that involve volatile compounds.
A. agilis UMCV2 and M. truncatula modify their volatile cocktail production during interaction with each other
To determine whether A. agilis UMCV2 and M. truncatula are able to interact with each other through their VOCs and examine the potential modulation of iron metabolism by these compounds, we used the separate compartment system described above. Four-day old plants were incubated with a small vial containing nutrient agar inoculated (or not inoculated in the case of controls) with A. agilis UMCV2 for 3 days. This technique prevented direct contact between the plant and bacterium while allowing the plants to perceive the bacterial VOCs, and vice versa. VOCs in axenic cultures of the bacterium and the plant were independently identified by GC-MS. The mixture of compounds emitted by the bacterium A. agilisUMCV2 comprised different types of VOCs: the ketones 2-butanone, 2-pentanone, 2-octanone, and 5-methyl 2-hexanone (Claeson and Sunesson 2005; Müller et al. 2008; Zou et al. 2007); the alcohols ethanol alcohol, benzyl alcohol, and phenylethyl alcohol (Kai et al. 2007; Thorn et al. 2011); 2.5-dimethylpirazyne pyrazyne, previously reported by Xu et al. (2004); the terpenes terpinolene and camphor (Dickschat et al. 2005; Wilkins and Schöller 2009);and the amine dimethylhexadecylamine (Velázquez-Becerra et al. 20011). Among acid VOCs, we only detected benzeneacetic acid ethyl ester (Table 1). In axenic cultures, we also detected 11 different VOCs produced by M. truncatula (Table 1) among them: 2-ethyl 1-hexanol, 1-dodecanol, eucalyptol (1,8-cineole), and 1-octen-3-ol (Table 1).
Furthermore, a series of 12 compounds, including nonanal and 3-octanone, were detected during plant-bacteria interactions, but not in axenic treatments. The VOC 3-octanone is commonly produced by fungi, and also by some leguminous plants (Boué et al. 2005). Additionally, dimethyl disulfide and dimethyl trisulfide compounds with antifungal (Kai et al. 2008; Zou et al. 2007) and anti-microbial effects (Bendimerad et al. 2005; Wang et al. 2009) have been reported among bacteria and plant VOCs. Unfortunately, we were unable to determine which of the 2 organisms produced the specific VOCs. The above data suggest that M. truncatula is able to perceive the A. agilis VOCs and respond by modifying its own VOC cocktail.
Dimethylhexadecylamine was previously reported as an A. agilis UMCV2-derived volatile compound capable of promoting plant growth (Velázquez-Becerra et al. 2011). Therefore, its detection in axenic cultures of A. agilis was not surprising. However, the concentration of dimethylhexadecylamine in the M. truncatula-A. agilis interaction system increased 12-fold, compared to that of axenic A. Agilis cultures (Fig. 2). This suggests that A. agilis may perceive M. truncatula through VOCs and respond with an increase in dimethylhexadecylamine emission.
Determination of dimethylhexadecylamine content in control, A. agilis UMCV2, and M. truncatula-A. agilis UMCV2 interaction systems by SPME-GC-MS. Total ion chromatogram of the pure standard at 0.50 nmol (Rt 42.3 min) obtained from control flasks (a), with bacteria (b), and from the plant-bacteria interaction system (c)
VOCs produced by A. agilis induce acidification of the M. truncatula rhizosphere
Considering that the VOCs produced by A. agilis UMCV2 elicited an increase in chlorophyll concentration of M. truncatula, and given that this measurement is considered as an indicator of the nutritional status of iron in plants, we analyzed rhizosphere acidification. In Strategy I plants, the first response to iron deficiency is an increased extrusion of protons from the roots into the surrounding environment (rhizosphere), thereby facilitating iron transport and mobility.
We tested the ability of A. agilis VOCs and pure dimethylhexadecylamine to induce acidification of the root environment using a colorimetric method (see Materials and methods). Seven-day-old M. truncatula plants were exposed to A. agilis VOCs or dimethylhexadecylamine for 48 h. After this time, a group of plants from each treatment was transferred to medium with iron sufficiency (100 μM) or iron deficiency (1 μM). Plants exposed to A. agilis VOCs and transferred to iron sufficiency exhibited a small change in rhizosphere acidification after 24 h (Fig. 3), whereas plants exposed to bacterial VOCs and transferred to iron deficiency showed a strong acidification as early as 24 h and more so at 48 h after iron stress. Plants exposed to dimethylhexadecylamine exhibited a similar pattern, acidifying the rhizosphere after 24 h of growth in iron-sufficient medium and producing a higher acidification area at 48 h after transfer to iron deficiency conditions. These data were compared with those of control experiments where the plants were not induced. Acidification of the medium was not observed when control plants were transferred to 100 μM iron, and only a slight acidification zone was observed at 48 h of growth in iron-deficient conditions (Fig. 3).
Acidification of the M. truncatula rhizosphere in response to A. agilis VOCs and dimethylhexadecylamine (DMHDA). Five-day-old plants were induced with A. agilis or DMHDA for 48 h and transferred to MS medium containing bromocresol purple with iron sufficiency (100 μM) or deficiency (1 μM). Photographs were taken at 24 and 48 h after transfer to these media, and are representative of 6 replicates
Acidification was evidently produced by the M. truncatula roots induced by bacterial VOCs and volatile dimethylhexadecylamine during plant-bacteria interactions, because this acidification corresponded to the area immediately around the roots and not to a general acidification that would be expected if acidification was directly caused by the bacterial VOCs or dimethylhexadecylamine. In a further experiment, we could not observe a direct change in the pH of the MS medium when the bacterium or dimethylhexadecylamine was grown or added in a separate compartment, respectively (Online Resource 1).
The results show that both the volatiles of A. agilis and dimethylhexadecylamine promote proton extrusion into the surrounding root area during interaction with M. truncatula plants, because both treatments elicited a change in pH from 6.5 to approximately 4 under conditions of iron deficiency; a change in pH was also observed in iron sufficiency but was less intense.
VOCs produced by A. agilis UMCV2 stimulate ferric chelate reductase activity in the roots of M. truncatula
After determining that the VOCs produced by A. agilis and dimethylhexadecylamine have the ability to induce the plant to acidify the root environment, we tested the effect of these same compounds on the activity of ferric chelate reductase, a second component of the iron uptake system in Strategy I plants.
Clearly, plants that were induced by A. agilis and dimethylhexadecylamine exhibited an increase in ferric reductase activity from 0 h (time of transfer) to 72 h after induction, compared to uninduced control plants in both conditions of iron (Fig. 4a and b). This increment in the ferric reductase activity ranged between 125 and 20 % depending on whether plants were induced with VOCs of A. agilis or dimethylhexadecylamine, and whether the plants were transferred to an iron deficiency or sufficiency condition after induction. Interestingly, a maximum reduction in activity was observed at 24 h after transfer, particularly in plants that were placed in iron deficiency. Under this condition, plants induced by A. agilis VOCs exhibited a 120 % increase in ferric reductase activity compared to controls; however, this peak of elevated activity was also present in plants transferred to conditions of iron sufficiency.
Inductive effect of A. agilis VOCs and DMHDA on ferric chelate reductase activity (a and b), biomass (c and d), and chlorophyll content (e and f) in iron-sufficient (a, c, and e) or -deficient (b, d, and f) media as indicated. M. truncatula seedlings were induced for 2 days with UMCV2 or DMHDA and were transferred to medium with or without iron. (♦) UMCV2, (▲) DMHDA, (■) Control
Plant growth, measured as total fresh weight, was also greater in the induced plants, even at time points as short as 3 days of iron stress. Thus, A. agilis VOCs promoted plant growth independently of the iron status (Fig. 4c and d). Similarly, chlorophyll content was quantified as an indirect measure of the nutritional status of iron in plants. As shown in Fig. 4e, plants grown in iron sufficiency exhibited a gradual increase in chlorophyll content; however, the amount of pigment was higher in plants that were induced with A. agilis. Under iron deficiency conditions, control (Fig. 4f) plants began to show slight signs of chlorosis after 72 h of iron stress, whereas the chlorophyll content of plants induced with A. agilis VOCs or dimethylhexadecylamine was similar to that of plants maintained in medium with iron sufficiency.
To determine whether this effect of A. agilis VOCs and dimethylhexadecylamine on M. truncatula plants was sustained over the long term, seedlings were grown under the same conditions but under iron-deficiency stress for 7 days. A. agilis VOCs induced ferric chelate reductase activity in M. truncatula seedlings in iron sufficiency and deficiency, with the activity being higher in iron deficiency. Similarly, dimethylhexadecylamine enhanced ferric reductase activity in iron-deficient plants compared to uninduced controls (Fig. 5a). Growth was also promoted in induced plants. The weights of plants induced by A. agilis VOCs and grown in iron sufficiency were higher; however, it is noteworthy that in iron deficiency, the weights of the plants induced with A. agilis and dimethylhexadecylamine were always higher than those grown in uninduced iron sufficiency conditions even when the induction occurred 7 days prior (Fig. 5b).
A. agilis and DMHDA increase iron deficiency responses in M. truncatula plants up to 7 days after induction. Plants were transferred to iron-sufficient medium (100 μM) or iron-deficient medium (1 μM). Bars represent mean (SE) values (n = 16) of iron reductase activity (a), biomass (b), acidification capacity (c), and chlorophyll content (d). Iron reductase activity was quantified using the ferrozine method. pH was directly measured in the solution of the root with a potentiometer. Chlorophyll content was measured using a spectrophotometric method, as described in Materials and methods. Lower-case letters indicate significant differences (p < 0.05; Duncan’s multiple range test)
Once the increase in iron reductase activity was identified, quantification of pH and chlorophyll content of plants were performed at 7 days post-induction. Figure 5c shows that plants exposed to A. agilis VOCs in iron deficiency produced a more intense acidification, reducing the pH from its initial value of 6.5 to 5.2, than plants from other treatments. Induced plants grown in iron sufficiency were also able to decrease the pH to 5.7, indicating an increased extrusion of protons into the medium by the induced plants.
Furthermore, plants exposed to dimethylhexadecylamine did not exhibit significant differences in acidification compared to control plants induced in both conditions (iron sufficiency and deficiency) (Fig. 5c). The differential effect of the bacterial VOCs mixture and dimethylhexadecylamine may be due to 2 reasons: a higher concentration of dimethylhexadecylamine in the VOC mixture or synergistic action of other VOCs with dimethylhexadecylamine.
Finally, the induced plants showed no symptoms of chlorosis at 7 days post-induction; the chlorophyll concentration of plants exposed to A. agilis VOCs was higher, irrespective of the iron treatment. Similarly, plants exposed to dimethylhexadecylamine exhibited a higher chlorophyll concentration even in iron deficiency, compared to uninduced plants grown in iron sufficiency (Fig. 5d).
These results demonstrate that VOCs produced by A. agilis UMCV2, and dimethylhexadecylamine in particular, have the ability to induce ferric chelate reductase activity and acidification of the rhizospheric environment, and that this induction persists even 7 days after removal of the stimulus. The increase in both activities resulted in a greater generation of biomass and higher chlorophyll content with time.
VOCs produced by A. agilis UMCV2 promote iron accumulation in M. truncatula
Because the plants induced with A. agilis and dimethylhexadecylamine showed an increase in chlorophyll content at 24 h after induction and up to 7 days, we analyzed the Fe content in plants. M. truncatula plants were grown under the same conditions as in the experiments for reductase activity. After 48 h of induction with A. agilis or dimethylhexadecylamine, the plants were transferred to medium with iron sufficiency or deficiency. After 7 days, plants were treated according to the AY-5 wet digestion method and the samples were analyzed by atomic absorption spectroscopy (Fig. 5).
Plants grown in iron-deficient medium and induced with A. agilis showed increased Fe levels in roots, compared to uninduced controls. Also, plants induced with dimethylhexadecylamine in iron-sufficient medium exhibited a 2.3-fold increase (per root basis; Fig. 6a) or a 1.5-fold increase (concentration basis; Fig. 6c) in Fe content, whereas plant roots induced by A. agilis VOCs showed a 1.6-fold increase (per root basis) or 1.3-fold increase (concentration basis) in Fe content compared to their respective controls.
Effect of A. agilis VOCs and DMHDA on total Fe content in M. truncatula plants. Bars represent mean (SE) values (n = 16) of Fe content in roots (a) and shoots (b), and Fe concentration in roots (c) and shoots (d) of 7-day-old plants after transfer to medium with iron sufficiency (100 μM) or deficiency (1 μM). Iron was quantified using an atomic absorption spectrometer, as described in Materials and methods. Lower-case letters indicate significant differences (p < 0.05; Duncan’s multiple range test)
The shoots of control and A. agilis UMCV2-treated plants grown in iron-deficient medium exhibited significantly different Fe content. In iron sufficiency conditions, the Fe content of dimethylhexadecylamine-treated shoots was between 3.7- and 2.5-fold that of controls (Fig. 6b and c). Similarly, A. agilis UMCV2 elicited a 3-fold increase in iron accumulation (Fig. 6b).
These data confirm that A. agilis VOCs and dimethylhexadecylamine induce iron acquisition responses, such as acidification of the rhizospheric area, reductase activity, and increased Fe content. These findings are consistent with the observed increase in chlorophyll content and biomass in M. truncatula.
Discussion
Growth promotion of M. sativa due to emission of A. agilis UMCV2 VOCs has been previously established (Velázquez-Becerra et al. 2011). Therefore, the promotion of M. truncatula growth was an expected result (Fig. 1). In this sense, it is noteworthy that M. truncatula plants grown in the presence of A. agilis UMCV2 had a chlorophyll concentration 35 % higher than that of plants grown in axenic cultures, because chlorophyll concentration is considered as an indicator of the iron nutritional status of plants. This result led us to test the hypothesis that the VOCs of A. agilis UMCV2 influence some of the Strategy I components of iron acquisition (i.e., rhizosphere acidification and induction of ferric chelate reductase) via the emission of VOCs that act as signal molecules.
In a pioneering work, Mathesius et al. (2003) showed that M. truncatula perceives its symbiont Sinorhizobium meliloti through bacterial N-acyl homoserine lactones and responds via changes in the accumulation of more than 150 proteins in the presence of each bacterial species. In this work, we presented evidence suggesting that M. truncatula can perceive the rhizobacterium A. agilis UMCV2 through VOCs and respond by modifying its own VOC profile. We detected 14 VOCs produced by A. agilis UMCV2 axenic cultures, and 11 VOCs produced by axenic M. truncatula plants; however, a group of 11 compounds was produced only when the bacterium and the plant were allowed to interact with each other through their respective VOC emissions. The latter indicates that at least one of the 2 organisms, if not both, can sense the other via VOC emission.
In parallel, we found that the axenic cultures of A. agilis UMCV2 produced the VOC dimethylhexadecylamine, as previously reported (Velázquez-Becerra et al. 2011). Interestingly, we also detected a 12-fold increase in dimethylhexadecylamine in the interacting atmosphere. This also suggests that the bacterium perceives the plant through volatile compounds and responds by increasing the emission of a compound that acts as a signal molecule between the 2 organisms.
The mechanism by which VOCs from A. agilis stimulate plant growth is unknown. In this study, we demonstrated the involvement of VOCs produced by the PGPR A. agilis UMCV2 and the specific compound dimethylhexadecylamine in the activation of iron deficiency stress response mechanisms. First, we found that acidification of the M. truncatula rhizospheric area was increased in plants induced with bacterial VOCs or dimethylhexadecylamine, compared to control uninduced plants (Fig. 3). However, neither A. agilis UMCV2 nor dimethylhexadecylamine alone acidified the culture medium, which indicates that rather than directly acidifying the medium, the VOCs of A. agilis and dimethylhexadecylamine act as a signal molecule from the bacterium to the plant, which responds by acidifying the rhizosphere in both iron-deficient and -sufficient conditions, with a clearly higher acidification in iron-deficient conditions.
The ability to acidify the rhizosphere has been linked primarily to the activation of one or more members of the ATPase AHA family, which have already been extensively described (Colangelo and Guerinot 2004; Santi et al. 2005). Plants induced with VOCs from A. agilis UMCV2 showed an increased capacity for medium acidification, generating a pH close to 4 at 48 h after induction and a pH of 5.2 after 7 days of induction. This suggests that in our experiments, M. truncatula may activate the ATPase AHA enzymes during rhizosphere acidification.
A similar acidification effect was reported by Zhang et al. (2009) using a separate compartment system, but employing the plant Arabidopsis thaliana and the commercial bacterium Bacillus subtilis GB03. The authors showed that the increase in chlorophyll concentration was due to an increase in the expression of the IRT1 and FRO2 genes (required for iron reduction and uptake by plants), which was related to the acidification of the medium by bacterial VOCs, including glyoxylic acid, methyl butanoic acid, and diethyl acetic acid. In addition, the authors showed that GB03 can acidify the medium through VOCs from a separate compartment. In our study employing a similar system of separate compartments, this effect was not observed with A. agilis UMCV2 or dimethylhexadecylamine in the other compartment (Online Resource 1). Therefore, A. agilis may induce the acidification of M. truncatula rhizosphere via emission of different VOCs. Additionally, benzeneacetic acid ethyl ester, the only organic acid found in the VOC profile of axenic A. agilis UMCV2 cultures, was not found during interaction with M. truncatula. However, it cannot be ruled out that the increase in the chlorophyll concentration of M. truncatula plants was probably due to variations in the expression of iron acquisition genes induced by VOCs.
Rhizosphere acidification promotes iron solubility (Römheld 1987); however, an acidic environment is not sufficient for iron uptake. Iron is a transition metal and its reduction is necessary before its transport into root hairs through the plasma membrane. Therefore, given the idea that the VOCs of A. agilis and particularly dimethylhexadecylamine promote the nutritional status of M. truncatula, we measured the activity of the ferric chelate reductase enzyme after inducing M. truncatula plants with bacterial VOCs and pure dimethylhexadecylamine.
Interestingly, induction with A. agilis VOCs and dimethylhexadecylamine increased the activity of ferric chelate reductase immediately after induction, in both iron-deficient and -sufficient conditions (Fig. 4). This was particularly evident at 24 h after transferring plants to iron sufficiency; the enzyme activity was between 2.25- and 1.8-fold higher than that in the uninduced controls. Enzyme activity in induced plants transferred to iron-deficient medium was between 2- and 1.6-fold higher than that of controls. The inductive effect diminished over time, with the ferric reductase activity in induced plants being between 1.7- and 1.3-fold higher than that of uninduced plants at 72 h after induction. Although the induction with VOCs or dimethylhexadecylamine increased the ferric reductase activity in conditions of iron deficiency and sufficiency, it was clear that the ferric reductase activity was higher in iron-deficient conditions, as seen with the acidification.
However, during short periods of iron deficiency stress, we did not observe clear signs of chlorosis, even in the uninduced controls. Andaluz et al. (2009) showed that between 3 and 5 days of iron stress, M. truncatula seedlings begin to exhibit yellow patches as symptoms of iron chlorosis. They noted that the ferric reductase activity decreased dramatically after the seventh day of iron stress. Consistent with their results, control plants exhibited clear signs of iron deficiency, including decreased reductase activity, up to 7 days post-induction (Fig. 5a). However, the plants induced with VOCs from A. agilis showed a 2.4-fold higher activity compared to the uninduced controls, in either condition of iron sufficiency or deficiency. A similar effect was reported for the A. thaliana ferric reductase activity in, which remained high after 7 days of treatment with a mixture of VOCs from B. subtilis GB03 (Zhang et al. 2009). Thus, we show an inductive effect of A. agilis VOCs on M. truncatula ferric reductase activity, which was sustained even after a week of growth in iron-deficient medium with a smaller effect observed in iron-sufficient conditions.
Additionally, in all cases the induced plants exhibited higher chlorophyll concentrations in the presence of bacterial VOCs compared with untreated controls (Fig. 1c), or when the plants were induced 72 h before the chlorophyll quantification (Fig. 4e–f). The effect was observed as late as 7 days post-induction in plants maintained on iron deficiency. Chlorophyll synthesis requires many iron-dependent enzymatic reactions (Hansen et al. 2003; Lin et al. 2000), including thylakoid synthesis and chloroplast development (Buchanan et al. 2000). The development and growth of M. truncatula was promoted by A. agilis-derived VOCs and dimethylhexadecylamine, whereas uninduced plants showed a photosynthetic capacity that was vulnerable to iron deficiency (Varsano et al. 2006). This situation may at least partially explain the elevated chlorophyll concentration and increased biomass generation observed in induced plants.
A general observation in our experiments was the greater effect of the A. agilis VOCs cocktail compared to pure dimethylhexadecylamine. This may be explained by the concentration of dimethylhexadecylamine in the VOC cocktail. The concentration of dimethylhexadecylamine in the plant-bacteria interaction system was 3-fold higher than the concentration of the pure standard used in the induction experiments (Fig. 2a vs. c). A previous logarithmic scan with different concentrations showed that a higher concentration of dimethylhexadecylamine provoked a deleterious effect on plant growth (Online resource 2). Therefore, an optimal concentration of dimethylhexadecylamine is important for its plant growth-promoting effects. An alternative explanation is that A. agilis UMCV2 produces VOCs other than dimethylhexadecylamine, and one or more of these VOCs may contribute to the inductive effect. Furthermore, it has been demonstrated that CO2 produced by bacteria significantly promote plant growth in vitro (Kai and Piechulla 2009). Therefore, the possible participation of A. agilis-emitted CO2 in plant growth induction cannot be ruled out.
A second general observation in our experiments was the higher inductive effect observed in plants grown in iron deficiency, compared to those grown in iron-sufficient conditions. This observation suggest that the plants grown in iron deficiency switch on strategy I mechanisms for iron uptake, with an additive effect observed in the presence of the A. agilis UMCV2 VOCs or pure dimethylhexadecylamine. However, iron sufficiency does not prevent the inductive effect of the bacterial VOCs or dimethylhexadecylamine.
The inductive effect of VOCs on iron-acquisition mechanisms of Strategy I plants were initially described in Arabidopsis. In that system, bacterial VOCs modulate genes encoding both the ATPase AHA7 responsible for acidification of the rhizosphere, and ferric chelate reductase (FRO), which is activated by the transcriptional regulator FIT1 (Colangelo and Guerinot 2004; Zhang et al. 2009). Thus, it is possible that the volatile compound dimethylhexadecylamine and is a potential homolog of FIT1 in M. truncatula, and acts as a signal initiator to induce the expression and activity of FRO and AHA genes (Robinson et al. 1999). Experiments are currently underway to show that dimethylhexadecylamine induces the expression of FRO genes in the roots of M. truncatula.
Previously, our group showed that A. agilis UMCV2 increases iron levels in Phaseolus vulgaris plants (Valencia-Cantero et al. 2007). Phaseolus plants grown in alkaline soil and inoculated with A. agilis UMCV2 exhibited a 1.76-fold increase in Fe content in whole plants compared to control plants. Consistent with these data, A. agilis increased the Fe content in M. truncatula shoots. Interestingly, the Fe content in the roots of M. truncatula grown in iron-deficient medium was higher than that of control plants. These results together with the observed increase in chlorophyll content suggest that A. agilis UMCV2 and dimethylhexadecylamine facilitate the uptake and storage of iron, mainly in roots. The iron is then redistributed to the aerial parts where it can be used in processes such as photosynthesis. To our knowledge, this is the first study to elucidate the modulation of rhizosphere acidification capacity and ferric reductase activity by a bacterial volatile compound.
References
Aguirre E, Leménager D, Bacaicoa E, Fuentes M, Baigorri R, Zamarreño AM, García-Mina JM (2009) The root application of a purified leonardite humic acid modifies the transcriptional regulation of the main physiological root responses to Fe deficiency in Fe-sufficient cucumber plants. Plant Physiol Biochem 47:215–223. doi:10.1016/jplaphy.2808.11.013
Andaluz S, Rodríguez-Chelma J, Abadía A, Abadía J, López-Millán AF (2009) Time course induction of several key enzymes in Medicago truncatula roots in response to Fe deficiency. Plant Physiol Biochem 47:1082–1088. doi:10.1016/j.plaphy.2009.07.009
Bendimerad N, Bendiab SAT, Benabadji AB, Fernandez X, Valette L, Cuvelier LL (2005) Composition and antibacterial activity of Pseudocytisus integrifolius (Salisb.) essential oil from Algeria. J Agr Food Chem 53:2947–2952. doi:10.1021/jf047937u
Boisson-Dernier A, Andriankaja A, Chabaud M, Niebel A, Journet E-P, Barker D, de Carvalho-Neibel F (2005) MtENOD11 gene activation during rhizobial infection and mycorrhizal arbuscule development requires a common AT-rich-containing regulatory sequence. Mol Plant-Microbe Interact 18:1269–1276. doi:10.1094/MPMI-18-1269
Boué S, Shih BY, Carter-Wientjes CH, Cleveland TE (2005) Effect of soybean lipoxygenase on volatile generation and inhibition of Aspergillus flavus mycelial growth. J Agr Food Chem 53:4778–4783. doi:10.1021/jf058038o
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville
Claeson AS, Sunesson AL (2005) Identification using versatile sampling and analytical methods of volatile compounds from Streptomyces albidoflavus grown on four humid building materials and one synthetic medium. Indoor Air 15:41–47. doi:10.1111/j.1600-0668.2005.00343.x
Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT is required for the iron deficiency response. Plant Cell 16:3400–3412. doi:10.1105/tpc.104.024315
Dickschat JS, Helmke E, Schulz S (2005) Volatile organic compounds from arctic bacteria of the Cytophaga-Flavobacterium-Bacteroides group: a retrobiosynthetic approach. Chemotaxonomic Investigations. Chemi Biodivers 2:318–353. doi:10.1002/cbdv.200590014
Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron–regulated metal transporter from plants identified by functional expression in yeast. Proc Nat Acad Sci USA 93:5624–5628
Gutiérrez-Luna FM, López-Bucio J, Altamirano-Hernández J, Valencia-Cantero E, Reyes de la Cruz H, Macías-Rodríguez L (2010) Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 51:75–83. doi:10.1007/s13199-010-0066-2
Hansen NC, Schmitt MA, Anderson JE, Strock JS (2003) Iron deficiency in soybean in the upper midwest and associated soil properties. Agron J 95:1595–1601. doi:10.2134/agronj2003.1595
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216:541–551. doi:10.1007/s00425-002-0920-4
Kai M, Piechulla B (2009) Plant growth promotion due to rhizobacterial volatiles – An effect of CO2? FEBS Lett 583:3473–3477. doi:10.1016/j.febslet.2009.09.053
Kai M, Effmert U, Berg G, Piechulla B (2007) Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol 87:351–360. doi:10.1007/s00203-006-0199-0
Kai M, Vespermann A, Piechulla B (2008) The growth of fungi and Arabidopsis thaliana is influenced by bacterial volatiles. Plant Signal Behav 3:1–3. doi:10.4161/psb.3.7.5681
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. doi:10.1042/bst0110591
Lin SF, Grant D, Cianzio S, Shoemaker R (2000) Molecular characterization of iron deficiency chlorosis in soybean. J Plant Nutr 23:1591–1606. doi:10.1080/01904160009382154
Masalha J, Kosegarten H, Elmaci O, Mengel K (2000) The central role of microbial activity for iron acquisition in maize and sunflower. Biol Fertil Soils 30:433–4399. doi:10.1007/s003740050021
Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anollés G, Rolfe BG, Bauer WD (2003) Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Nat Acad Sci USA 100:1444–1449. doi:10.1073_pnas.262672599
Müller H, Westendorf C, Leitner E, Chernin L, Riedel K, Schmidt S, Eberl L, Berg G (2008) Quorum-sensing efects in the antagonistic rhizosphere bacterium Serratia plymuthica HRO-C48. FEMS Microbiol Ecol 67:468–478. doi:10.1111/j.1574-6941.2008.00635.x
Ortíz-Castro R, Valencia-Cantero E, López-Bucio J (2008) Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav 3:263–265. doi:10.1094/MPMI-20-2-0207
Ortíz-Castro R, Díaz-Pérez C, Miguel Martínez-Trujillo, del Río RE, Campos-García J, López-Bucio J (2011) Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc Nat Acad Sci USA 108:7253–7258. doi:10.1073/pnas.1006740108
Perkin-Elmer Corp. (1996) Analytical methods for atomic absorption spectroscopy. The Perkin-Elmer, USA, pp 141–143
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397:694–697. doi:10.1038/17800
Römheld V (1987) Different strategies for iron acquisitionin higher plants. Physiol Plant 70:231–234. doi:10.1111/j.1399-3054.1987.tb06137.x
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80:175–180. doi:0032-0889/86/80/0175/06
Santi S, Cesco S, Varanini Z, Pinton R (2005) Two plasma membrane H+−ATPase genes are differentially expressed in iron-deficent cucumber plants. Plant Physiol Biochem 43:287–292. doi:10.1016/j.plaphy.2005.02.007
Schmidt W (1999) Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol 141:1–26. doi:10.1046/j.1469-8137.1999.00331.x
Spaepen S, Vanderleyden J, Okon Y (2009) Plant growth-promoting actions of rhizobacteria. In: van Loon LC (ed) Advances in botanical research 51. Academic Press, Burlington, pp 283–320
Statsoft Inc. (2001) STATISTICA (data analysis software system), 6th edn. Tulsa, USA
Susín S, Abían J, Peleato ML, Sanchez-Baeza F, Abadía A, Gelpí E, Abadía J (1994) Flavin excretion from roots of iron-deficient sugar-beet (Beta vulagris L). Planta 193:514–519. doi:10.1007/BF02411556
Terry N, Abadia J (1986) Function of iron in chloroplasts. J Plant Nutr 9:609–646. doi:10.1080/01904168609363470
Thorn RMS, Reynolds DM, Greenman J (2011) Multivariate analysis of bacterial volatile compound profiles for discrimination between selected species and strains in vitro. J Microbiol Meth 84:258–264. doi:10.1016/j.mimet.2010.12.001
Valencia-Cantero E, Hernández-Calderón E, Velázquez-Becerra C, López-Meza JE, Alfaro-Cuevas R, López-Bucio J (2007) Role of dissimilatory fermentative iron-reducing bacteria in Fe uptake by common vean (Phaseolus vulgaris L.) plants grown in alkaline soil. Plant Soil 291:263–273. doi:10.1007/s11104-007-9191-y
Varsano T, Wolf SG, Pick U (2006) A chlorophyll a/b binding protein homolog which is induced by iron deficiency is associated with enlarged photosytem I units in the eukaryotic alga Dunaliella salina. J Biol Chem 281:10305–10315. doi:10.1074/jbc.M511057200
Velázquez-Becerra C, Macías-Rodríguez LI, López-Bucio J, Altamirano-Hernández J, Flores-Cortez I, Valencia-Cantero E (2011) A volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro. Plant Soil 339:329–340. doi:10.1007/s11104-010-0583-z
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233. doi:10.1105/tpc.001388
Wang D, Rose C, Kinkel L, Cao A, Tharayil N, Gerik J (2009) Production of methyl sulfide and dimethyl disulfide from soil-incorporated plant materials and implications for controlling soilborne pathogens. Plant Soil 324:185–197. doi:10.1007/s11104-009-9943-y
Welkie GW, Miller GW (1988) Riboflavin excretion from roots of iron‐stressed and reciprocally grafted tobacco and tomato plants. J Plant Nutr 11:691–700. doi:10.1080/01904168809363834
Wilkins K, Schöller C (2009) Volatile organic metabolites from selected Streptomyces strains. Actinomycetologica 23:27–33. doi:10.3209/saj.SAJ230202
Xu C, Mo M, Zhang L, Zhang K (2004) Soil volatile fungistasis and volatile fungistatic compounds. Soil Biol Biochem 36:1997–2004. doi:10.1016/j.soilbio.2004.07.020
Yi Y, Guerinot ML (1996) Genetic evidence that induction of root Fe (III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J 10:835–844. doi:10.1046/j.1365-313X.1996.10050835.x
Zhang H, Sun Y, Xie X, Kim MS, Dowd SE, Paré PW (2009) A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J 58:568–577. doi:10.1111/j.1365-313X.2009.03803.x
Zou CS, Mo MH, Gu YQ, Zhou JP, Zhang KQ (2007) Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol Biochem 39:2371–2379. doi:10.1016/j.soilbio.2007.04.009
Acknowledgments
We thank the Consejo Nacional de Ciencia y Tecnología, México (Grant 128341) and Coordinación de la Investigación Científica-Universidad Michoacana de San Nicolás de Hidalgo (Grant 2.22) for financial support. MCOM received the PhD Scholarship 21559 from Consejo Nacional de Ciencia y Tecnología, México.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Online Resource 1
(JPEG 295 kb)

Online Resource 2
Logarithmic scanning of the effect of DMHDA on M. truncatula plants. Vernalized seeds were placed on 1 side of a divided Petri dish and after 4 days, 0, 0.05, 0.5., and 5 nmol of DMHDA were spotted on the other side. At day 6, plant length and fresh weight were determined. The panels show the plant fresh weight (a), plant length (b), and representative images of 6-day-old plants at different concentrations of DMHDA. Bars represent mean (SE) (n = 16). Lower-case letters indicate significant differences (p < 0.05; Duncan’s multiple range test) (JPEG 505 kb)
Rights and permissions
About this article
Cite this article
del Carmen Orozco-Mosqueda, M., Velázquez-Becerra, C., Macías-Rodríguez, L.I. et al. Arthrobacter agilis UMCV2 induces iron acquisition in Medicago truncatula (strategy I plant) in vitro via dimethylhexadecylamine emission. Plant Soil 362, 51–66 (2013). https://doi.org/10.1007/s11104-012-1263-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1263-y