Abstract
Salt tolerance of sorghum varieties in terms of fresh weight, ion accumulations, proline content and peroxidase activity was analyzed in this study. Three sorghum varieties, Payam, Kimia, and Jambo, differing in salt tolerance, were grown in a greenhouse-hydroponic culture with a complete nutrition solution to which 0, 50, 100, 150 and 200 mM NaCl was added. Plant roots and leaves were harvested at 15 and 30 days after treatment and subjected to analysis. Clear decline in K+ and Ca2+ concentrations and increase in Na+ and proline contents were observed in the root and leaf tissues at each NaCl concentration in all varieties during the NaCl treatment. The Ca2+ concentration in leaves was higher than in roots, and had the following order in the tested cultivars: Jambo, Kimia, and Payam. Total peroxidase activity increased under salinity stress and it was proportional with the salt concentration. Payam had the largest decrease (46.95%) in fresh weight caused by NaCl, while Jambo had the lowest decrease, 28.63%. Linear regression analysis revealed significant relationships between the estimated factors and fresh weight. The profiles of isoperoxidases were modified under stress conditions. Two isoforms, A1 and A2, were detected in all three varieties with different intensities. Under NaCl stress, isoperoxidases were strongly expressed and a third isoform, A3, was specifically found in variety Jambo suggesting that A3 is implicated in salt adaptation of this variety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sorghum (Sorghum bicolor [L.] Moench) is often grown in areas of relatively low rainfall, high temperatures and saline soils (Boursier and Läuchli 1990). Because salinity stress is becoming one of the major constrains in agricultural productivity particularly in arid and semiarid areas of the world, breeding for plant tolerance to salinity stress should be given a high research priority in future research programs (Arzani 2008). Therefore, the development of salt tolerant plants using physiological, biochemical and molecular markers are recommended and may offer mechanistic understanding of tolerance (El-Baz et al. 2003).
Many metabolic changes are known to occur in plants subjected to salt stress, among which physiological parameters, such as ionic contents, are salinity tolerance indicators (Ashraf and Waheed 1993; El-Baz et al. 2003; Netondo et al. 2004). Nevertheless, generalizations of plant responses to salinity are difficult and could not be adequate. The deleterious effects of salinity on plant growth are associated with the decreased osmotic potential of the growing medium, specific ion toxicity, and nutrient ion deficiency (Hasegawa et al. 2000; Luo et al. 2005; Netondo et al. 2004). The control of ion accumulations under salt stress in higher plants are usually caused by either ion exclusions at the root cortex (Jeschke 1984) or redistribution of excess ions to senescing leaves (Yeo and Flowers 1984).
One of the symptoms of salt stress is a substantial K+ efflux from cells reducing the intracellular K+ pool, affecting the cytosolic K+ homeostasis (Cuin et al. 2003; Shabala et al. 2006), and therefore the growth and survival of the plant. Reduction of this efflux correlates with increased salt tolerance (Flowers and Hajibagheri 2001; Chen et al. 2005). Metabolic toxicity of Na+ is largely a result of its ability to compete with K+ for binding sites essential for cellular function. More than 50 enzymes are activated by K+, and Na+ cannot substitute K+ in this role (Bhandal and Malik 1988). Thus, high levels of Na+, or high Na+/K+ ratios can disrupt various enzymatic processes in the cytoplasm. Moreover, protein synthesis requires high concentrations of K+, due to the K+ requirement for the binding of tRNA to ribosomes (Blaha et al. 2000) and probably due to other aspects of ribosome function (Wyn Jones et al. 1979). The disruption of protein synthesis by elevated concentrations of Na+ is an important cause of damage by Na+ as suggested by Tester and Davenport (2003) and Bavei et al. (in press).
An early response to the sudden addition of extracellular Na+ is a temporary rise in cytosolic Ca2+ (Knight et al. 1997). Salinity-induced changes in activity of Ca2+ transporters and components of Ca2+-related signal transduction pathways suggest that Ca2+ plays a physiologically relevant role in plant responses to salinity (Tester and Davenport 2003). Especially, it was observed in many plants that Ca2+ inhibition of initial Na+ influx is directly correlated with the Ca2+-induced decrease in shoot Na+ accumulation, and these are correlated with a reduction in growth inhibition by Na+ upon addition of Ca2+ (Cramer 2002). Calcium has been known for some time to alleviate the effects of salt (LaHaye and Epstein 1969), possibly by maintaining the ion selectivity of membranes (Greenway and Munns 1980). It has been shown, however, that the high ionic strength of saline solutions displaces Ca2+ from the membranes of root cells (Cramer et al. 1985; Lynch and Läuchli 1985).
Proline is a very influential regulator of K+ permeable ion channels and, hence, has direct impact on intracellular K+/Na+ homeostasis (Cuin and Shabala 2005, 2007a) and reduces the levels of reactive oxygene species (ROS) generated during osmotic stress (Hong et al. 2000). Furthermore, in addition to the conventional osmoprotective role of proline in plants, a beneficial effect of over-expressed levels of proline in salt-stressed plants may be the result of protecting the integrity and transporter proteins of plasma membranes (Cuin and Shabala 2007b). Moreover, proline also protects thylakoid membranes against free radical-induced photodamage by stabilizing the photosystem II complex and preserving the structure of enzymes and proteins (Sivakumar et al. 2000). Activities of the enzymes catalase, peroxidase, and polyphenoloxidase were promoted by proline in vivo. Accumulations of free proline under salt, drought, and freezing stresses have been reported for several plants (Potluri and Devi Prasad 1996). While some researchers have reported positive correlations between the capacity for proline accumulation and salinity tolerance (Aspinall and Paleg 1981; Almansouri et al. 1999), others have challenged the value of this solute as positive indicator for resistance to salt stress (Delauney and Verma 1993; Heuer 2003). Thus, it is controversial that hyper accumulation of proline is essential for improving salinity tolerance, or it is just a symptom of salt stress. In addition, it cannot be excluded that both mechanisms may coexist, providing some effective ROS scavenging in sensitive cultivars or species, while indicating a symptom of salt stress in tolerant ones (Chen et al. 2007).
Active oxygen radicals (O •−2 , OH•, and H2O2) are commonly generated under salt stress, and considered as a major peroxidative damaging factor and they need to be scavenged for maintenance of normal growth (El-Baz et al. 2003). Plant cells possess different antioxidant enzymes such as catalase, peroxidase (POD) and superoxide dismutase, which eliminate these reactive free radicals or suppress their formation. Apparently, the high value of antioxidant enzymes activity in NaCl tolerant plant could be related to salt adaptation process (Piqueras et al. 1996; Sreenivasulu et al. 1999).
There are various reports on the different ionic contents, accumulation of amino acids and their contributions to salinity tolerance among genotypes of particular species, and, hence, high degree of diversity of plants’ responses to salinity. For example, results from some studies suggest that there is a positive correlation between the capacity for proline accumulation and salinity tolerance. Nevertheless, some researchers reported a negative correlation between proline accumulation and salt tolerance, or showed that a highly salt-tolerant wild relative of tomato, Lycopersicon peruvianum, accumulates higher concentrations of Na+ than the salt-sensitive domesticated tomato, L. esculentum (Tal 1971; Santa-Cruz et al. 1999). There are some more inconsistent results too. In Arabidopsis thaliana there is no relationship between Na+ accumulation and salt sensitivity (Tester and Davenport 2003), while in some brassicas, Na+ accumulation is correlated with toxicity (Ashraf and Naqvi 1992; Cramer 2002; Rengel 1992; Schmidt et al. 1993). Thus it seems likely that plants respond to salinity in diverse form that necessitates the discovery of individual metabolic acclimation strategies at species or even at genotype level. Taken all these into consideration we have studied salinity tolerance in sorghum with relevance to ion accumulations, proline content and peroxidase activity.
Materials and methods
Three varieties of sorghum differing in salt tolerance, Payam (sensitive), Kimia (moderately tolerant), and Jambo (tolerant), previously investigated by Bavei et al. (in press), were used in this study. These varieties were evaluated under five concentrations of salinity (0, 50, 100, 150, and 200 mM) and at two time points (sampling at 15 and 30 days after salt application). Seeds were grown in plastic pots containing a peat-sand-perlite mix in three replications in 2007 in the greenhouse of the University of Shahrekord, Iran. Treatments were arranged according to a split-split plot design with randomized complete blocks layout.
After 7 days of seed planting, the seedlings were thinned to one plant per pot. During the first 8 days, pots were watered twice daily with deionized water. Thereafter, seedlings were irrigated with Hoagland nutrient solution (Hoagland and Arnon 1959). Application of nutrients started at low concentrations (10%) and increased gradually to the full concentration by 10 days after planting, at which time the seedlings were treated with NaCl. Day and night temperatures, natural illumination and the relative humidity in the greenhouse were set to similar to field conditions. To avoid osmotic shock, saline treatments were initiated by adding 50 mM of NaCl per liter of culture solution per every second day. Plants were harvested at 15 and 30 days after the final salinity level was reached. The control was irrigated with NaCl-free nutrient solution, whose electrical conductivity was 0.01 dS m−1.
Analysis of minerals
Inorganic solute concentrations were measured in three replicates of the roots and leaves, which were harvested at 15 and 30 days after salt application. From each plant, three leaves of different ages were used for elemental analysis. Samples were weighed, carefully rinsed with distilled water, and then dried at 75°C for 72 h. After drying, 0.2 g of dried sample was put into porcelain crucible inside a furnace at 550°C for 2 h. After removing samples from the furnace and before completely cooling, 10 ml of 2 N HCl was added and heated for several minutes for completely dissolve the ash. After filtering the solution with ash-less filter paper, the filtered solution volume was diluted to 100 ml with distilled water and the resulting solution was analyzed for Na+, K+ and Ca2+ with flame photometer (Genway PFP-7 model). Results were expressed as mg g−1 dry weight of leaf or root tissue.
Proline content
Proline accumulation was determined as described by Bates et al. (1973). 0.5 g of fresh leaf tissues from each treatment were homogenized in 10 ml of 3% w/v sulphosalicylic acid and the homogenate was filtrated. The resulting solution was treated with 2.5% ninhidrine solution and glacial acetic acid. In test tubes, the reaction mixtures were kept in a water bath at 100°C for 60 min to develop the colors. Soon after removal from the water bath, the test tubes were cooled in ice bath and toluene was added to separate chromophores. Optical density was read at 520 nm using UV–VIS spectrophotometer. Proline content was expressed as mg g−1 fresh weight of leaf tissue.
Enzyme extraction
Leaf and root tissues of each variety were homogenized separately in 50 mM Tris–HCl buffer pH 7.4 at 4°C. The homogenate was centrifuged at 9,000×g for 20 min in a refrigerated high-speed centrifuge. The pellet was washed with the same extraction buffer and centrifuged in the same way. The resultant supernatants were assayed for peroxidase activity.
Assay of peroxidase activity
Peroxidase (POD) activity (EC 1.11.1.7) was determined by the absorbance at 430 nm of pyrogallol according to Amako et al. (1994). Reaction mixtures contained 1.5 ml of 100 mM K-phosphate buffer (pH 6.8), 1 ml of 60 mM pyrogallol, 0.48 ml of 0.6 mM H2O2 and 20 μ1 of the enzyme extract. The increase in ΔA430 was measured for 3 min and POD activity expressed as ΔA430 min−1 mg−1 protein. Proteins in the extracts were quantified by the method of Bradford (1976) using BSA as standard.
Peroxidase electrophoresis
Non-denaturing discontinuous polyacrylamide gel electrophoresis (PAGE) was done in 10% separating and 5% stacking gels according to Davis (1964). Enzyme extracts (30 μg protein) from leaf tissues were loaded into the slots. After electrophoresis, the gels were stained with benzidine for peroxidase isozymes as described by Schrauwen (1966).
Statistical analysis
The experiment was carried out in a split-split plot design with completely randomized blocks layout of five salt levels (0, 50, 100, 150, and 200 mM NaCl), three varieties (Payam, Kimia and Jambo), two sampling times (15 and 30 days after salt application), and three replicates. Analysis of variance (ANOVA) was performed by Minitab statistical program (Minitab Inc., State College, PA). Means were separated using the least significant difference (LSD) test at 5% level.
Results
Plant fresh weight
Fresh weight of the plants grown under control conditions ranged from 46.26 (Kimia) to 48.00 g (Jambo). Under these conditions, there were no significant differences in fresh weights of the three varieties. In all plants, fresh weight decreased under salt stress that ranged from 46.94 (50 mM) to 22.48 g (200 mM) in Jambo, from 41.09 (50 mM) to 14.89 g (200 mM) in Kimia, and from 42.27 (50 mM) to 9.51 g (200 mM) in Payam. The greatest decrease, 46.95%, was observed with Payam, while Jambo had the lowest decrease, 28.63%. These differences were significant at P ≤ 0.5 level (Table 1).
Effect of salinity stress on the concentration of mineral ions
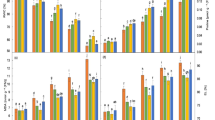
An immediate and primary effect of the imposition of salt stress is a perturbation in tissue cation levels. To determine how Na+ accumulated under our experimental conditions, and how the concentrations of other cations (such as Ca2+ or K+) might be affected by Na+ accumulation, cation contents of sorghum roots and leaves were measured at 15 and 30 days after NaCl treatment. The Na+ concentration was higher in roots than in leaves (Fig. 1). The root Na+ concentration of var. Kimia and Jambo became saturated at about 150 mM external NaCl concentration and did not substantially increase at higher salt concentration. Jambo accumulated significantly (P ≤ 0.01) less Na+ in roots and leaves than did other varieties at each NaCl concentration. Increasing levels of NaCl led to a significant (P ≤ 0.01) rise in Na+ concentration of leaves with no indication of reaching a saturation level as it was found in roots.
There was a significant (P ≤ 0.01) decrease in K+ concentration in roots and leaves of all varieties with increasing salinity (Fig. 2). Jambo accumulated more K+ at all levels of NaCl concentration both in roots and leaves than the other two varieties. Concentrations of K+ in leaves of all varieties were higher than in roots under stress conditions.
There was a clear proportionate decline of Ca2+ in roots and leaves with increasing NaCl concentration in the growth medium (Fig. 3). The Ca2+ concentration in leaves was higher than in roots in the following sequence: Jambo, Kimia, and Payam.
Clear declines in K+ and Ca2+ concentrations with advancing time of NaCl treatment were observed in the root and leaf tissues at each NaCl concentration in each variety (Table 2).
Effect of NaCl on proline accumulation
The accumulation of proline in response to salt stress in leaves of sorghum plants is presented in Fig. 4. The var. Jambo had significantly higher proline concentration compared to var. Kimia and var. Payam in all salt treatments. Increasing concentrations of NaCl from 50 to 200 mM progressively increased the proline concentration in the leaf tissue of var. Payam and var. Kimia by about fourfold, while in var. Jambo, the increase was about sixfold compared to the non-treated control.
Effect of NaCl on peroxidase activity
The activity of the antioxidative peroxidase enzyme in leaves and roots of different sorghum varieties was estimated. Total peroxidase activity of the crude enzyme extracts by using pyrogallol, which reacts with all peroxidases, was measured in root and leaf tissues at 15 and 30 days after application of different NaCl concentrations. The time course of appearance of peroxidase was similar in all varieties. As shown in Fig. 5, the activity of peroxidase was significantly enhanced by NaCl treatments. POD activity in leaves of var. Jambo and Kimia was higher than in leaves of var. Payam at each salinity level. The activity of peroxidase in root tissue was more pronounced than in leaves. The pattern of POD activity in roots was similar to that in leaves (Fig. 5).
Differential expression of peroxidase isoforms
Peroxidase isoforms were detected in the extracts of both control and NaCl-treated seedlings of sorghum varieties by using 10% PAGE. Since the same amounts of soluble proteins (30 μg protein) from each preparation was loaded on gel, the intensity of isoform bands reflects the amounts of individual peroxidase isoforms in each variety. Two isoperoxidases, designated A1 and A2, were detected in non-treated control extract of all varieties (Fig. 6). The amounts of these isoperoxidases were progressively enhanced with increasing NaCl concentrations and were intensified especially at the second time point. The amounts of isoperoxidases A1 and A2 were relatively higher in the var. Jambo than in the two other varieties. One isoperoxidase, designated A3, was expressed only in the var. Jambo. It appeared at the first time point at 200 mM NaCl concentration, but expressed with higher intensity at the second time point both at 150 and 200 mM NaCl concentrations.
Discussion
By increasing salinity in root medium, sodium was the major cation that accumulated in plant tissues. In this study, the preferential accumulation of Na+ in roots over leaves may be interpreted as a mechanism of tolerance in at least two ways: (1) plants need to maintain internal water potential below that of the soil to maintain turgor and water uptake into the roots for growth and (2), restricting Na+ transport to shoot (Renault et al. 2001). Moreover, the less Na+ accumulation in leaves could be due either to minimized Na+ loading to the xylem because of reduced transpiration (Wang 2001) or to maximized retrieval before reaching the leaves (Tester and Davenport 2003). This implies that roots and leaves experience different primary stresses at the initial stage of high salinity stress. We propose that within short time of salt stress at moderate severity (100 mM NaCl) during the day, leaves mainly experience osmotic stress, whereas roots experience both osmotic and ionic stresses. The conclusion is supported by the analysis of K+ content, which had significantly decreased level in root even at the first time point of salt stress, while it was not significantly different in leaves compared to the non-treated control at this time point (data not shown).
According to our results, high Na+ concentration strongly inhibited the uptake and accumulation of K+ and Ca2+ in roots. Because K+ is a macronutrient involved in turgor control, inhibition of K+ uptake should inhibit growth (Renault et al. 2001) and changes in the cytosolic K+/Na+ ratio that induce early senescence by triggering programmed cell death (Shabala 2009). Salinity decreased root Ca2+ concentration as reported by Colmer et al. (1996). High Na+ levels in the external medium greatly reduce the physicochemical activity of dissolved calcium and may thus displace Ca2+ from the plasma membrane of root cells (Cramer and Läuchli 1986). In turn, displacement of Ca2+ from root membranes by Na+ affects Na+/K+ uptake selectivity in favor of sodium (Boursier and Läuchli 1990; Netondo et al. 2004). High extracellular Ca2+ inhibits the plasma membrane inwardly directed Na+-permeable nonselective cation channels (NSCCs) that mediate toxic Na+ influx, and apoplastic Ca2+ also prevents K+ loss from the cell by regulating K+ efflux channels (Shabala et al. 2006). Our results indicate that Na+ preferentially accumulated to higher concentrations in older leaves and roots (the leaves and roots harvested after 30 days salt treatment), especially in treatments of 100 mM NaCl and above. This phenomenon is common in glycophytes (Nakamura et al. 1996) and could be explained by the fact that older leaves have been exposed to salt for a longer period than the younger ones. In addition, their vacuoles are bigger and thus can accumulate more Na+ than those of younger leaves (Netondo et al. 2004). Increased NaCl concentrations in our experiment reduced K+ and Ca2+ contents in leaves and roots of all varieties. However, overall K+ and Ca2+ concentrations in leaf tissues were higher than in roots. Therefore, severe potassium deficiency is unlikely to occur, at least in sorghum leaves, at low to moderate salt concentrations. The results also show that there was a significant difference in K+ concentrations both in leaves and roots of all varieties and at both time points var. Jambo had the highest amount of K+ (Fig. 2). This difference would imply that var. Jambo had limited Na+ transmission to leaf and it is more selective for K+ than Na+ uptake. A strong correlation has been observed between NaCl-induced K+ efflux and salt tolerance based on variety of physiological and agronomical indices (Chen et al. 2005, 2007). This led to the suggestion of using K+ retention as an indicator for salt tolerance (Ashraf and Tufail 1995; Munns et al. 2003; Santa-Maria and Epstein 2001).
Reduction in Ca2+ content was significantly higher at long-term than at short-term salinity stress. This is characteristic of Ca2+ whose transport is known to be affected by NaCl. The Ca2+ content of Jambo tissues was less affected compared with the other two varieties. The ionic interaction, particularly with Na+, leads to low Ca2+ concentration in the xylem fluid, and in turn, to a lower supply of Ca2+ to the leaf tissues. It seems that the intact roots of var. Jambo was capable to increase the translocation of Ca2+ ions to the leaves.
Under salt stress, retention of shoot development is observed as delayed leaf emergence and expansion, and decreased leaf size. The mechanism of this increased sensitivity of the shoot to salt stress is not known. However, it is hypothesized that the plant’s reduction in leaf growth is an adaptive response to save water in soils with reduced osmotic potential, i.e. dry and saline soils (Munns and Tester 2008). Considering the data obtained on fresh weight reduction under salt stress, Jambo was able to tolerate salt stress better (Table 1). The genotype Payam showed higher fresh weight reduction than Jambo under salt stress and, hence, is considered salt sensitive (Table 3).
The difference in salt tolerance was accompanied by a large variation in organic and inorganic solutes, which consequently promoted differences in the fresh weights of these three sorghum genotypes. This study showed a negative significant relationship between leaf and root content of Na+ and salt tolerance in term of plant fresh weight. The significant relationships between the plant fresh weight and K+ and Ca2+ contents (Table 4) indicate significant contributions of these ions to fresh weight of sorghum under salt stress. Although leaf K+ content had the lowest R 2 value, it was still significantly correlated with fresh weight. This could be related to the fact that Ca2+ inhibition of initial Na+ influx is directly correlated with the Ca2+-induced decrease in shoot Na+ accumulation (Cramer 2002). The highly significant relationships between these ion contents and the rankings among genotypes for fresh weight also confirmed the importance of K+ and Ca2+ selectivity to the salt tolerance.
As shown in Fig. 4, proline content in sorghum leaves ranged from 0.78 to 5.08 mg g−1 of fresh tissue and considering this amino acid, all genotypes were affected by salt stress. In Brassica juncea grown under salt stress conditions, proline content increased more than 19-fold compared to control plants (Madan et al. 1994). When the var. Jambo was grown in 200 mM NaCl, its proline content increased to 5.08 mg g−1, while proline content in Kimia and Payam were 3.58 and 3.02 mg g−1, respectively (Fig. 4). This change in proline content in var. Jambo could be correlated with its capacity to tolerate and adapt to salinity condition. Our findings corroborated the results presented in previous reports demonstrating that total free amino acids in the leaves are higher in salt tolerant than in salt sensitive lines of alfalfa (Petrusa and Winicov 1997) sunflower (Ashraf and Tufail 1995), safflower (Ashraf and Fatima 1995), Eruca sativa (Ashraf 1994), and Lens culinaris (Hurkman et al. 1991). In contrast, some reports showed a negative correlation between proline accumulation and salt tolerance (Aziz et al. 1998; Parida et al. 2004). Alarcon et al. (1994) found low contribution of this amino acid to the increase of cell osmotic potential of tomato plants under salt stress condition. Therefore, some researchers assumed that the increase in proline content is rather a salt stress effect than a cause of tolerance (Widholm 1988; Ashraf 1989). According to Chen et al. (2005) and Cuin and Shabala (2007a), by decreasing the extent of the NaCl-induced K+ efflux, proline could substantially mitigate the effects of salt-stress on optimal K+/Na+ homeostasis in the cell cytosol and, ultimately, enhance plant adaptation to salinity. They reported that increased pools of amino acids may eventually contribute to cell osmotic adjustment via regulating the cellular content of inorganic solutes, specifically that of K+, which would contribute to osmotolerance. This indicates that the common assumption that increases in free amino acid under abiotic stress is merely a symptom of damage may need reconsidering. Linear regression revealed positive relationships between proline content and fresh weight of sorghum plants under salinity. Therefore, we suggest that proline accumulation is not merely a symptom of salt-stress injury in sorghum, but rather it can be used as a criterion for salt tolerance. However, proline accumulation cannot be used as an exclusive criterion for salt tolerance, as it also accumulates under other stresses such as high temperature, drought and starvation (Hong et al. 2000). Proline content in sorghum leaves harvested at 30 days after salt treatment was significantly higher than in leaves sampled earlier (Table 3). This is in agreement with the observation of Colmer et al. (1995) that solute accumulation in leaf blades is highly dependent on the plant and leaf age and is higher in older leaves than in younger ones.
In the present study, a significant increase in the activity of peroxidase was recorded in all varieties under NaCl stress conditions, and roots had higher activity than leaves (Fig. 5). Jambo had higher peroxidase activity in both roots and leaves at all NaCl concentration than the other two varieties (Fig. 5). The qualitative and quantitative changes in the activity of peroxidase activity isolated from plants subjected to salinity stress were reported to play a key role in salt tolerance (Heath and Packer 1968; Sreenivasulu et al. 1999). The greater activity of peroxidase in salt-adapted cells may reflect the changed mechanical properties of the cell wall (Heath and Packer 1968). There was also a significant relationship between peroxidase activity and fresh weight. The salt tolerance of Jambo, in term of fresh weight compared to other genotypes, is hypothesized to be the result of better protection from ROS.
The peroxidase system of higher plants exists in multiple isoforms that are developmentally regulated and highly reactive in response to exogenous stimuli (Gaspar et al. 1982). In the present study, NaCl stress caused an induction of isoperoxidases, namely A1, A2, and A3, with different Rf values (Fig. 6). Two bands, A1 and A2, were displayed with different densities and intensities in salt treated plants of all varieties, whereas A3 was specifically found only in the var. Jambo. Similar increases in two isoperoxidases were reported in tobacco associated with resistance to blue mold and were assigned a role in catalyzing cross linking of the cell wall extensions (Ye et al. 1990). As well as POD activity, the amounts of POD isoforms were increased in all varieties by salinity. The highest increase was detected in the salt tolerant var. Jambo (Fig. 6). These peroxidases might be involved in maintenance of cell membrane integrity and regulation of seedling growth under salt stress conditions as demonstrated earlier in some other plant species (Gaspar et al. 1991). This can be further substantiated by the occurrence of a specific isoperoxidase (A3) only in the var. Jambo subjected to 150 and 200 mM NaCl stress (Fig. 6), which could be related to salt adaptation of this variety. Our results are in agreement with the finding of Sreenivasulu et al. (1999) who reported that high peroxidase activity and additional isoperoxidase isoforms were found in a tolerant cultivar compared to a salt susceptible cultivar of Setaria italica.
The isoform A3 of var. Jambo belongs to the category of enzymes detected in the leaves of the tolerant cultivar of Haliminone portulacoides (Kalir et al. 1984), Setaria italica (Sreenivasulu et al. 1999) and Cucums sativus (El-Baz et al. 2003) subjected to salinity stress. The present data reveal that the relatively tolerant nature of Jambo variety may be related to the induction of the specific peroxidase isozyme A3.
References
Alarcon JJ, Sanchez-Blanco MJ, Bolarin MC, Torrecillas A (1994) Growth and osmotic adjustment of two tomato cultivars during and after saline stress. Plant Soil 166:75–82
Almansouri M, Kinet JM, Lutts S (1999) Compared effects of sudden and progressive impositions of salt stress in three durum wheat (Triticum durum Desf.) cultivars. J Plant Physiol 154:743–752
Amako A, Chen K, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Arzani A (2008) Improving salinity tolerance in crop plants: a biotechnological view. In Vitro Cell Dev Biol Plant 44:373–383
Ashraf M (1989) The effect of NaCl on water relations, chlorophyll, protein. and proline contents of two cultivars of black gram (Vigna mungo L.). Plant Soil 119:205–210
Ashraf M (1994) Organic substances responsible for salt tolerance in Eruca sativa. Biol Plant 36:255–259
Ashraf M, Fatima H (1995) Responses of some salt tolerant and salt sensitive lines of safflower (Carthamus tinctorius L.) to salt stress. Acta Physiol Plant 17:61–71
Ashraf M, Naqvi MI (1992) Growth and ion uptake of 4 Brassica species as affected by Na/Ca ratio in saline sand culture. Z Pflanz Bodenk 155:101–108
Ashraf M, Tufail M (1995) Variation in salinity tolerance in sunflower (Helianthus annuus L.). J Agron Crop Sci 174:351–362
Ashraf M, Waheed A (1993) Responses of some genetically diverse lines of chickpea (Cicer orietinum L.) to salt. Plant Soil 154:257–266
Aspinall B, Paleg LG (1981) Proline accumulation: physiological aspects. In: Paleg LG, Aspinall D (eds) The Physiology and Biochemistry of Drought Resistance in Plants. Academic Press, New York, pp 205–241
Aziz A, Martin-Tanguy J, Larher F (1998) Stress induced changes in polyamine and tyramine levels can regulate proline accumulation in tomato leaf discs treated with sodium chloride. Physiol Plant 104:195–202
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bavei V, Shiran B, Khodambashi M, Ranjbar A (2011) Protein electrophoretic profiles and physiochemical indicators of salinity tolerance in sorghum (Sorghum bicolor L.). Afr J Biotech (in press)
Bhandal IS, Malik CP (1988) Potassium estimation, uptake and its role in the physiology and metabolism of flowering plants. Int Rev Cytol 110:205–254
Blaha G, Stelzl U, Spahn CMT, Agrawal RK, Frank J, Nierhaus KH (2000) Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods Enzymol 317:292–309
Boursier P, Läuchli A (1990) Growth responses and mineral nutrient relations of salt-stressed Sorghum. Crop Sci 30:1226–1233
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28:1230–1246
Chen Z, Zhou M, Newman I, Mendham N, Zhang G, Shabala S (2007) Potassium and sodium relations in salinized barley tissues as a basis of differential salt tolerance. Func Plant Biol 34:150–162
Colmer TD, Epstein E, Dvorak J (1995) Differential solute regulation in leaf blades of various ages in salt-sensitive wheat and a salt-tolerant wheat × Lophopyrum elongatum (Host) A. Love Amphiploid. Plant Physiol 108:1715–1724
Colmer TD, Fan TWM, Higashi RM, Läuchli A (1996) Interactive effects of Ca2+ and NaCl salinity on the ionic relations and proline accumulation in the primary root tip of Sorghum bicolor. Physiol Plant 97:421–424
Cramer GR (2002) Sodium-calcium interactions under salinity stress. In: Läuchli A, Lättge U (eds) Salinity. Environment-Plants-Molecules. Kluwer Academic Press, Dordrecht, Netherlands, pp 205–227
Cramer GR, Läuchli A (1986) Displacement of Ca by Na from the plasmalemma of root cells. J Exp Bot 37:321–330
Cramer GR, Läuchli A, Polito VS (1985) Displacement of Ca2+ by Na+ from the plasmalemma of root cells. A primary response to salt stress? Plant Physiol 79:207–211
Cuin TA, Shabala S (2005) Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol 46:1924–1933
Cuin TA, Shabala S (2007a) Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 225:753–761
Cuin TA, Shabala S (2007b) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30:875–885
Cuin TA, Miller AJ, Laurie SA, Leigh RA (2003) Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot 54:657–661
Davis BJ (1964) Disc electrophoresis-II method and application to human serum proteins. Ann New York Acad Sci 121:404–427
Delauney AJ, Verma DP (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
El–Baz FK, Mohamed AA, Aly AA (2003) Development of biochemical markers for salt stress tolerance in cucumber plants. Pak J Biol Sci 6:16–22
Flowers TJ, Hajibagheri MA (2001) Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil 231:1–9
Gaspar T, Penel CL, Thorpe T, Greppin H (1982) Peroxidases. A survey of their biochemical and physiological roles in higher plants. Universite de Geneve, Geneve, pp 89–112
Gaspar T, Penel CL, Hagage D, Greppin H (1991) Peroxidases in plant growth, differentiation and developmental processes. In: Lobarzewsky JH, Greppin H, Penel C, Gaspar T (eds) Biochemical. Molecular, and Physiological aspects of Plant Peroxidases. University de Geneve, Geneve, pp 249–280
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31:149–190
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Mol Biol 51:463–499
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. 1. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heuer B (2003) Influence of exogenous application of proline and glycine betaine on growth of salt-stressed tomato plants. Plant Sci 165:693–699
Hoagland DR, Arnon DI (1959) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 307:32
Hong ZL, Lakkineni K, Zhang ZM, Verma DPS (2000) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Hurkman WJ, Rao HP, Tanaka CK (1991) Germinlike polypeptides increase in barley roots during salt stress. Plant Physiol 97:366–374
Jeschke WD (1984) K+–Na+ exchange at cellular membranes, intracellular compartmentation of cations and salt tolerance. In: Staples RC, Toemniessen GH (eds) Salinity Tolerance in Plants. Wiley and Sons, New York, pp 37–66
Kalir A, Omri G, Poljakoff-Mayber A (1984) Peroxidase and catalase activity in leaves of Halimione portulacoides exposed to salinity. Physiol Plant 62:238–244
Knight H, Trewavas AJ, Knight MR (1997) Calcium signaling in Arabidopsis thaliana in responding to drought and salinity. Plant J 12:1067–1078
LaHaye PA, Epstein E (1969) Salt tolerance by plants: enhancement with calcium. Science 166:395–396
Luo Q, Yu B, Liu Y (2005) Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J Plant Physiol 162:1003–1012
Lynch J, Läuchli A (1985) Salt stress disturbs the calcium nutrition of barley (Hordeum vulgare L.). New Phytol 99:345–354
Madan S, Nainawatee HS, Jain RK, Malik MS, Chowdhury JB (1994) Leaf position-dependent changes in proline, pyrroline-5-carboxylate reductase activity and water relations under salt-stress in genetically stable salt-tolerant somaclones of Brassica juncea L. Plant Soil 163:151–156
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Munns R, Rebetzke GJ, Husain S, James RA, Hare RA (2003) Genetic control of sodium exclusion in durum wheat. Aust J Agric Res 54:627–635
Nakamura T, Ishitani M, Harinasut P, Nomura M, Takabe T, Takabe T (1996) Distribution of glycine betaine in old and young leaf blades of salt-stressed barley plants. Plant Cell Physiol 37:873–877
Netondo GW, Onyango JC, Beck E (2004) Sorghum and Salinity: I. Response of growth, water relations, and ion accumulation to NaCl salinity. Crop Sci 44:797–805
Parida AK, Das AB, Mohanty P (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some anti oxidative enzymes. J Plant Physiol 161:531–542
Petrusa LM, Winicov I (1997) Proline status in salt-tolerant and salt-sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol Biochem 35:303–310
Piqueras AJ, Olmos Hernandez E, Hellin E, Sevilla F (1996) Changes in antioxidant enzymes and organic solutes associated with adaptation of citrus cells to salt stress. Plant Cell Tiss Org Cult 45:53–60
Potluri SD, Devi Prasad PV (1996) Influence of salinity on auxiliary bud cultures of six lowland tropical varieties of potato (Salanum tuberosum L.). Plant Cell Tiss Org Cult 32:185–191
Renault S, Croser C, Franklin JA, Zwiazek JJ (2001) Effect of NaCl and Na2SO4 on red-osier dogwood (Cornus stolonifera Michx). Plant Soil 233:261–268
Rengel Z (1992) The role of calcium in salt toxicity. Plant Cell Environ 15:625–632
Santa-Cruz A, Acosta M, Rus A, Bolarin MC (1999) Short-term salt tolerance mechanisms in different salt tolerant tomato species. Plant Phyiol Biochem 37:65–71
Santa-Maria GE, Epstein E (2001) Potassium/sodium selectivity in wheat and amphiploid cross wheat × Lophopym elongation. Plant Sci 160:523–534
Schmidt C, He T, Cramer GR (1993) Supplemental calcium does not improve growth of salt-stressed Brassicas. Plant Soil 156:415–418
Schrauwen J (1966) Nachweis Von Enzymen nach electrophoretischer Trennung an polyacrylamid sauren. J Chromatogr 23:177–180
Shabala S (2009) Salinity and programmed cell death: unraveling mechanisms for ion specific signaling. J Exp Bot 60:709–712
Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141:1653–1665
Sivakumar P, Sharmila P, Saradhi PP (2000) Proline alleviates salt-stressed induced enhancement in ribulose-1, 5-bisphosphate oxygenase activity. Biochem Biophysic Res Commun 279:512–515
Sreenivasulu N, Ramanjulu S, Ramachandra-kini K, Prakash HS, Shekar-Shetty H, Savithri HS, Sudhakar C (1999) Total peroxidase activity and peroxidase isoforms as modified by salt stress in two cultivars of fox-tail millet with differential salt tolerance. Plant Sci 141:1–9
Tal M (1971) Salt tolerance in the wild relatives of cultivated tomato: responses of Lycopersicon sculentum, L. pruvianum and L. sculentum minor to sodium chloride solution. Aust J Agric Res 24:353–361
Tester M, Davenport RJ (2003) Na+ transport and Na+ tolerance in higher plants. Ann Bot 91:503–527
Wang H (2001) Regulation of the plant Cl metabolic pathway and global gene responses in maize under salt stress, Ph.D. Thesis, University of Arizona
Widholm JM (1988) In vitro selection with plant cell and tissue cultures. Iowa State J Res 62:587–595
Wyn Jones RG, Brady CJ, Spears J (1979) Ionic and osmotic relations in plant cell. In: Liadman DL, Wyn Jones RG (eds) Recent advances in the biochemistry of cereals. Academic Press, London, pp 63–103
Ye XS, Pan SQ, Kuc J (1990) Activity, isozyme pattern, and cellular localization of peroxidase as related to systemic resistance to tobacco to blue mold (Peronospora tobaciana) and to tobacco mosaic virus. Phytopathology 80:1295–1299
Yeo AR, Flowers TJ (1984) Mechanisms of salinity resistance in rice and their role as physiological criteria in plant breeding. In: Staples RC, Toemniessen GH (eds) Salinity Tolerance in Plants. Wiley, New York, pp 37–46
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bavei, V., Shiran, B. & Arzani, A. Evaluation of salinity tolerance in sorghum (Sorghum bicolor L.) using ion accumulation, proline and peroxidase criteria. Plant Growth Regul 64, 275–285 (2011). https://doi.org/10.1007/s10725-011-9568-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9568-z