Abstract
We demonstrate that the exogenous application of salicylic acid (SA) mitigated salt stress in Sorghum bicolor by improving morphological and biochemical parameters. Salt treatment to Sorghum seedlings resulted in reduced growth, maximum quantum yield of photosystem II (Fv/Fm ratio), photochemical quenching (qP), PSII efficiency (ɸPSII) and CO2 assimilation. The application of SA in the range of 0.1–0.5 mM to 0–150 mM salt-stressed 14–day–old Sorghum seedlings mitigated salt stress by increasing endogenous levels of SA which may be responsible for effectively increasing RWC (up to 17%), and synthesis of organic osmoticum, proline (up to 53%) and better ion homeostasis by reducing Na+ (up to 25%) while increasing K+ uptake (up to 38%) to increase the K+/Na+ ratio in plants. The application of SA increased the activity and expression of antioxidant enzymes superoxide dismutase (SOD) and ascorbate peroxidase (APX), leading to a reduction in ROS, consequently preventing lipid peroxidation (MDA, up to 27%) and electrolyte leakage (up to 9%) leading to prevention of salt-induced damage. We conclude that foliar application of SA significantly increased the endogenous level of SA, causing better plant water status, ion homeostasis and mitigating oxidative damage, reversing salt-induced growth inhibition. Our results suggest the application of SA may help in bringing moderately saline lands into agriculture to improve food production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is among the significant environmental stresses and prime concerns of modern agriculture. There are 424 million hectares of topsoil and 833 million hectares of subsoil that are damaged by salt throughout the globe with more than 20% of all irrigated agricultural land, severely impacted by salinity (Negacz et al. 2022). Salinity alters soil physicochemical characteristics, affecting the functions of the plant by rapidly inducing osmotic stress due to physiological drought and slowly building up ion-specific toxicity followed by oxidative damage as a result of reactive oxygen species (ROS) production (Munns and Tester 2008; Kohli et al. 2019; Ahmad et al. 2019; Mansoor et al. 2022).
Osmotic stress as a primary component of salt stress occurs several consequent times if plants are exposed to gradually increasing NaCl concentrations. High concentrations of sodium in saline soils limit water uptake and the absorption of nutrients in the plant (Gong 2021). Osmotic stress causes water deficiency in the plant, which produces numerous transient biophysical changes causing serious structural and metabolic ramifications such as reduction of cell turgor pressure, shrinkage of the plasma membrane and physical alteration of the cell wall (Zhao et al. 2021). The osmotic adjustment refers to the alteration of osmotic pressure inside plant cells in response to osmotic stress if plants are to survive the effects of salt (osmotic) shock (Shavrukov 2013). A valuable measure of soil water stress and the water condition of the whole plant is the leaf water potential (LWP) in plants. Plants acquire tolerance mechanisms to maintain high LWP associated with the dehydration avoidance mechanism in response to osmotic stress, due to excessive salt in the soil (Diatta et al. 2021). Osmotic regulation in plants under osmotic stress can be achieved in one of three ways: by reducing intracellular water, by reducing cell volume, or by increasing cell osmoticum to lower the free energy of water bound inside the cell. This active osmotic regulation contributes to the maintenance of the difference in water potential between inside and outside the cell, allowing the cell to absorb water under lower external water potential conditions (Yang et al. 2021).
Slow accumulation of soluble salts in high concentrations in soil mainly Na+ and Cl− slowly develops Na+ toxicity and nutrient deficiency of other essential ions by limiting the intake of K+, Ca2+, and Mg2+ into the plant (Hussain et al. 2019). Ionic toxicity besides affecting the hydration shell of the other molecules also disrupts the non-covalent interaction between amino acids, causing damage to the cell wall, decreasing concentrations of cations such as potassium and calcium (Johnson and Puthur 2021) and disturbing the K+/Na+ ratio (Shabala et al. 2015). Both osmotic and ion stress lead to secondary oxidative stress (ROS accumulate) due to over reduction of photosynthetic membrane triggering disruption of the metabolic balance of cells, causing further biochemical and molecular damage to cell organelles and membrane components (Mahmud et al. 2017; Martinière et al. 2019; Zhao et al. 2020).
Plant’s adaptive systems against salinity stress include salt exclusion and compartmentalization, osmolyte biosynthesis (Khan et al. 2015), ion homeostasis (Munns and Tester 2008) and coordinated antioxidants response (Yancey 2005) to create an equilibrium between ROS production, oxidative damage to biomolecule and activity and expression level of the antioxidant system (Kohli et al. 2019; Ahmad et al. 2019; Mansoor et al. 2022). Maintenance of cytosolic K+ homeostasis with the ability of various tissues to retain K+ under stress conditions confers tolerance in plants (Shabala et al. 2016). Under stressed conditions, there is a need to redirect a large pool of ATP towards defence reactions, even though its production is drastically low, decreasing the cytosolic K+ to sub-threshold levels inactivate numerous metabolic reactions, allowing redistribution of ATP pool (Shabala 2017).
Organic osmolytes proline, multifunctional amino acid, mitigate salt stress-induced adverse effects directly by lowering the water potential of the cells to facilitate water uptake for maintaining membrane structure or indirectly by scavenging ROS (Ghosh et al. 2021). The increase in osmoprotectants can be accomplished by increased biosynthesis and/or decreased degradation or increased uptake and/or decreased export) depending on the type of stress and species under consideration (Singh et al. 2017). The antioxidant system, another effective defence mechanism, comprised of several antioxidant enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), guaiacol peroxidase (GPX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) keeps the optimal concentration of reactive oxygen species (ROS) and protects it from oxidative stress (Sharma et al. 2012).
Developing strategies for strengthening existing tolerance mechanisms to mitigate the deleterious effect of salt stress in plants through conventional and biotechnological approaches have received considerable attention, which has significant implications in agriculture. However, differences in the response of the plant to salt stress at a cellular and whole plant level, complexity in tolerance mechanism and involvement of other environmental factors with a lack of efficient criteria for selection make these approaches less effective in overcoming salt stress (Jini and Joseph 2017). Exogenous application of phytohormones such as salicylic acid to strengthen tolerance against abiotic stresses to improve plant growth and yield has received much attention in recent years (Jini and Joseph 2017; Ahanger et al. 2019; Kaya et al. 2020; Pai and Sharma 2022) to facilitate saline soil under agriculture. Salicylic acid (SA) is a phenolic endogenous growth regulator with multifaceted functions in the alleviation of biotic stress (Loake and Grant 2007) and abiotic stress (Emamverdian et al. 2020; Liu et al. 2022), including salt stress (Jini and Joseph 2017; Dehnavi et al. 2022; Jangra et al. 2022; Pai and Sharma 2022).
SA is reported to reduce the effects of salinity by improving growth (Ahanger et al. 2019; El-Taher et al. 2021) and photosynthesis (Mahmud et al. 2017; Bukhat et al. 2020; El-Taher et al. 2021) along with maintainence of ion homeostasis (Dehnavi et al. 2022). SA is known to induce the synthesis of proline and glycine betaine, molecules with multiple functions such as compatible solutes, antioxidants and in maintaining plant water relations under stress conditions thereby protecting the plant (Jaiswal et al. 2014; Jini and Joseph 2017; Dehnavi et al. 2022). An increase in proline on application of SA reduced MDA content and alleviated membrane damage (Li et al. 2014; Samadi et al. 2019). SA is also reported to lower values of electrolyte leakage (Yildirim et al. 2008; Jangra et al. 2022) and improve membrane stability index (MSI) resulting in the repair of plasmalemma injury caused by ROS with an increase in the antioxidants (Hayat et al. 2010; Bukhat et al. 2020; Kaur et al. 2022).
Sorghum bicolor (L.) Moench (Poaceae), a C4 plant, is the fifth most cultivated economic crop growing in arid and semiarid regions. Sorghum is a staple food for millions of people and is primarily grown for its seeds, fodder, sugar, and fibre or for the generation of bioenergy (Bakari et al. 2023). It is moderately tolerant to soil salinity of up to 6.8 dSm–1 and water salinity of up to 4.5 dSm–1 of electrical conductivity, above which a 16% yield loss is reported per soil salinity unit increase (Calone et al. 2020). Sorghum serves as a suitable potential model for growing on salt-affected lands to conserve freshwater, reduce fossil fuel pollution, and secure food safety for humans and animals by bringing saline soils into agriculture. The application of exogenous SA may be a convenient and viable option to improve the salt tolerance of Sorghum in salt-affected areas. Hence, in the present work, Sorghum was identified to study the role of exogenous SA in the mitigation of salt stress to increase crop productivity and ensure food security. Earlier studies on the effect of SA in Sorghum genotypes like Yajin 13, 71 (Nimir et al. 2016), Haryana jowar HJ 513 and HJ541 (Jangra et al. 2022) and speedfeed cultivar (Dehnavi et al. 2022) are reported, but this is the first report in cv. MSH–51. The ameliorative effect of SA depends on several factors, including plant species, plant developmental stage, the mode of application and concentration; however, the definite underlying physiological mechanism is not fully understood (Klessig et al. 2016). In addition to confirming valuable results, our work adds to the effect of SA on light reaction and CO2 fixation and relates the impact of exogenous SA on parameters studied with endogenous levels of SA.
Materials and methods
Plant material and growth conditions
Sorghum bicolor cv. MSH–51 was obtained from Goa Bagayatdar Sahakari Kharedi Vikri Saunstha Maryadit, a cooperative body for seed distribution in India. After surface sterilization with 4% sodium hypochlorite (Merck A.R. Grade), seeds were soaked for 6 h before sowing in vermiculite-containing plastic pots in a plant growth room with 16 h photoperiod at a temperature of 25 oC ± 2 oC with a light intensity of ≈ 500 µmol m–2 s–1 and watered regularly. The experiment was conducted with 0 (non-saline control) and 80, 100, 120 and 150 mM NaCl (saline controls) dissolved in Hoagland’s solution. SA (Merck, Tissue culture grade) of 0.1, 0.25 and 0.5 mM was dissolved in water (aqueous solubility of SA being 2.2 mg mL–1) and sprayed exogenously on 7th days after sowing (DAS) for three consecutive days (60 sprays/day) using an atomizer. Plants were harvested for analysis five days after the final treatment (14th DAS). The randomized method was used in the experimental design.
Shoot root length and biomass
The shoot and root length of ten randomly selected plants from each treatment was measured manually and their mass was recorded as fresh mass (FM) biomass, dried at 75 oC for 72 h to measure dry mass (DM) biomass.
External leaf morphology using SEM
Scanning Electron Microscopy (JSM 5800 LV, JEOL, Japan) was used to characterize stomata and trichomes, according to Da Costa and Sharma (2016).
Relative water content (RWC)
The RWC was determined according to Barrs and Weatherley (1962) using the second leaf of randomly selected plants and the formula:
Sodium and potassium concentration in shoot and root
The sodium and potassium concentrations in the sample were determined using a Digital Flame Photometer (Esico Model 381), according to Chapman and Pratt (1962). Fresh tissue (0.5 g) was oven-dried for 72 h at 60 oC. Dried tissue was placed in a muffle furnace in a crucible for 4–5 h at 500 oC to convert it to ash, which was then dissolved in 0.1 N HNO3 and filtered through Whatman Paper No.1. The amount was calculated using standard solutions.
Chlorophyll fluorescence
Chlorophyll fluorescence was measured using a fluorescence monitoring system (PAM 101, Walz, Germany) according to Sharma et al. (1997). The dark-adapted leaf was exposed to a weak modulated beam to measure initial fluorescence (F0), after which it was exposed to a saturating pulse of white light to obtain maximum fluorescence (Fm). Steady-state fluorescence (Fs) was measured when leaves were exposed to actinic light. Leaves were exposed to another pulse of saturated light to obtain Fm’, followed by infrared radiation to get F0’. Variable fluorescence (Fv) was calculated as Fm – F0 to obtain Fv/Fm ratio. Photochemical quenching (qP) was calculated as (Fm’ – Fs)/(Fm’ – F0) and PSII efficiency (ΦPSII) measured as (Fm’ – Fs)/ Fm’ according to Schreiber et al. (1986).
Photosynthetic pigments
Photosynthetic pigments were determined using HPLC (Waters, USA), according to Sharma and Hall (1996). Leaf tissue (0.2 g) was ground in 100% acetone (Merck, HPLC grade) and incubated overnight at 4 ºC. The homogenate was centrifuged at 4 ºC for 10 min at 4,000 g and filtered through 0.2 μm Ultipor®N66®Nylon membrane filter (PALL Life Sciences, USA). Samples of 10 µl were analyzed. The pigments were separated using 10 ul sample through a C18 column (Waters Spherisorb ODS2–250 mm×4.6 mm×5 μm) with a linear gradient of ethyl acetate and acetonitrile: water (9:1) at a flow rate of 1.2 ml min–1 for 30 min. Waters 2996 photodiode array detector was used to obtain the sample spectra at 445 nm. Spectral peaks were identified based on RT and spectral characteristics. β–carotene was used as an external standard for the relative quantification of pigments.
IRGA measurements
Photosynthetic rate (PN), transpiration rate (E) and stomatal conductance (gs) were measured using a portable infrared gas analyzer (IRGA, ADC Bio scientific, LCi–SD) according to Sharma and Hall (1996). These photosynthetic parameters were measured at ambient temperature and carbon dioxide concentration with a light intensity of 1,200 µmol m–2 s–1 emitted by a detachable light source by a diachronic lamp (Hansatech, UK).
Proline concentration
Proline concentration was measured spectrometrically according to Bates et al. (1973). Leaf tissue was homogenized with 3% sulfosalicylic acid and reacted with ninhydrin in GAA. The mixture was incubated in a dry bath at 95 oC for 60 min and added with toluene, leading to the formation of a red chromophore measured at 520 nm. The L-proline standard was used for the calculation, expressed as µmol g–1 FM.
Lipid peroxidation and membrane leakage
Lipid peroxidation was estimated spectrophotometrically according to Sankhalkar and Sharma (2002) by determining 2–thiobarbituric acid-malondialdehyde (TBA-MDA) formation at 532 nm with subtraction for non-specific turbidity at 600 nm. The MDA concentration was calculated using the MDA-TBA extinction coefficient (155 mM–1 cm–1).
Membrane leakage was determined by Gong et al. (1998). Plant tissue was suspended in 25 ml of Milli–Q® water and incubated for 2 h in a water bath at 30 °C; this was used to measure the initial electrical conductivity (EC1). The same was then boiled for 15 min at 100 °C to release electrolytes, cooled, and measured with a conductivity meter (Eutech instruments Multi-parameter PCTestrTM 35) to determine the final electrical conductivity (EC2). Electrolyte leakage (%) was measured according to the following formula.
H2O2, •OH and O2
The total H2O2 concentration was measured spectrophotometrically according to Sagisaka (1976) at 480 nm by homogenizing tissue with 5% TCA and reacting the supernatant with potassium thiocyanate and iron ammonium sulfate. Standard H2O2 was used for calculation.
•OH concentration was measured according to Liu et al. (2009). The tissue was homogenized with 50 mM phosphate buffer and added with 25 mM potassium phosphate buffer containing 2.5 mM 2–deoxyribose; after an hour of dark incubation, it was reacted with 1% TBA in GAA. The OD at 532 nm was measured after boiling this mixture for 10 min and immediately cooling it on ice. The amount of •OH expressed as absorbance units (absorbance X 1000) per gram sample fresh mass.
O2– was determined using nitroblue tetrazolium (NBT, Sigma) according to Kumar et al. (2014). The leaf sample was cut to 1 cm and immersed in tubes containing 0.2% NBT in 50 mM potassium phosphate buffer (pH 7.5) staining solution and left overnight at room temperature in dark. The solution was drained and the leaf sample was heated with absolute ethanol in a dry bath (WiseTherm HB-48P) for 10 min with intermittent shaking to remove chlorophyll for proper visualisation. The leaf surface mounted in 70% glycerol was examined and photographed under a light microscope (Nikon eclipse E200). A dark blue stain of formazan is produced by the reaction between NBT and endogenous O2– within the leaf.
Antioxidant enzyme activity
Superoxide dismutase (SOD) activity was measured spectrophotometrically according to Giannopolitis and Ries (1977) method. The leaf tissue was homogenized with phosphate buffer, and the supernatant was reacted with a mixture of 13 mM methionine, 75 µM NBT, 2 µM riboflavin and 0.1 mM EDTA. The amount of enzyme required to cause 50% inhibition of NBT reduction, monitored at 560 nm, was defined as one unit of SOD activity.
The ascorbate peroxidase (APX) activity was estimated according to Nakano and Asada (1981) method. Ascorbate oxidation was followed at 290 nm with a reaction mixture of 50 mM phosphate buffer (pH 7), 0.5 mM ascorbate, 0.1 mM EDTA, 1.2 mM H2O2, and 0.1 mL enzyme extract. The amount of oxidized ascorbate was calculated using its extinction coefficient (ε = 2.8 mM− 1 cm− 1) with one unit of APX defined as 1mmol ml–1 ascorbate oxidized per minute.
catalase activity was determined according to Aebi (1984), using 50 mM phosphate buffer homogenized leaf tissue. The supernatant was added to a mixture of 50 mM K2HPO4 and 10 mM H2O2. A kinetic study of the H2O2 decomposition was carried out at 240 nm, and the difference in absorbance per unit of time was measured as catalase activity. The catalase concentration was calculated using the extinction coefficient (ε = 39.4 mM− 1 cm− 1).
Gene expression
RNA was extracted for gene expression studies from nitrogen ground tissue using RNA purification reagent (Invitrogen) using the protocol provided with the kit and quantified using a nano-drop spectrophotometer at 260 and 280 nm to check for its purity. cDNA synthesis was done using a PCR kit (Invitrogen Superscript III Reverse Transcriptase) following the protocol provided with the kit. RNA equivalent of 5 µg in PCR vials was added with 1 µl random primer, 1 µl of the dNTPs mix (10 mM) and made to 12 µl with standard distilled water, incubated at 65 oC for 5 min, cooled quickly on the ice, and briefly centrifuged. Reaction buffer (5X) and 0.1 M DTT was added, mixed gently, and incubated at 25 oC for 2 min. Superscript III-RT was added, and the total volume was made to 20 µl using sterile distilled water. The cDNA reaction was carried out at three consecutive temperatures of 25, 42 and 50 oC for 10, 50 and 15 min, respectively, as per the instructions in the Invitrogen protocol. cDNA template (4 µl) was added with 10 µl Supermix (SsoAdvanced SYBR Green, Bio-Rad), 2 µl of 10 µM each of specific primers for SOD, APX, CAT (Forward and reverse in 1:1 ratio) and made to 20 µl with nuclease-free water and amplified. Bio-Rad CFX manager software was used for data analysis and calculated using Livak and Schmittgen (2001).
SA concentration within the plants
Endogenous SA, both free and conjugated, was determined using HPLC (Agilent Technologies) with fluorescence detector (Agilent technologies 1260FLD) and MS detector (Agilent Technologies 6460 Triple Quad LC/MS) according to Allasia et al. (2018) briefly, 0.4 g leaf tissue was homogenized with 70% aqueous ethanol (v/v), vortexed and centrifuged at 10,000 g for 10 min at RT. The supernatant was transferred to a fresh centrifuge tube and the pellet was reextracted with methanol, centrifuged and the supernatant was pooled and concentrated to 500 ul in a vacuum concentrator, and added with 65 µL of 20% aqueous TCA (w/v) and 650 µL of 1:1 (v/v) ethyl acetate and cyclohexane, vortexed and centrifuged at 10,000 g for 2 min at RT and the upper organic phase was collected and evaporated to dryness in a vacuum concentrator for the analysis of free SA and extraction of conjugated SA. For conjugated SA, the dried sample was added with 37% HCl and heated at 80 oC for 1 h in a dry bath; after cooling to RT, ethyl acetate and cyclohexane (1:1 v/v) were added, vortexed and centrifuged at 10,000 g for 2 min at RT, the upper phase was collected containing SA resulted from the acidic hydrolysis of SA-conjugates which was evaporated to dryness in a vacuum concentrator. The dried sample of free and conjugated SA was solubilized in 100 µL of 10% aqueous methanol (v/v) with 0.1% aqueous trifluoracetic acid (TFA, v/v, Sigma-Aldrich). The solubilized samples were filtered through a 0.2 μm Nylon membrane filter (Ultipor®N66®, PALL Life Sciences, USA) and analysed using HPLC (Agilent Technologies) with a fluorescence detector (Agilent Technologies 1260FLD) and MS detector (Agilent Technologies 6460 Triple Quad LC/MS) with reverse phase C18 column (Agilent, Zorbax SB-C18, 2.1 × 50 mm, 1.8 microns) with excitation at 305 nm and emission at 407 nm with a linear aqueous methanol gradient from 10 to 82% (v/v, Merck, HPLC grade) at a flow rate of 1 mL min–1 for 10 min. The quantity of SA was calculated from the peak area value using SA (Sigma) as an external standard and identification of SA was carried out using an MS detector and RT of the peak.
Statistical analysis
Experiments were repeated thrice with three replicates and data were expressed as mean ± SE (n = 3). Using IBM SPSS (version 26), ANOVA was performed to confirm the variability of the results and Duncan’s multiple range test to determine significant (p ≤ 0.05) differences between treatment groups. The correlation between physiological parameters was assessed using Karl Pearson correlation coefficients that were significant at p ≤ 0.05 and p ≤ 0.01 (as indicated in Supplementary Material 3). Principal Component Analysis (PCA) was performed using OriginPro software (OriginLab Corporation, Northampton, MA, USA).
Results
Shoot-root length and biomass
Growth was determined by measuring shoot-root length and its fresh and dry mass (Supplementary Material 1). The shoot and root length decreased in the range of 37–70% and 19–65%, respectively, with varying salt concentrations (80–150 mM). Non-saline control sprayed with all concentrations of SA showed no significant change (p > 0.05) in shoot length. Plants grown with 150 mM NaCl and sprayed with 0.25 mM SA showed a maximum increase of 59% (p < 0.05) in the shoot length. Similarly, root length improved significantly (p < 0.05) on spraying all three SA concentrations to salt-grown plants, with a maximum increase of up to 28% observed on spraying 0.25 mM SA to plants grown with 150 mM NaCl.
Shoot-root fresh mass (FM) and dry mass (DM) showed a parallel trend of reduction with salt stress, which was reversed on the spraying of SA. Shoot FM improved significantly (p < 0.05) in salt stress plants sprayed with 0.1, 0.25 and 0.5 mM SA up to 34, 47 and 35%, respectively, compared to saline controls (Supplementary Material 1). Plants grown at 150 mM NaCl and sprayed with 0.25 mM SA showed an increase in the shoot DM of up to 39% compared to its saline controls. Likewise, root FM and DM also significantly improved (p < 0.05) in plants grown at all concentrations of NaCl (80–150 mM) and sprayed with all three SA concentrations, with the highest upsurge of root FM and DM up to 61% and 40%, respectively, observed on spraying 0.25 mM SA compared to unsprayed saline control.
External leaf morphology
External leaf morphology of Sorghum showed a deformed structure of the stomata with an increase in salt stress, mainly at a high NaCl concentration of 150 mM; however, application of 0.25 mM SA to salt-stressed plants reverted the deformities. The length of the trichomes decreased and shrank with increased salt stress over control, but spraying with 0.25 mM SA to salt-stressed plants prominently increased the trichome length (Supplementary Material 2).
Relative water content (RWC)
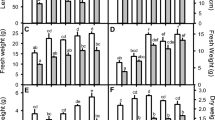
With an increase in salt stress, the relative water content significantly decreased (p < 0.05) by 9, 22, 28 and 42% in 80, 100, 120, and 150 mM NaCl-grown plants, respectively (Fig. 1a) compared to non-saline control. Salt-stressed plants treated with SA showed improved RWC compared to unsprayed salt-stressed plants (saline control). Among the different SA concentrations, 0.25 mM SA was observed to have a maximum improvement in RWC (7–17%) to NaCl-grown plants. SA concentrations of 0.1 and 0.5 mM applied to salt-stressed plants showed a significant (p < 0.05) but a relatively lesser upsurge in the RWC content up to 9 and 13%, respectively, compared to the saline controls. In plants grown with NaCl and sprayed with SA, RWC had a significant positive correlation with growth and biomass (Supplementary Material 3).
Effect of exogenous SA foliar application on (a) relative Water Content (RWC) (b) proline (c) MDA (d) electrolyte leakage (EL) in Sorghum seedlings 14 DAS. C represents non–saline control (0 mM NaCl); saline controls represent plants grown with 80, 100, 120 and 150 mM NaCl without SA spray and 0.1, 0.25 and 0.5 represent SA concentrations sprayed on three consecutive days (7th – 9th DAS). Data represent the means of three independent experiments (n = 3) with five replicates and vertical bars represent standard error; different alphabets in the figure indicate significant differences (p < 0.05) according to Duncan’s multiple range tests
Sodium and potassium concentration in shoot and root
Our results showed a greater accumulation of Na+ in the roots than in the shoots of Sorghum seedlings grown with NaCl. The Na+ concentration increased gradually with increasing NaCl concentration in both shoot and root, reaching 453 and 749%, respectively, at the highest NaCl concentration compared to the non-saline control (Table 1). A spray of 0.25 mM SA significantly reduced (p < 0.05) the Na+ amount in shoot and roots by up to 25 and 18%, respectively, compared to saline controls; however, there was no such significant difference on spraying SA to the non-saline plants. In roots, all three concentrations of SA significantly reduced (p < 0.05) the Na+ concentration, with maximum reduction observed on spraying 0.25 mM SA. Exogenous application of 0.25 mM SA reduced the Na+ accumulation in the root by 13, 15, 18 and 14% in 80, 100, 120 and 150 mM NaCl-grown seedlings, respectively, compared to their saline controls.
K+ concentration, in contrast to Na+, decreased with increasing NaCl treatment but was significantly improved (p < 0.05) on foliar application of SA in both shoot and root of Sorghum seedlings grown under salt stress. The foliar spray of 0.1, 0.25 and 0.5 mM SA enhanced the K+ concentration in shoots up to 32, 38 and 31%, respectively, compared to their saline controls. However, in roots, 0.25 mM SA showed a small but significant increase (p < 0.05) in K+ of 4, 6 and 4% at 80, 100 and 120 mM NaCl, respectively, compared to their saline controls. Our data with Na+ and K+ indicate that the application of SA improved the K+/Na+ ratio both in the shoot and root (Table 1), which was positively correlated with growth, biomass and RWC (Supplementary Material 3).
Photosynthetic pigments, chlorophyll fluorescence, IRGA measurements
In plants grown with 80 to 150 mM NaCl, total chlorophyll and carotenoid content decreased by 13–49% and 4–57%, respectively, compared to non-saline control (Table 2). This salt-induced decline was reversed by spraying SA, with maximum recovery observed on applying 0.25 mM SA in all NaCl-grown seedlings. The application of 0.25 mM SA significantly improved (p < 0.05) total chlorophylls by up to 34% and total carotenoids by up to 92% in 150 mM NaCl plants compared to saline controls. Application of 0.1 and 0.5 mM SA to salt-stressed plants also increased the chlorophylls and carotenoid content; however, the observed increase was lesser than in 0.25 mM SA-sprayed plants. In SA foliar sprayed plants, total chlorophyll and total carotenoids were significantly positively correlated to growth, biomass, and RWC. In addition, total chlorophyll was also positively correlated to the shoot-root K+/Na+ ratio (Supplementary Material 3).
The photosynthetic efficiency (Fv/Fm) of Sorghum seedlings grown at 80, 100, 120 and 150 mM NaCl reduced by 16, 23, 30 and 39%, respectively, compared to non-saline controls. A spray of SA to non-saline plants had no significant effect (p > 0.05) on Fv/Fm ratio, but the application of 0.1, 0.25 and 0.5 mM SA to salt-stressed plants increased the Fv/Fm ratio up to 11, 18 and 14% respectively (Table 2). A similar ameliorating effect in the salt-induced decrease of PSII quantum efficiency (ΦPSII) was observed on spraying SA. Applying 0.1, 0.25 and 0.5 mM SA to 150 mM salt-grown Sorghum seedlings increased the PSII quantum efficiency (ΦPSII) up to 162, 160, and 158%, respectively, compared to saline control. Photochemical quenching (qP) did not decrease significantly up to 100 mM NaCl; however, it reduced by 11.1% and 29.3% at higher NaCl concentrations of 120 and 150, respectively, compared to non-saline control. A significant recovery of 33% in qP was observed in plants grown with 150 mM NaCl and sprayed with 0.25 mM SA. The light reaction parameters were positively correlated to growth, biomass, RWC, Shoot K+/Na+ ratio, and pigments in SA-sprayed plants (Supplementary Material 3).
Salinity stress declined net photosynthetic (PN), transpiration rate (E) and stomatal conductance (gs) significantly (p < 0.05) up to 37, 44 and 49%, respectively, in plants treated with the highest NaCl concentration of 150 mM over non-saline control (Fig. 2a–c). The application of SA mitigated the salt-induced decrease in the gas exchange parameters of PN, E and gs. Maximal amelioration of PN up to 19% was noticed in seedlings sprayed with 0.25 mM SA over unsprayed saline control. Similarly, the application of 0.1 and 0.5 SA showed a significant (p < 0.05) but minor increase of 6% over saline control. The spray of 0.25 mM SA also showed maximum enhancement (p < 0.05) in E and gs up to 30 and 52%, respectively. The parameters related to the dark reactions of photosynthesis showed a high, significantly positive correlation with growth, biomass, RWC, shoot-root ion homeostasis, pigments and light reaction (Supplementary Material 3).
Effect of exogenous SA foliar application on (a) photosynthetic rate (PN) (b) transpiration rate (E) (c) stomatal conductance (gs) in Sorghum seedlings 14 DAS. C represents non-saline control (0 mM NaCl); saline controls represent plants grown with 80, 100, 120 and 150 mM NaCl without SA spray and 0.1, 0.25 and 0.5 represent SA concentrations sprayed on three consecutive days (7th – 9th DAS). Data represent the means of three independent experiments (n = 3) with five replicates and vertical bars represent standard error; different alphabets in the figure indicate significant differences (p < 0.05) according to Duncan’s multiple range tests
Proline concentration
Proline concentration in Sorghum leaves increased with increasing salt stress and further improved with all concentrations of SA spray. Plants grown with 80 to 150 mM NaCl contained 135–428% (p < 0.05) higher proline concentration than non-saline control, which on the application of 0.1, 0.25 and 0.5 mM SA further increased significantly (p < 0.05) up to 25, 46, and 53%, respectively (Fig. 1b).
Lipid peroxidation and membrane leakage
Lipid peroxidation measured as TBA-MDA adduct increased gradually with the upsurge of salt stress from 80 to 150 mM in the range of 19.8–91.5% compared to non-saline control (Fig. 1c). A spray of SA significantly reduced (p < 0.05) the MDA concentration compared to saline control in all salt concentrations. Application of 0.25 mM SA to 80, 100, 120 and 150 mM NaCl-grown seedlings reduced the MDA concentration by 18, 17, 21, and 27%, respectively, compared to their saline controls. The application of 0.1 and 0.5 mM SA also decreased the lipid peroxidation but to a lesser extent than observed for plants sprayed with 0.25 mM SA. Non-saline plants sprayed with SA showed no significant difference (p > 0.05) in MDA concentration.
A gradual significant upsurge of up to 25.7% (p < 0.05) in membrane leakage was also observed with increased NaCl (Fig. 1d). Spraying of SA reduced electrolyte leakage (EL) significantly in saline plants but had no effect on non-saline plants. A spray of 0.1, 0.25 and 0.5 mM SA reduced (p < 0.05) the EL in 80 mM NaCl stressed plants by 6, 8, and 8%, respectively, compared to saline control. Likewise, in 100, 120 and 150 mM NaCl stressed plants, the application of 0.25 mM SA reduced (p < 0.05) the EL by 9, 5, and 6%, respectively, compared to saline control. Application of 0.1 and 0.5 mM SA also mitigated the EL but to a lesser extent than observed with 0.25 mM SA. Lipid peroxidation and EL positively correlated with each other and negatively correlated with growth, biomass, RWC, shoot-root K+/Na+, pigments and photosynthesis (Supplementary Material 3).
H2O2, •OH and O2
The amount of ROS (H2O2 and •OH) significantly increased (p < 0.05) with an increase in the NaCl compared to non-saline control and attenuated when sprayed with SA (Fig. 3a–b). The application of SA reduced the ROS production at all salt concentrations used in our study; however, the maximum reduction in ROS was observed at 80 mM NaCl. The most effective concentration of SA that showed maximum ROS reduction at all NaCl concentrations was 0.25 mM which reduced (p < 0.05) H2O2 and •OH generation up to 28% and 30%, respectively, over saline control. Furthermore, sprays of 0.1 and 0.5 mM SA to salt-stressed plants also attenuated (p < 0.05) H2O2 up to 19 and 23%, respectively, and •OH up to 19% compared to saline controls. Generation of ROS was negatively correlated with growth, biomass, RWC, shoot-root ion homeostasis, pigments, photosynthesis and positively correlated with lipid peroxidation and membrane damage (Supplementary Material 3). NBT staining showed maximum dark blue colouration on the leaf surface in plants treated with NaCl indicative of higher production of O2–. Application of SA lowered the production of O2– seen as a decrease in dark blue colouration. Non-saline control showed minimum staining, indicating the lowest production of O2– (Fig. 3c).
Effect of exogenous SA foliar application on ROS generation (a) hydrogen peroxide (H2O2) (b) hydroxyl radical (•OH) (c) superoxide (O2–) anion detected with nitroblue tetrazolium (NBT) on leaf surface under light microscopy (10 X) (i) non-saline control (ii) 120 mM saline control (iii) 120 mM NaCl with 0.25 mM SA (Inset images are obtained using ImageJ (Java based) to indicate the extent of NBT staining). Antioxidants (d) superoxide dismutase (SOD) (e) ascorbate peroxidase (APX) (f) catalase (CAT) in Sorghum seedlings 14 DAS. C represents non-saline control (0 mM NaCl); saline controls represent plants grown with 80, 100, 120 and 150 mM NaCl without SA spray and 0.1, 0.25 and 0.5 represent SA concentrations sprayed on three consecutive days (7th – 9th DAS). Data represent the means of three independent experiments (n = 3) with five replicates and vertical bars represent standard error; different alphabets in the figure indicate significant differences (p < 0.05) according to Duncan’s multiple range tests
Antioxidant enzyme activity and gene expression
The activity of antioxidant enzymes SOD, APX, and CAT increased up to 77, 195, and 87%, respectively, in 150 mM NaCl plants compared to non-saline control (Fig. 3d–f). Activities of SOD and APX further improved, whereas CAT reduced with the application of SA. Plants grown at 80, 100, 120, and 150 mM concentrations of NaCl and sprayed with 0.5 mM SA showed maximum SOD activity with an increase (p < 0.05) of 29, 36, 24, and 24%, respectively, compared to saline control. APX activity also increased in NaCl-grown plants up to 24, 31 and 39% on spraying 0.1, 0.25, and 0.5 mM SA respectively, compared to saline control. In contrast, CAT activity reduced in NaCl plants on spraying 0.1, 0.25, and 0.5 mM SA up to 11, 27, and 37%, respectively, compared to saline control.
Gene expression of antioxidant enzymes SOD, APX, and CAT increased with an increase in the salt concentration (Fig. 4a–c). Expression of SOD and APX further increased on the application of SA however, expression of CAT declined. SOD expression increased significantly by 2.4 and 6.8 folds in 80 and 120 mM NaCl-grown plants, respectively, compared to non-saline control, which on the application of 0.5 mM SA further increased by 3.2 and 4.4 folds, respectively, compared to saline plants. Expression of APX increased by 2.2 and 5.8 folds in 80 and 120 mM NaCl plants, respectively, compared to non-saline plants, which on the application of 0.5 mM SA further increased by 2.2 and 2.4 folds, respectively, in the same concentration of salt-grown plants compared to saline control. Furthermore, salt-induced increase in CAT expression was reversed (p < 0.05) on the application of all concentrations of SA compared to saline control.
Effect of exogenous SA foliar application on gene expression of antioxidants (a) superoxide dismutase (SOD) (b) ascorbate peroxidase (APX) (c) catalase (CAT) in Sorghum seedlings 14 DAS. C represents non-saline control (0 mM NaCl); Saline controls represent plants grown with 80 and 120 mM NaCl without SA spray, and 0.1, 0.25 and 0.5 represent SA concentrations sprayed on three consecutive days (7th – 9th DAS). Data are expressed as the mean of fold changes of three independent experiments (n = 3) with five replicates and vertical bars representing standard error; different alphabets in the figure indicate significant differences (p < 0.05) according to Duncan’s multiple range tests
SA concentration within the plants
The salt stress caused an increase in the free SA concentration within the plants. Plants grown with 120 mM NaCl showed an upsurge in free SA of 280% compared to the non-saline control (Fig. 5a). The non-saline control sprayed with SA showed an increase in the endogenous SA of 1016.67%; however, there was no difference in the physiological response in these plants compared to non-saline control. Saline plants sprayed with SA showed a rise of 125.43% in their free endogenous form. The conjugated SA showed a slight decline (25.58%) as a result of spraying of SA to non-saline plants; however, conjugated SA increased (22.58%) on the application of SA to 120 mM salt-stressed plants (Fig. 5b).
Effect of exogenous SA foliar application on salicylic acid within Sorghum seedlings 14 DAS (a) free SA (b) conjugated SA. Control represents non-saline control (0 mM NaCl); Control + SA (non-saline plants sprayed with 0.25 mM SA); NaCl (120 mM NaCl stressed plants); NaCl + SA (120 mM NaCl stressed plants sprayed with 0.25 mM SA). Data represent the means of three independent experiments (n = 3) with three replicates and vertical bars represent standard error; different alphabets in the figure indicate significant differences (p < 0.05) according to Duncan’s multiple range tests
Principle component analysis
A Principle Component Analysis (PCA) plot was performed to identify the factors affecting salt-stressed plants with different concentrations of salicylic acid spray (Fig. 6). The biplot of the two principal components collectively is 89.5% of the total variation, with PC1 explaining 83.21% and PC2 explaining 6.28% variation. Plants grown with salt stress were closely related to oxidative damage caused by increased ROS (H2O2 and •OH) leading to electrolyte leakage, whereas plants sprayed with SA were positively correlated to growth and photosynthetic parameters, ion homeostasis and increased antioxidant levels. Karl Pearson’s correlation coefficient was also performed between the studied parameters and R2 values are shown in Supplementary Material 3.
Principle Component analysis (PCA) biplot representing scores of sample (points) and parameters studied (vectors). The biplots were created using the first two components (PC1 and PC2) that explained the maximum differences between the datasets. C represents non–saline control (0 mM NaCl); saline controls represent plants grown with 80, 100, 120 and 150 mM NaCl without SA spray (pink triangles) and 0.1 (black dots), 0.25 (blue stars) and 0.5 (purple squares) represent SA concentrations (mM) sprayed on three consecutive days (7th – 9th DAS). APX – ascorbate peroxidase; CAT – catalase; DM – dry mass; E – transpiration rate; EC – electrical conductivity; FM – fresh mass; Fm – maximum fluorescence; Fv – variable fluorescence; gs – stomatal conductance; PN –photosynthetic rate; ΦPSII – PSII efficiency; qP – photochemical quenching; RWC – relative water content; Endo SA – endogenous Salicylic acid; SOD – superoxide dismutase; Total Chl – total chlorophyll; Total Car – total carotenoids
Discussion
The study presents a correlation of SA-sprayed and unsprayed salt-stressed plants with growth, light and dark relations of photosynthesis, ion homeostasis, oxidative damage and antioxidant system that holistically aids in mitigating salt stress (Fig. 6, Supplementary Material). We observed that the response of SA to salt stress was concentration-dependent, with the maximum mitigating effect observed at the exogenous application of 0.25 mM SA, for most parameters, attributed to a change in its endogenous level for effectively mitigating salinity in Sorghum seedlings. No significant mitigating effect of SA was seen in non-saline plants, suggesting its role in stress conditions probably by acting as a signalling molecule.
Results demonstrated that increased salinity reduced growth, which was improved on the application of SA (Supplementary Material), clearly indicating a role for SA in mitigating salt stress. Observed changes in the stomatal morphology and increased size of trichomes suggested better plant water status (RWC) and adaptational changes against stress. Our SEM data (Supplementary Material 2) suggested SA reduced damage to the stomatal structure, thereby minimizing transpiration, water loss and sustaining turgor to maintain the development and productivity of plants (El-Taher et al. 2021). The observed decrease in RWC (Fig. 1a) with increased salt stress could be due to impaired water absorption as a result of the low water potential, indicating turgor loss and limited water availability for cell metabolism, whereas spraying SA restored the RWC to some extent (Fig. 1a). This is possibly by increasing leaf diffusive resistance (Karlidag et al. 2009) due to morphological adaptation allowing relatively higher gs compared to their saline control (Fig. 2c) in order to facilitate better intake of CO2 for higher photosynthesis (PN, Fig. 2a) at the cost of slightly higher water vapours loss (E, Fig. 2b) without improving the water use efficiency.
This suggests the potential role of SA in maintaining cell turgor, thereby bettering plant-water relationships and improving plant growth and biomass (Khoshbakht & Asgharei 2015). Ahanger et al. (2019) in Vigna angularis (Willd.) Ohwi & H.Ohashi showed similar improvement in RWC on SA application. Increased size of trichomes due to the application of SA in our study; in addition to improving plant water status, it also provided storage for Na+, thereby reducing the ion toxicity to cells Peng et al. (2016) and may also help in increasing the boundary layer resistance to slow down the water loss rate (Shabala 2013).
The observed decrease in growth and biomass, probably due to the osmotic and ionic effect (Table 1) of salinity, also led to declined cellular division and proliferation (West et al. 2004). SA overturned osmotic and ionic effects to increase growth and shoot-root length (Supplementary Material 1). Increased levels of photosynthetic pigments due to the application of SA to salt-stressed plants (Table 2) also led to better Pn (Fig. 2a) and, thus, higher biomass. Furthermore, improvement in growth and biomass can be related to the retained structure of stomata and increased length of trichomes in SA-sprayed plants, assisting with the prevention of water loss (Mo et al. 2016) and salt accumulation (Shabala 2013; Peng et al. 2016, Supplementary Material) as suggested by our results. Our results of improved growth in Sorghum on the application of SA are in accordance with Dehnavi et al. (2022) in speedfeed cultivar and Jangra et al. (2022) in HJ 513 and HJ 541. Similar results were observed by Tahjib-Ul-Arif et al. (2018) in maize, Ahanger et al. (2019) in Vigna angularis and Miao et al. (2020) in cucumber seedlings suggesting better photosynthesis and water use efficiency when applied with SA under salt stress.
Observed reversal of significant accumulation of Na+ and depreciation of K+ ions in plants treated with salt stress on application by SA suggested its role in maintaining cytosolic K+/Na+ homeostasis by neutralizing ion toxicity critical to the salinity tolerance mechanism, which may depend on the ability of SA to aid through its effect on transporter channels (Rubio et al. 2020), improving the plant water status, as evidenced by a positive correlation between the K+/Na+ ratio and RWC (Supplementary Material 3). SA also lessens salinity by reducing the uptake of the ions by roots, most likely through a signalling action (Kaur et al. 2022) thereby preventing an excessive buildup of Na+, preserving the ionic balance in plant leaves. SA facilitates binding of Ca2+ over Na+ which in turn enhances the absorption of K+ and Ca2+ (Xu et al. 2022). It is reported that enhancing endogenous SA also increases the activity of antiporters and/or pumps, which causes Na+ to accumulate in cell vacuoles and lessen the toxicity of salt in the upper sections of plants (Abdoli et al. 2020). Our results are in accordance with Dehnavi et al. (2022) showing an increased K+/Na+ ratio in salt stress Sorghum plants on the application of SA. Jini and Joseph (2017) also demonstrated an increase in the K+/Na+ ratio on SA-sprayed salt-stressed rice. The energy requirement for the transport of salt ions across the membrane may be small as long as there is no leakage; however, our results with lipid peroxidation (Fig. 1c) indicated a significant leakage/damage to the membrane under salt stress, which was significantly mitigated on the application of SA suggesting lowering of salt leakage across tonoplast and plasma membrane, as a delicate balance is required to prevent its toxic level, thereby reducing the energy cost of excluding the salt from the physiologically active site as the osmotic adjustment cost of salt transport or accumulation is small (Munns et al. 2020).
The observed increase in proline content in a dose-dependent manner with increasing salt stress indicates the accumulation of osmolytes as one of the most frequently reported salt-stress-induced adjustments that are involved in maintaining water content (Li et al. 2014). Proline, in addition to being an excellent osmolyte, plays a role as an antioxidative defence molecule, chemical chaperone, metal chelator and signal molecule, maintaining the cell turgor balance, scavenging free radicles and stabilizing membranes and subcellular structure, thereby preventing electrolyte leakage (Sangwan et al. 2022). We observed a further increase in the accumulation of proline with the application of SA up to 53%, whereas in a similar study by Jangra et al. (2022) only an increase up to 20% was reported in Sorghum seedling cv HJ513 indicating variation in tolerance between Sorghum cultivars. Although the energy cost associated with carbon in organic osmoticum like proline (Fig. 1b) is high, but this may be compensated by reduced leakiness of the tonoplast and plasma membrane (reduced lipid peroxidation) as a result of its coordinated synthesis in the chloroplast and cytoplasm and degradation in the mitochondria (Verslues and Sharma 2010). The application of SA reduced the accumulation of Na+, which still remained at a level high enough to cause adverse effects, but increased proline concentration can mitigate the toxic effect of Na+ during osmotic adjustment (Noreen et al. 2011).
The observed reduction in photosynthetic pigments with salt stress occurred primarily due to the production of ROS, as observed in our study (Fig. 3a–c) causing pigment oxidation along with reduction in RWC limiting water further affecting chloroplast structure, while a spray with SA reversed the pigment damage (Table 2) on account of higher activity and expression of antioxidant enzymes SOD (Figs. 3d and 4a) and APX (Figs. 3e and 4b). SA application is reported to protect the degradation of pigments under light stress by better energy regulation between Light-harvesting complexes (Chen et al. 2020) and increased activity of pigment synthesis (higher SPAD value) (Tahjib-Ul-Arif et al. 2018) in salt stress. The chlorophyll content is a biochemical marker of plant salt tolerance and we noticed improved chlorophyll concentration in SA-sprayed plants protecting pigments from phytotoxic effect of ions and oxidative stress which was correlated to improved growth, biomass, RWC and reduced Na+/K+ (Supplementary Material 3). Our results corroborate with the findings of Nazar et al. (2015), who showed SA improved chlorophyll content, water use efficiency and growth of mustard. According to reports, SA may enhance the accumulation of Mg2+ contributing to a protective impact on chlorophyll since Mg2+ is its constituent element (Xu et al. 2022). Moreover, exogenous SA can stimulate the production of cytokinins, which promote the growth of chloroplasts and the synthesis of chlorophyll (Abdoli et al. 2020).
Our results also showed that SA improved the photosynthetic efficiency (Fv/Fm), quantum efficiency of PSII (ΦPSII) and qP, suggesting its role in effectively utilizing the harvested light in a photochemical reaction. SA protects chlorophyll pigments, including carotenoids, as seen in this study (Table 2), from the phytotoxic effect of ions and provides oxidative protection to the chloroplast, thus assisting photosynthesis (Foyer and Noctor 2005). Ionic imbalance within the chloroplast, due to salt stress, is reported to change chlorophyll structure, including its arrangement and orientation, resulting in poor efficiency of light and dark reactions (Bose et al. 2017). These parameters improved on the application of SA, which may be due to improved chlorophyll content and improvement in the thylakoid membrane by scavenging ROS produced during salt stress. In addition, SA may also aid in effectively utilizing the harvested light in a photochemical reaction. An increase in chlorophyll pigments on spraying SA plays a key role in light capturing for photosynthesis and has a direct impact on the intensity of photosynthesis (Bose et al. 2017). Chen et al. (2020) reported that the application of SA increased Fv/Fm, indicating the role of SA in efficiently transferring excitation energy from the light-harvesting complex to the reaction center. Similar results were observed by Tahjib-Ul-Arif et al. (2018) in maize and Miao et al. (2020) in cucumber seedlings, suggesting better photosynthesis and water use efficiency when SA was applied under salt stress.
Photosynthesis is hindered due to salt stress by effects on stomatal closure, which reduces the CO2 intake and, thus, the photosynthetic rate, depicted in our results with reduced PN, E and gs in salt-stressed plants (Fig. 2); which was increased on the application of SA suggesting its role in photosynthesis by maintaining stomatal structure (Supplementary Material 2), regulation of water (Fig. 1a) and pigments (Table 2). Szalai et al. (2005) suggested that SA may mobilize reserved material by increasing physiological activities to promote growth by reversing the reduction in meristem activity and cell elongation. Our results are in accordance with Jangra et al. (2022) depicting an increase in PN up to 34% in Sorghum cv. HJ513 which was much higher compared to the recovery rate of 19% observed in our study on application of SA. Ahanger et al. (2019) observed improved total chlorophyll, carotenoids, Fv/Fm, PN, E, and gs in salt-stressed Vigna angularis. Studies by Zheng et al. (2018) observed improvement in Dianthus superbus growth and photosynthesis under 0.3 and 0.6% salt stress after SA treatment, which could be due to an increase in palisade and spongy cells enabling better CO2 diffusion. Contrary to our observation, Moharekar et al. (2003) observed reduced chlorophyll concentration in wheat and moong seedlings, suggesting the variable role of SA depending on plant species, mode of SA application, tissue type and developmental stage.
We demonstrated an increase in ROS (H2O2, •OH and O2–) with increased salt stress, which was counteracted by the application of SA, reducing the production of ROS, thereby minimizing oxidative stress caused by the ROS (Fig. 3a–c). This is corroborated by our result on the increased MDA (Fig. 1c) with salt stress, suggesting oxidation of polyunsaturated lipids caused by ROS leading to increased electrolyte leakage (EL) (Fig. 1d), which was reduced with the application of SA. When H2O2 is accumulated in high concentrations, toxicity in plants caused by the production of reactive hydroxyl radicals can lead to greater oxidative stress that eventually disturbs plant metabolism causing lipid peroxidation. High salt concentration can disrupt the balance of ion movement, compromising cell membrane integrity in addition to peroxidation of unsaturated fatty acids in cellular membranes, allowing the leakage of electrolytes such as potassium, magnesium and calcium. Ca2+ ions are the initial signal activating the Salt-Overlay-Sensitive pathway, promoting the extrusion of Na+ from the cytosol (Halfter et al. 2000). Further, excess salt in the cell wall and apoplast can lead to the generation of salt crystals that can physically damage the cell wall, leading to the loss of cell membrane integrity. However, the application of SA reduced MDA content and EL, suggesting its role in protection against oxidative damage. Reduction in the ROS accumulation on the application of SA may have a direct contribution to the protection of key cellular organelles like mitochondria and chloroplasts, aiding their smooth functioning (Ahanger et al. 2019). The preservation of the integrity of cell membrane is a critical component of salt tolerance and damage caused to membrane integrity by salt stress is seen as a positive correlation with increased ROS production and degree of membrane damage, all of which were reduced on application of SA.
Emamverdian et al. (2020) suggested that low/optimal concentrations of SA can reduce ROS production, improve plant tolerance and increase plant defence mechanisms by inducing antioxidant enzyme activities, leading to reduced oxidative stress. SA is reported to inhibit the production of ROS, mainly hydroxyl radical, to decrease the concentration of MDA (Dong et al. 2015) and EL with an increase in proline level. Krantev et al. (2008) demonstrated a reduction in MDA and EL in SA-applied cadmium-treated maize plants. These observations are further supported by the SA-induced increase in the activity and expression of SOD, indicating its role in modulating the cell redox balance by reduced production of ROS and protecting plants against oxidative damage, seen as reduced MDA and membrane leakage. Similar results of reduced membrane permeability were observed by Nimir et al. (2016) in Sorghum cv. Yajin 13 and Yajin 17. Anaya et al. (2017) reported similar results in Vicia faba that salinity induced oxidative stress in shoots and roots, while seeds soaked in SA reduced the ROS concentration. In addition, SA aids in modulating the ROS signalling pathway in a concentration-dependent manner by influencing the activity of NADPH oxidase (Poór 2020; Liu et al. 2021).
We reported an increase in the activity (Fig. 3d–f) and expression (Fig. 4a–c) of antioxidant enzymes with salt stress, which is known to be a tolerance mechanism to detoxify ROS by plants however, the oxidative damage with increased ROS generation, lipid peroxidation and membrane leakage still persisted. It has been reported that plants with higher antioxidant levels, either constitutive or induced, are more resilient to stress (Sreenivasulu et al. 2000). The activity and gene expression of SOD and APX were further enhanced with the application of SA, increasing the ROS scavenging property; however, the activity and gene expression of CAT were reduced. Dehnavi et al. (2022) observed an increased CAT activity on application of SA under salinity stress in Sorghum cv. speedfeed whereas Jangra et al. (2022) did not observe a significant change in CAT activity at all SA concentrations in Sorghum cv. HJ513 and HJ541 show variations in CAT activity between Sorghum cultivars in reponse to SA. An increase in SOD activity usually must lead to increased H2O2 content as SOD converts superoxide to H2O2, but we notice a reduction of H2O2 with a spray of SA. This can be correlated with an increase in activity and gene expression of APX and a reduction of CAT. It seems like most of the H2O2 is produced in the chloroplast and cytosol, where APX is an active scavenger, hence lesser need for the antioxidant CAT to detoxify this ROS in the peroxisomes and lower the concentration of H2O2 with SA despite increased levels of SOD. Jini and Joseph (2017) reported that the activity of the antioxidant enzyme SOD significantly increased with the NaCl treatment compared to the control and also showed that the application of a high concentration of SA (1mM) resulted in a decrease in SOD activity. Studies by Abdoli et al. (2020) in Trachyspermum ammi L. reported reduced oxidative stress on the application of SA due to enhanced antioxidant enzymes and reduction of leaf Na+ content and enhanced K+ uptake. SA priming was reported by Kaur et al. (2022) to suppress the production of ROS and decrease MDA by increasing the activities of antioxidant enzymes like SOD, APX and CAT, indicative of SA-induced protection of cells and subcellular systems from toxic ROS impact. Kim et al. (2018) even reported inhibition of CAT and APX activity and a higher level of H2O2 on the application of SA in excess > 0.1 mM in rice.
Our results showed that endogenous free SA levels increased while conjugated SA declined on the application of SA to non-saline control plants without any significant difference in growth (Fig. 5a–b), but free SA and conjugated SA both increased in salt-stressed plants mitigating stress effect suggesting a fine-tuning of endogenous SA and stress level for the hormonal effect. The increase in endogenous free SA under salt stress may be due to its crucial role as a signalling molecule in the activation of plant defence responses, with its accumulation acting as a signal to activate defence-related genes and pathways. Kim et al. (2017) showed that 0.1 mM exogenous salicylic acid alleviates salt stress damage in cucumber by controlling endogenous salicylic acid levels. Alonso-Ramírez et al. (2009) exhibited that the 0.05 mM exogenous application of phytohormones and gibberellic acid also increases the SA level in Arabidopsis, reversing the inhibitory effect of salt oxidation on germination and seedling establishment. Studies by Abdoli et al. (2020) also revealed a rise in endogenous SA with salt stress in Trachyspermum ammi L. (ajowan) owing to salt-induced SA biosynthesis enzyme benzoic acid 2-hydroxylase. Our results with the application of SA on various parameters in non-saline control and salt-stressed plants suggest that the concentration of SA in mitigating salt stress varies between plant species, mode of application, tissue type and developmental stage (Pai and Sharma 2022).
The PCA plot (Fig. 6) provides insight into the possible mechanisms of salt tolerance of Sorghum by application of SA. We show a correlation between the studied parameters, which displayed a close association of salt-stressed plants with oxidative stress, with increased ROS content (H2O2 and •OH) leading to increased electrolyte leakage and MDA with reduced growth, biomass, net photosynthesis, pigments, K+/Na+. SA-sprayed plants showed a positive correlation with improved growth and biomass, water status, pigments, light and dark reaction of photosynthesis with small angles between them in the PCA plot, suggesting its role in mitigating salt stress to improve productivity. We observe that all parameters such as biomass, RWC, Total Chl, carotenoids, Chlorophyll fluorescence parameters, photosynthetic rate and K+/Na+ ratio negatively influenced by salt stress and positively influenced by SA are placed together. Parameters that were increased by salt stress such as ROS (H2O2 and •OH), EL, MDA and antioxidant CAT and reduced by SA are placed together in the PCA graph. Salt stress enhanced a few parameters including proline, antioxidants: SOD and APX and endogenous SA, as a natural adaption strategy which was further enhanced on the application of SA to confer tolerance and are placed together in the PCA graph.
Transitioning from lab to field involves incremental investments hence, here we attempt to elucidate the cost-benefit of the application of SA to mitigate salt stress and increase productivity. A plant population of 300–400 thousand plants per hectare is recommended for Sorghum (Medina-Méndez et al. 2021). The mitigating effect against salt stress, in this study, was observed on utilization of 12 mL (0.012 L) of SA per plant, resulting in a requirement of 4800 L of SA per hectare of 0.25 mM concentration requiring approximately 165 g per 4800 L which will cost < 300 Indian rupees in addition of the cost of spraying and labour which is feasible compared to loss of production due to salinity. We showed an increase of ~ 177% in the number of seeds (productivity, data not shown here) in SA-sprayed plants as compared to salt stress, which is a good return on investment.
Conclusion
Salinity is a major abiotic stress affecting crop productivity and, hence, food security. This study emphasizes the remarkable potential of SA as a mitigating agent against salt stress in Sorghum seedlings. Our study involved a comprehensive analysis of physiological and biochemical parameters making it evident that SA application contributes significantly to the alleviation of salt-induced damage. The results show that SA acts as a regulator in maintaining ion homeostasis by effectively reducing sodium uptake while promoting potassium retention, thereby aiding to protect the cellular integrity and minimize osmotic stress which in return improved the plant water relationship seen as increased RWC. The increase in osmolyte-like proline also contributed towards maintaining the plant-water relationship. Further, SA also improved the photosynthetic efficiency under salt stress along with an increase in the chlorophyll content indicating its positive role in sustaining optimal carbon assimilation and energy production even during stress, which is likely to be due to the ability of SA to maintain stomatal conductance minimizing stress-induced water deficits. The role of SA in reinforcing the activity and expression of antioxidant enzymes (SOD and APX) is also noteworthy, leading to oxidative stress combat by reducing ROS scavenging. Practically, the outcome of this study holds great promise for sustainable agriculture in salinity-stressed regions as a feasible strategy to combat salinity stress. In conclusion, this study accentuates the multifaceted benefits of SA (Fig. 7) in alleviating salt stress and enhancing resilience in plants by a holistic approach of increasing plant water status, improving the K+/Na+ ratio and decreasing oxidative stress by increasing activity and expression of antioxidants to minimize membrane leakage and enhance the growth and photosynthesis of the plant; hence SA can be used as a potent tool to grow Sorghum in salt-stressed areas to foster food security and environmental sustainability in the years to come.
Model depicting the possible mechanism of SA-induced salt stress mitigation in Sorghum plants. Upward arrows indicate enhancement in the parameters and the down arrow represents a decrease. Salt stress triggers osmotic stress and ionic stress which led to oxidative stress by production of reactive oxygen species (ROS). Salt stress enhances the production of endogenous SA as an adaptive strategy which is further enhanced by application of exogenous SA to reach the threshold level necessary to mitigate salt stress and provide protection to the Sorghum plants. This is seen as increased growth, biomass, RWC, ion homeostasis (K+/Na+), total pigments (Chlorophyll and carotenoids), maximum quantum yield of photosystem II (Fv/Fm ratio), photochemical quenching (qP), PSII efficiency (ɸPSII), photosynthetic rate (PN), transpiration rate (E), and stomatal conductance (gs). These changes were linked to reduced lipid peroxidation and membrane damage due to decreased ROS generation due to SA-induced further increased in proline, antioxidants (SOD and APX) which was modulated with increase in free endogenous SA level
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DAS:
-
Days after sowing
- DM:
-
Dry mass
- E:
-
Transpiration rate
- EC:
-
Electrical conductivity
- EL:
-
Electrolyte leakage
- FM:
-
Fresh mass
- Fo:
-
Initial fluorescence
- F′o:
-
Initial fluorescence in actinic light
- Fm:
-
Maximum fluorescence
- F′m:
-
Maximum fluorescence in actinic light
- Fs:
-
Steady–state fluorescence
- Fv:
-
Variable fluorescence
- GAA:
-
Glacial acetic acid
- gs:
-
Stomatal conductance
- HR:
-
Hypersensitive response
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- PCA:
-
Principle component analysis
- PN :
-
Photosynthetic rate
- PR:
-
Pathogenesis–related genes
- ɸPSII:
-
PSII efficiency
- qP:
-
Photochemical quenching
- RWC:
-
Relative water content
- SA:
-
Salicylic acid
- SAR:
-
Systemic acquired resistance
- SOD:
-
Superoxide dismutase
- TBA:
-
2–thiobarbituric acid
- TM:
-
Turgour mass
References
Abdoli S, Ghassemi-Golezani K, Alizadeh-Salteh S (2020) Responses of ajowan (Trachyspermum ammi L.) to exogenous salicylic acid and iron oxide nanoparticles under salt stress. Environ Sci Pollut Res 27:36939–36953. https://doi.org/10.1007/s11356-020-09453-1
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Ahanger MA, Aziz U, Alsahli AA, Alyemeni MN, Ahmad P (2019) Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 10:42. https://doi.org/10.3390/biom10010042
Ahmad P, Tripathi DK, Deshmukh R, Pratap Singh V, Corpas FJ (2019) Revisiting the role of ROS and RNS in plants under changing environment. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2019.02.017
Allasia V, Ponchet M, Quentin M, Favery B, Keller H (2018) Quantification of salicylic acid (SA) and SA-glucosides in Arabidopsis thaliana. Bio-protocol 8(10):e2844–e2844. https://doi.org/10.21769/BioProtoc.2844
Alonso-Ramírez A, Rodríguez D, Reyes D et al (2009) Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol 150(3):1335–1344. https://doi.org/10.1104/pp.109.139352
Anaya F, Fghire R, Wahbi S, Loutfi K (2017) Antioxidant enzymes and physiological traits of Vicia faba L. as affected by salicylic acid under salt stress. J Mater Environ Sci 8(7):2549–2563
Bakari H, Djomdi, Ruben ZF, Roger DD, Cedric D, Guillaume P, Pascal D, Philippe M, Gwendoline C (2023) Sorghum (Sorghum bicolor L. Moench) and its main parts (By-Products) as promising sustainable sources of value-added ingredients. Waste Biomass Valor 14:1023–1044. https://doi.org/10.1007/s12649-022-01992-7
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428. https://doi.org/10.1071/BI9620413
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–208. https://doi.org/10.1007/BF00018060
Bose J, Munns R, Shabala S, Gilliham M, Pogson B, Tyerman SD (2017) Chloroplast function and ion regulation in plants growing on saline soils: lessons from halophytes. J Exp Bot 68(12):3129–3143. https://doi.org/10.1093/jxb/erx142
Bukhat S, Manzoor H, Athar HUR, Zafar ZU, Azeem F, Rasul S (2020) Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J Plant Growth Regul 39:809–822. https://doi.org/10.1007/s00344-019-10024-z
Calone R, Sanoubar R, Lambertini C, Speranza M, Antisari LV, Vianello G, Barbanti L (2020) Salt tolerance and na allocation in Sorghum bicolor under variable soil and water salinity. Plants 9(5):561. https://doi.org/10.3390/plants9050561
Chapman HD, Pratt PF (1962) Methods of analysis for soils, plants and waters. Soil Sci 93:68. https://doi.org/10.1097/00010694-196201000-00015
Chen YE, Mao HT, Wu N, Mohi Ud Din A, Khan A, Zhang H, Yuan S (2020) Salicylic acid protects photosystem II by alleviating photoinhibition in Arabidopsis thaliana under high light. Int J Mol Sci 21(4):1229. https://doi.org/10.3390/ijms21041229
Da Costa MVJ, Sharma PK (2016) Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54(1):110–119. https://doi.org/10.1007/s11099-015-0167-5
Dehnavi RA, Zahedi M, Ludwiczak A, Piernik A (2022) Foliar application of salicylic acid improves salt tolerance of sorghum (Sorghum bicolor (L.) Moench). Plants 11(3):368. https://doi.org/10.3390/plants11030368
Diatta AA, Min D, Jagadish SK (2021) Drought stress responses in non-transgenic and transgenic alfalfa—current status and future research directions. Adv Agron 170:35–100. https://doi.org/10.1016/bs.agron.2021.06.002
Dong YJ, Wang ZL, Zhang JW, Liu S, He ZL, He MR (2015) Interaction effects of nitric oxide and salicylic acid in alleviating salt stress of Gossypium hirsutum L. J Soil Sci Plant Nutr 15(3):561–573. https://doi.org/10.4067/S0718-95162015005000024
El-Taher AM, El-Raouf A, Osman HS, Azoz NA, Omar SN, Elkelish MA, Abd El-hady A MAM (2021) Effect of salt stress and foliar application of salicylic acid on morphological, biochemical, anatomical, and productivity characteristics of cowpea (Vigna unguiculata L.) plants. Plants 11(1):115. https://doi.org/10.3390/plants11010115
Emamverdian A, Ding Y, Mokhberdoran F (2020) The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal Behav 15:1777372. https://doi.org/10.1080/15592324.2020.1777372
EOS Data (nd): Crop management Sorghum. https://shorturl.at/msB35
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28(8):1056–1071. https://doi.org/10.1111/j.1365-3040.2005.01327.x
Ghosh UK, Islam MN, Siddiqui MN, Khan MAR (2021) Understanding the roles of osmolytes for acclimatizing plants to changing environment: a review of potential mechanism. Plant Signal Behav 16(8):1913306. https://doi.org/10.1080/15592324.2021.1913306
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gong Z (2021) Plant abiotic stress: new insights into the factors that activate and modulate plant responses. J Integr Plant Biol 63(3):429. https://doi.org/10.1111/jipb.13079
Gong M, Li YJ, Chen SZ (1998) Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153:488–496
Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci 97(7):3735–3740. https://doi.org/10.1073/pnas.97.7.3735
Hayat S, Hasan SA, Hayat Q, Irfan M, Ahmad A (2010) Effect of salicylic acid on net photosynthetic rate, chlorophyll fluorescence, and antioxidant enzymes in Vigna radiata plants exposed to temperature and salinity stresses. Plant Stress 4:62–71
Hussain S, Bai Z, Huang J, Cao X, Zhu L, Zhu C, Khaskheli MA, Zhang C, Jin Q, Zhang J (2019) 1-Methylcyclopropene modulates physiological, biochemical, and antioxidant responses of rice to different salt stress levels. Front Plant Sci 10:124. https://doi.org/10.3389/fpls.2019.00124
Jaiswal A, Pandurangam V, Sharma SK (2014) Effect of salicylic acid in soybean (Glycine Max L. Meril) under salinity stress. Bioscan 9(2):671–676
Jangra M, Devi S, Kumar N, Goyal V, Mehrotra S (2022) Amelioration effect of salicylic acid under salt stress in Sorghum bicolor L. Appl Biochem Biotechnol 194(10):4400–4423. https://doi.org/10.1007/s12010-022-03853-4
Jini D, Joseph B (2017) Physiological mechanism of salicylic acid for alleviation of salt stress in Rice. Rice Sci 24(2):97–108. https://doi.org/10.1016/j.rsci.2016.07.007
Johnson R, Puthur JT (2021) Seed priming as a cost-effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol Biochem 162:247–257. https://doi.org/10.1016/j.plaphy.2021.02.034
Karlidag H, Yildirim E, Turan M (2009) Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci Agric 66:180–187. https://doi.org/10.1590/S0103-90162009000200006
Kaur H, Hussain SJ, Kaur G, Poor P, Alamri S, Siddiqui H, Khan MIR (2022) Salicylic acid improves Nitrogen fixation, growth, yield and antioxidant defence mechanisms in chickpea genotypes under salt stress. J Plant Growth Regul 41:2034–2047. https://doi.org/10.1007/s00344-022-10592-7
Kaya C, Ashraf M, Alyemeni MN, Corpas FJ, Ahmad P (2020) Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J Hazard Mater 399:123020. https://doi.org/10.1016/j.jhazmat.2020.123020
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462. https://doi.org/10.3389/fpls.2015.00462
Khoshbakht D, Asgharei MR (2015) Influence of foliar–applied salicylic acid on growth, gas–exchange characteristics, and chlorophyll fluorescence in citrus under saline conditions. Photosynthetica 53:410–418. https://doi.org/10.1007/s11099-015-0109-2
Kim Y, Kim S, Shim IS (2017) Exogenous salicylic acid alleviates salt–stress damage in cucumber under moderate nitrogen conditions by controlling endogenous salicylic acid levels. Hortic Environ Biotechnol 58(3):247–253. https://doi.org/10.1007/s13580-017-0111-7
Kim Y, Mun BG, Khan AL, Waqas M, Kim H, Shahzad R, Imran M, Yun B, Lee I (2018) Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS ONE 13(3):e0192650. https://doi.org/10.1371/journal.pone.0192650
Klessig DF, Tian M, Choi HW (2016) Multiple targets of salicylic acid and its derivatives in plants and animals. Front Immunol 7:206. https://doi.org/10.3389/fimmu.2016.00206
Kohli SK, Khanna K, Bhardwaj R, Abd-Allah EF, Ahmad P, Corpas FJ (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8(12):641. https://doi.org/10.3390/antiox8120641
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165(9):920–931. https://doi.org/10.1016/j.jplph.2006.11.014
Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB (2014) Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-protocol 4(8):e1108–e1108
Li T, Hu Y, Du X, Tang H, Shen C, Wu J (2014) Salicylic acid alleviates the adverse effects of salt stress in Torreya Grandis Cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE 9:e109492. https://doi.org/10.1371/journal.pone.0109492
Liu Y, Zhao Z, Si J, Di C, Han J, An L (2009) Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora Bungeana. Plant Growth Regul 59(3):207–214. https://doi.org/10.1007/s10725-009-9405-9
Liu J, Zhang W, Long S, Zhao C (2021) Maintenance of Cell Wall Integrity under High Salinity. Int J Mol Sci 22:3260. https://doi.org/10.3390/ijms22063260
Liu J, Qiu G, Liu C, Li H, Chen X, Fu Q, Lin Y, Guo B (2022) Salicylic acid, a multifaceted hormone, combats Abiotic stresses in plants. Life 12(6):886. https://doi.org/10.3390/life12060886
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Loake G, Grant M (2007) Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol 10(5):466–472. https://doi.org/10.1016/j.pbi.2007.08.008
Mahmud S, Sharmin S, Chowdhury BL, Hossain MA (2017) Effect of salinity and alleviating role of Methyl Jasmonate in some Rice varieties. Asian J Plant Sci 16:87–93. https://doi.org/10.3923/ajps.2017.87.93
Mansoor S, Ali Wani O, Lone JK, Manhas S, Kour N, Alam P, Ahmad A, Ahmad P (2022) Reactive oxygen species in plants: from source to sink. Antioxidants 11(2):225. https://doi.org/10.3390/antiox11020225
Martinière A, Fiche JB, Smokvarska M, Mari S, Alcon C, Dumont X, Hematy K, Jaillais Y, Nollmann M, Maurel C (2019) Osmotic stress activates two reactive oxygen species pathways with distinct effects on protein nanodomains and diffusion. Plant Physiol 179(4):1581–1593. https://doi.org/10.1104/pp.18.01065
Medina-Méndez J, Soto-Rocha JM, Hernández-Pérez M, Gómez-Tejero J (2021) Technology for the production of sorghum for grain in the Vertisols of Campeche, Mexico. https://doi.org/10.4236/as.2021.126043
Miao Y, Luo X, Gao X, Wang W, Li B, Hou L (2020) Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci Hortic 272:109577. https://doi.org/10.1016/j.scienta.2020.109577
Mo Y, Yang R, Liu L, Gu X, Yang X, Wang Y, Zhang X, Li H (2016) Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering. Plant Growth Regul 79(2):229–241. https://doi.org/10.1007/s10725-015-0128-9
Moharekar ST, Lokhande SD, Hara T, Tanaka R, Tanaka A, Chavan PD (2003) Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica 41:315–317. https://doi.org/10.1023/B:PHOT.0000011970.62172.15
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Munns R, Day DA, Fricke W, Watt M, Arsova B, Barkla BJ, Bose J, Byrt C, Chen Z, Foster K, Gilliham M, Henderson SW, Jenkins CLD, Kronzucker HJ, Miklavcic SJ, Plett D et al (2020) Energy costs of salt tolerance in crop plants. New Phytol 225(3):1072–1090. https://doi.org/10.1111/nph.15864
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nazar R, Umar S, Khan NA, Sareer O (2015) Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S Afr J Bot 98:84–94. https://doi.org/10.1016/j.sajb.2015.02.005
Negacz K, Malek Ž, de Vos A, Vellinga P (2022) Saline soils worldwide: identifying the most promising areas for saline agriculture. J Arid Environ 203:104775. https://doi.org/10.1016/j.jaridenv.2022.104775
Nimir NEA, Zhou G, Guo W, Ma B, Lu S, Wang Y (2016) Effect of foliar application of GA3, kinetin, and salicylic acid on ions content, membrane permeability, and photosynthesis under salt stress of sweet sorghum [Sorghum bicolor (L.) Moench]. Can J Plant Sci 97(3):525–535. https://doi.org/10.1139/cjps-2016-0110
Noreen S, Ashraf M, Akram NA (2011) Does exogenous application of salicylic acid improve growth and some key physiological attributes in sunflower plants subjected to saltstress? J Appl Bot Food Qual 84:169–177
Pai R, Sharma PK (2022) Exogenous application of salicylic acid mitigates salt stress in rice seedlings by regulating plant water status and preventing oxidative damage. Environ Exp Biol 20:193–204. https://doi.org/10.22364/eeb.20.18
Peng Z, He S, Sun J, Pan Z, Gong W, Lu Y, Du X (2016) Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci Rep 6(1):1–14. https://doi.org/10.1038/srep34548
Poór P (2020) Effects of Salicylic Acid on the metabolism of mitochondrial reactive oxygen species in plants. Biomolecules 10(2):341. https://doi.org/10.3390/biom10020341
Rubio F, Nieves-Cordones M, Horie T, Shabala S (2020) Doing ‘business as usual’ comes with a cost: evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol 225(3):1097–1104. https://doi.org/10.1111/nph.15852
Sagisaka S (1976) The occurrence of peroxide in a perennial plant, Populus Gelrica. Plant Physiol 57:308–309. https://doi.org/10.1104/pp.57.2.308
Samadi S, Habibi G, Vaziri A (2019) Effects of exogenous salicylic acid on antioxidative responses, phenolic metabolism and photochemical activity of strawberry under salt stress. Iran J Plant Physiol 9(2):2685–2694
Sangwan S, Shameem N, Yashveer S, Tanwar H, Parray JA, Jatav HS, Sharma S, Punia H, Sayyed RZ, Almalki WH, Poczai P (2022) Role of salicylic acid in combating heat stress in plants: insights into modulation of vital processes. Front Biosci (Landmark Ed) 27(11):310. https://doi.org/10.31083/j.fbl2711310
Sankhalkar S, Sharma PK (2002) Protection against photooxidative damage provided by enzymatic and non-enzymatic antioxidant system in sorghum seedlings. Indian J Exp Biol 40:1260–1268
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51. https://doi.org/10.1007/BF00024185
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot 112(7):1209–1221. https://doi.org/10.1093/aob/mct205
Shabala S (2017) Signalling by potassium: another second messenger to add to the list? J Exp Bot 68(15):4003–4007. https://doi.org/10.1093/jxb/erx238
Shabala S, Wu H, Bose J (2015) Salt stress sensing and early signalling events in plant roots: current knowledge and hypothesis. Plant Sci 241:109–119. https://doi.org/10.1016/j.plantsci.2015.10.003
Shabala L, Zhang J, Pottosin I, Bose J, Zhu M, Fuglsang AT, Velarde-Buendia A, Massart A, Hill CB, Rosessner U, Bacic A, Wu H, Azzarello E, Pandolfi C, Zhou M, Poschenrieder C, Mancuso S, Shabala S (2016) Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol 172(4):2445–2458. https://doi.org/10.1104/pp.16.01347
Sharma PK, Hall DO (1996) Effect of photo inhibition and temperature on carotenoids in sorghum leaves. Indian J Biochem Biophys 33:471–477
Sharma PK, Shetye R, Bhonsle S (1997) Effect of supplementary ultraviolet–B radiation on young wheat seedlings. Curr Sci 72:400–405
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26. https://doi.org/10.1155/2012/217037
Shavrukov Y (2013) Salt stress or salt shock: which genes are we studying? J Exp Bot 64(1):119–127. https://doi.org/10.1093/jxb/ers316
Singh A, Sharma MK, Sengar RS (2017) Osmolytes: Proline metabolism in plants as sensors of abiotic stress. J Appl Nat Sci 9(4):2079–2092. https://doi.org/10.31018/jans.v9i4.1492
Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol Plant 109(4):435–442. https://doi.org/10.1034/j.1399-3054.2000.100410.x
Szalai G, Páldi E, Janda T (2005) Effect of salt stress on the endogenous salicylic acid content in maize (Zea mays L.) plants. Acta Biol Szeged 49(1–2):47–48
Tahjib-Ul-Arif M, Siddiqui M, Sohag AAM, Rahman M, Polash MAS, Tran LSP (2018) Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J Plant Growth Regul 37(4):1318–1330
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. The Arabidopsis Book. https://doi.org/10.1199/tab.0140
West G, Inzé D, Beemster GTS (2004) Cell cycle modulation in the response of the primary Root of Arabidopsis to Salt stress. Plant Physiol 135:1050–1058. https://doi.org/10.1104/pp.104.040022
Xu L, Chen H, Zhang T, Deng Y, Yan J, Wang L (2022) Salicylic acid improves the salt tolerance capacity of Saponaria officinalis by modulating its photosynthetic rate, osmoprotectants, antioxidant levels, and ion homeostasis. Agronomy 12(6):1443. https://doi.org/10.3390/agronomy12061443
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830. https://doi.org/10.1242/jeb.01730
Yang X, Lu M, Wang Y, Wang Y, Liu Z, Chen S (2021) Response mechanism of plants to drought stress. Horticulturae 7(3):50. https://doi.org/10.3390/horticulturae7030050
Yildirim E, Turan M, Guvenc I (2008) Effect of Foliar Salicylic Acid Applications on Growth, Chlorophyll, and Mineral Content of Cucumber grown under salt stress. J Plant Nutr 31(3):593–612. https://doi.org/10.1080/01904160801895118
Zhao C, Zhang H, Song C, Zhu JK, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. Innov 1(1):100017. https://doi.org/10.1016/j.xinn.2020.100017
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021) Regulation of plant responses to salt stress. Int J Mol Sci 22(9):4609. https://doi.org/10.3390/ijms22094609
Zheng J, Ma X, Zhang X, Hu Q, Qian R (2018) Salicylic acid promotes plant growth and salt-related gene expression in Dianthus superbus L.(Caryophyllaceae) grown under different salt stress conditions. Physiol Mol Biol Plants 24(2):231–238. https://doi.org/10.1007/s12298-017-0496-x
Acknowledgements
The authors are grateful to the University Grant Commission-Special Assistant Program (UGC-SAP) F.3–9/2015/DRS-III (SAP-II) for the support and Goa University for providing financial assistance to RP through Research studentship GU/DTT/Ph.D/Res.Stud/2022–23/627.
Author information
Authors and Affiliations
Contributions
RP performed the experiments and analyzed the data under the supervision of PKS. Both authors drafted and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pai, R., Sharma, P.K. Exogenous supplementation of salicylic acid ameliorates salt-induced membrane leakage, ion homeostasis and oxidative damage in Sorghum seedlings. Biologia 79, 23–43 (2024). https://doi.org/10.1007/s11756-023-01554-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01554-9