Abstract
An experiment was conducted to study the effects of salinity (control, 50, 100, 150, and 200 mM NaCl) on antioxidants and osmolytes in Kimia (grain) and Pegah (sweet-forage) cultivars of sorghum at vegetative and reproductive stages. There were significant differences for potassium/sodium ratio and relative water content (RWC) at vegetative and reproductive stages, as the values were higher at vegetative stage, while leaf stomatal conductance (gs), lipid peroxidation in terms of malondialdehyde content, hydrogen peroxide content, antioxidants and osmolytes were higher at reproductive stage. Salinity enhanced leaf antioxidative defense and decreased leaf K+/Na+ ratio and cell membrane stability at both stages. However, the changes were greater at the highest saline level at vegetative stage. Although RWC and gs were not influenced by salinity at vegetative stage, they significantly decreased at reproductive stage. Further, the sweet-forage cultivar was more salt-tolerant than the grain cultivar at both the stages. It seemed that assaying oxidative and antioxidative characteristics of leaves at different phenological stages could assist in evaluating cultivars’ tolerance to salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crop plants in arid and semi-arid areas are frequently exposed to varying environmental factors such as salinity. More than 20 % of irrigated agricultural lands are salt-affected around the world and it is predicted that soil and water salinity will further increase in future (Mudgal et al. 2010). Cell dehydration, ionic toxicity, nutritional stress, osmotic stress, drought stress, and oxidative stress are some effects of salinity stress (Zhu 2002). Under saline circumstance, ionic toxicity is resulted due to replacement of K+ by Na+ in cellular reactions (Chinnusamy et al. 2005). Zhu (2002) noticed that Na+ and Cl− ions could cross the hydration shells and interfered with non-covalent interactions between amino acids in protein structures. Mudgal et al. (2010) reported that growth reduction in crops was related to various biochemical mechanisms. Nevertheless ion distribution is energetically more efficient than accumulation of osmolytes for osmotic adjustment. Several plants prefer to synthesize organic compounds namely proline, soluble proteins, and soluble sugars to tolerate the osmotic stress following the salinity stress (Vaseva et al. 2012). In addition, changes in leaf water potential, related to relative water content (RWC) and stomatal conductance to H2O (gs) have been reported to participate in the plant’s responses to salinity stress (Sundar et al. 2004).
It is now known that salinity stress induces oxidative stress (Zhu 2002). Reactive oxygen species (ROS), i.e., superoxide radical (O2•–) and hydrogen peroxide (H2O2), produce hydroxyl radical (OH•), which is highly reactive and starts the peroxidation of membrane lipids, mutations, breakage of DNA strands, chlorophyll degradation, proteins destruction, etc. (Jaleel et al. 2009). Hydrogen peroxide is also able to cross biomembranes and attack biomolecules but its reactivity is limited and most of the oxidative damages are consequences of its conversion into other more reactive species (Jaleel et al. 2009). Lipid peroxidation, quantified by malondialdehyde (MDA), indicates the organelle and cell membrane stability (Weisany et al. 2011).
Antioxidative defense is a combination of low molecular mass antioxidants namely ascorbate, glutathione, tocopherols, carotenoids, phenols as well as antioxidative enzymes, viz., superoxide dismutase (SOD), peroxidase (POX), ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR) (Jaleel et al. 2009). Capability of antioxidative defense has an essential role in decreasing ROS damages (Sundar et al. 2004). On the other hand, synthesis and accumulation of osmolytes like free proline, soluble proteins, and soluble carbohydrates is one of the strategies in some plants against salinity (Vaseva et al. 2012).
Stress-induced activities of antioxidants depend on species, cultivar and age (Jaleel et al. 2009). Hence, this investigation was planned to study the leaf oxidative, antioxidative and osmotic responses of grain and sweet-forage cultivars of sorghum under NaCl stress during vegetative and reproductive stages.
Materials and methods
Plant materials and treatments
A pot-culture study was conducted with two commercial sorghum cultivars, viz., Kimia, a grain cultivar and Pegah, a sweet-forage cultivar in a climate-controlled greenhouse under 14/10 h day/night photoperiod, 30/25 °C day/night temperature, 40/50 % day/night RH and 1500 µmol m−2 s−1 midday light intensity at the College of Agriculture and Natural Resources, University of Tehran, Karaj, Iran in 2011. Additional lighting was supplied by tungsten (100 W m−2) and white light fluorescent (23 W m−2) lamps. The experimental design was factorial completely randomized design with four replications.
Salinity stress was applied 2 weeks after cultivation by supplying a mixture of sodium chloride and calcium chloride (10:1 M Na+:Ca2+) at 50, 100, 150, and 200 mM, and persisted up to the end of the growing period. Control plants were supplied equal volume of water. In order to impose salinity levels higher than 100 mM, saline concentrations were progressively increased by supplying 50 mM NaCl every 3 days toward the final saline concentration and the soil electrical conductivities (ECs) tested after the trial. Soil and water characteristics are reported in Electronic supplementary material.
Measurements were performed on the youngest fully expanded leaf of three plants at vegetative (the developmental stage 3, growing point differentiation) and reproductive (the developmental stage 7: soft dough) stages as suggested by Kansas State University.
Sodium and potassium contents, relative water content, and stomatal conductance
Sodium (Na+) and potassium (K+) content were determined by flamephotometery. For RWC, fresh leaf samples were saturated in 10 ml of water for 3 h and their turgid weights were recorded. Subsequently they were oven dried at 70 °C for 48 h and their dry weights were recorded. RWC was calculated as: RWC [%] = [(fresh weight − dry weight)/(turgid weight − dry weight)] × 100.
The stomatal conductance (gs) was assessed using a portable leaf porometer (Decagon SC-1, Decagon Devices, USA) between 10:00 and 14:00 h on a sunny day.
Hydrogen peroxide and lipid peroxidation
For hydrogen peroxide (H2O2) estimation, 0.2 g fresh leaf samples were homogenized in liquid nitrogen, mixed with 2 ml trichloroacetic acid (TCA; 0.1 % w/v), and centrifuged at 12,000g for 15 min at 4 °C. To 0.4 ml supernatant, 0.4 ml ice-cold potassium phosphate buffer (10 mM, pH 7.0) and 0.8 ml of potassium iodide (1 M) were added. The absorbance of the reaction mixture was measured at 390 nm (Turan and Tripathy 2012).
Level of lipid peroxidation in terms of MDA content was determined as per the method of Radyukina et al. (2009). Fresh leaf samples (0.1 g) were homogenized in liquid nitrogen, mixed with 1.5 ml TCA (20 % w/v), and centrifuged at 10,000g for 15 min at 4 °C. To 0.3 ml of supernatant, 1.2 ml, thiobarbituric acid reagent (TBA, 0.5 % w/v) was added and heated at 95 °C for 30 min, cooled, and centrifuged at 10,000g for 10 min at 4 °C. The absorbance was read at 532 and 600 nm.
Antioxidative enzyme activities
For enzymatic assays, frozen leaf samples were homogenized in liquid nitrogen, extracted with 50 mM of ice-cold potassium phosphate buffer (pH 7.0), centrifuged at 20,000g for 30 min at 4 °C. The supernatant was used as the crude enzyme extracts.
Superoxide dismutase (SOD) activity was assayed according to Maia et al. (2010). The 3 ml of the reaction mixture contained 2.5 ml crude enzyme extract, 50 mM of potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 13 mM methionine, 63 μM nitro-blue tetrazolium, and 1.3 μM riboflavin. The reaction was allowed to proceed in tubes covered with a black cloth in a dark room at 25 °C for 15 min, then illuminated with two 20 W fluorescent tubes at 25 °C for 15 min. The absorbance was read at 560 nm.
Peroxidase (POX) was assayed according to Shamsi et al. (2008). Reaction mixture consisted of 0.3 ml of the crude enzyme extract, 0.2 ml of potassium phosphate buffer (50 mM, pH 7.0), guaiacol (1 %), H2O2 (0.4 %). The absorbance was measured at 470 nm.
Ascorbate peroxidase activity (APX) was assayed as described by Dinakar et al. (2010). The 2.5 ml of reaction mixture consisted of 0.1 ml of the crude enzyme extract, 50 mM potassium phosphate buffer (pH 7.0), 0.2 mM EDTA, 0.5 mM sodium ascorbate, and 0.25 mM H2O2. The reaction was started by adding H2O2 at 25 °C. The absorbance was recorded at 290 nm for 1 min.
Catalase (CAT) activity was assayed according to the method of Zhang et al. (2012). The 2.5 ml of reaction mixture consisted of 0.2 ml of crude enzyme extract, 50 mM potassium phosphate buffer (pH 7.0) and 10 mM H2O2. The absorbance was measured at 240 nm for 3 min.
Glutathione reductase (GR) activity was assayed according to Turan and Tripathy (2012). The 1 ml reaction mixture consisted of 0.3 ml of the crude enzyme extract, 100 mM Tris–HCl buffer, (pH 7.8), 21 mM EDTA, 5 µM NADPH, 0.5 mM oxidized glutathione (GSSG). NADPH was added to start the reaction. The absorbance was recorded at 340 nm.
Osmolyte content
For estimation of free proline, 0.5 g fresh leaf samples were homogenized in 10 ml of sulfosalicylic acid (3 % w/v), centrifuged at 3000g for 5 min at 4 °C. To 2 ml of the supernatant, 2 ml of ninhydrin reagent and 2 ml of glacial acetic acid were added, and mixture was heated in water bath at 100 °C for 1 h. Reaction was stopped by keeping the tubes in an ice bath and the reaction mixture was extracted with 4 ml of toluene, and the absorbance was recorded at 520 nm (Vaseva et al. 2012).
Soluble protein content was estimated according to Bradford (1976), using bovine serum albumin (BSA) as a standard. Fresh leaf samples (0.6 g) was homogenized in 12 ml of ice-cold potassium phosphate buffer (50 mM, pH 7.8) containing EDTA (0.1 mM), and centrifuged at 15,000g for 20 min at 4 °C. The 0.02 ml of the supernatant was mixed with 3 ml of solution containing 100 mg l−1 of Coomassie brilliant blue G-250, 50 ml of ethanol (95 %), and 100 ml of phosphoric acid (85 %). The absorbance was read at 595 nm after 2 min.
Soluble carbohydrate content was estimated using the anthrone reagent method (Khayyat et al. 2014). Fresh leaf samples (0.3 g) was homogenized in 5 ml of ethanol (95 %) and centrifuged at 4500g for 15 min. The supernatant was gathered and the residue was re-suspended in ethanol (5 ml, 95 %). This procedure was repeated four times to gather 20 ml supernatant, concentrated by heating to 5 ml. Reaction mixture consisted of 50 μl of the supernatant, 3 ml of anthrone-sulfuric acid solution and 150 μl of deionized water, stirred for 5 min, heated in a water bath (100 °C) for 20 min, and cooled in water (4 °C). The absorbance was read at 625 nm.
Statistical analysis
All data were analyzed using the general linear model (GLM) in SAS 9.1 system. Fisher’s protected least significant difference (LSD) test at P ≤ 0.05 level was used to define significant differences among means. The vegetative and reproductive stages were compared by the orthogonal contrast.
Results and discussion
Potassium/sodium ratio, relative water content, and stomatal conductance
Salinity stress significantly decreased leaf K+/Na+ ratio of sorghum cultivars at both vegetative and reproductive stages (Table 1). Although K+ selectivity of ion channels was markedly more in leaves rather than roots, sodium entry was still greater than potassium leading to decrease in leaf K+/Na+ ratio of sorghum (Dashti et al. 2009).
It appears that higher K+/Na+ ratio in the grain cultivar (Kimia) is associated with the ability of this cultivar to restrict Na+ mobilization to leaves at reproductive stage (Table 1). Lower significant K+/Na+ ratio at reproductive stage, in comparison with vegetative stage, represented greater sodium accumulation in leaves during this time (data not shown). Indeed, Na+ accumulation in leaves rose during the growth period while K+ absorption remained constant or sometimes it decreased (Dashti et al. 2009). It had been suggested that plant tolerance to salinity stress was dependent on limiting Na+ ions and accumulating K+ ions in cellular parts as K+/Na+ ratio played an important role in salinity tolerance (Heidari 2009).
Relative water content was not significantly changed at vegetative phase but it decreased significantly at the highest level of salinity at reproductive stage (Table 2). Sundar et al. (2004) reported that salinity directly influenced leaf water relations and osmotic potential. Heidari (2009) reported that negative water potential in salt-affected leaves resulted in stomatal closure either directly or indirectly via hormonal signals. Plants adapt to saline stress, followed by water deficit, by using a wide range of responses such as stomatal closure and different leaf cuticle characteristics (Heidari 2009).
Despite lower RWC in grain cultivar, gs did not change significantly in sweet-forage cultivar (Table 2). However, RWC and gs were significantly different at two phenological stages (data not shown). Buchanan et al. (2005) reported that stomatal conductance depended on changes in stomatal aperture; however, other mechanisms could also take part in changing water flows during salt stress. Furthermore, adaptation to specific environmental stresses varied within germplasms of a crop (Buchanan et al. 2005).
Hydrogen peroxide and lipid peroxidation
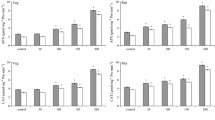
Salinity enhanced H2O2 production and lipid peroxidation at both vegetative and reproductive stages; however, the enhancement was notably high at the highest level of salinity at vegetative stage (Fig. 1a, b). It is clear that cell and organelle membranes are among the targets of H2O2, initiating lipid peroxidation by oxidizing polyunsaturated fatty acids of biomembranes (Weisany et al. 2011). Lipid components of membranes damaged due to lipid peroxidation, resulted in enhancement of membrane permeability (Heidari 2009).
Effect of salinity stress was more prominent at reproductive stage than vegetative stage (data not shown). At all salinity levels, grain sorghum cultivar was more sensitive than the sweet-forage cultivar. Lipid peroxidation of leaf cells, caused by salinity, has previously been reported in salt-sensitive cultivars of sorghum (Weisany et al. 2011). As a result, lipid peroxidation level has been considered as an indicator for evaluating salt tolerance of cultivars (Weisany et al. 2011).
Antioxidative enzyme activities
Salinity caused a significant increase in the activity of all the antioxidant enzymes at both phenological stages; however, the changes were much greater at the highest salinity level (Figs. 2a–c, 3a, b). Salinity induced increase in antioxidant enzymes activity in sorghum leaves have also been reported by Heidari (2009).
Owing to involvement of reactive oxygen species in salinity damages to macromolecules and cellular compartments, the role of enzymatic scavengers is quite important for preserving cellular integrity against oxidative damages (Vaseva et al. 2012). Superoxide dismutase dismutates superoxide radical to hydrogen peroxide and oxygen; therefore, it reduces the risk of hydroxyl radical production via the metal-catalyzed Haber–Weiss reaction (Batkova et al. 2008). Catalase reduces hydrogen peroxide into water and oxygen, while POX decomposes hydrogen peroxide by oxidation of co-substrates like phenolic compounds and/or antioxidants (Jaleel et al. 2009). Ascorbate peroxidase, a heme-protein utilizes ascorbic acid as an electron donor in the neutralization of hydrogen peroxide (Sundar et al. 2004). Glutathione reductase, participating in ascorbate–glutathione cycle, reduces the oxidized glutathione (GSSG) with NADPH as an electron donor to reduced glutathione (GSH) (Batkova et al. 2008).
Similar to hydrogen peroxide and lipid peroxidation, the antioxidative enzyme activities of the grain cultivar were greater than the sweet-forage cultivar (Figs. 2, 3). Batkova et al. (2008) reported that both cultivars and aging affect the antioxidative defense of plant cells.
Furthermore, it was observed that the antioxidative defense of leaves was higher at reproductive stage (data not shown). Sundar et al. (2004) reported that enzymatic antioxidants distributed between the bundle sheet and mesophyll compartments in sorghum leaves played a crucial role in the defense of sorghum cells to oxidative damages under stress. The fact that bundle sheet cells were more susceptible to oxidative damages than mesophyll cells was very important in determining the sensitivity of sorghum cultivars to severe conditions (Sundar et al. 2004).
Osmolyte accumulation
All the leaf osmolytes, viz., proline, total soluble proteins and total soluble carbohydrates increased by increasing salinity levels (Table 3). A slight enhancement in organic solutes, followed by salinity, has been previously reported in sorghum leaves by Chai et al. (2010). Soluble organic compounds had both osmoprotection and osmoregulatory roles under salt and drought stresses (Gill et al. 2003).
Proline, as an osmolyte, reduced the osmotic potential of the cell that led to decrease in toxic ion absorption (Chai et al. 2010). Vaseva et al. (2012) reported that proline accumulation in cells was associated with its osmoprotectant attributes against osmotic stress. The relationship between salinity tolerance and proline accumulation has been previously reported by Chinnusamy et al. (2005). Proline in free form was an effective molecule for preventing singlet oxygen formation (Vaseva et al. 2012).
Soluble carbohydrates participating in decreasing water potential contributed to the maintenance of the structure of proteins and membranes under osmotic stress (Heidari 2009). Further, carbohydrates acted as signaling molecules for sugar-responsive genes, which led to defense responses and turgor cell expansion (Heidari 2009).
Significant differences were not observed in grain and sweet-forage cultivars at vegetative stage (Table 3). But at reproductive stage, sweet-forage cultivar showed more osmolytes than the grain cultivar (Table 3). Further, all of these osmotic compounds were significantly higher at reproductive stage (data not shown). Variations in a crop’s response to stresses, such as the accumulation of compatible solutes were dependent on the diversity of germplasms contributing to cultivar adaptation (Buchanan et al. 2005).
Conclusion
The results showed that salinity stress decreased leaf K+/Na+ ratio and cell membrane stability, while it led to increase in antioxidant enzymes and osmotic compounds at both vegetative and reproductive stages. Further, both sweet-forage and grain cultivars of sorghum could well tolerate salinity stress up to 200 mM NaCl. Although sweet-forage cultivar of sorghum was found to be more salt-tolerant than the grain cultivar, more studies on other physiological parameters are still necessary.
References
Batkova, P., Pospisilova, J., & Synkova, H. (2008). Production of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biologia Plantarum, 52, 413–422.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Buchanan, C. D., Lim, S., Salzman, R. A., Kagiampakis, I., Morishige, D. T., Weers, B. D., et al. (2005). Sorghum bicolor’s transcriptome response to dehydration, high salinity and ABA. Plant Molecular Biology, 58, 699–720.
Chai, Y. Y., Jiang, C. D., Shi, L., Shi, T. S., & Gu, W. B. (2010). Effects of exogenous spermine on sweet sorghum during germination under salinity. Biologia Plantarum, 54, 145–148.
Chinnusamy, W., Jagendorf, A., & Zhu, J. K. (2005). Understanding and improving salt tolerance in plants. Crop Science, 45, 437–448.
Dashti, A., Ali Khan, A., & Collins, J. C. (2009). Effects of salinity on growth, ionic relations and solute content of Sorghum bicolor (L.) Monench. Journal of Plant Nutrition, 32, 1219–1236.
Dinakar, C., Abhaypratap, V., Yearla, S. R., Raghavendra, A. S., & Padmasree, K. (2010). Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta, 231, 461–474.
Gill, P. K., Sharma, A. D., Singh, P., & Bhullar, S. S. (2003). Changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Growth Regulation, 40, 157–162.
Heidari, M. (2009). Antioxidant activity and osmolyte concentration of sorghum (Sorghum bicolor) and wheat (Triticum aestivum) genotypes under salinity stress. Asian Journal of Plant Sciences, 8, 240–244.
Jaleel, C. A., Riadh, K., Gopi, R., Manivannan, P., Ines, J., Al-Juburi, H. J., et al. (2009). Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiologiae Plantarum, 31, 427–436.
Khayyat, M., Tehranifar, A., Davarynejad, G. H., & Sayyari-Zahan, M. H. (2014). Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of ‘Malas-e-Saveh’ and ‘Shishe-Kab’ pomegranates in response to salinity stress. Photosynthetica, 52(2), 301–312.
Maia, J. M., Costa De Macedo, C. E., Voigt, E. L., Freitas, J. B. S., & Silveira, J. A. G. (2010). Antioxidative enzymatic protection in leaves of two contrasting cowpea cultivars under salinity. Biologia Plantarum, 54(1), 159–163.
Mudgal, V., Madaan, N., & Mudgal, A. (2010). Biochemical mechanisms of salt tolerance in plants: A review. International Journal of Botany, 6, 136–143.
Radyukina, N. L., Mapelli, S., Ivanov, Y. V., Kartashov, A. V., Brambilla, I., & Kuznetsov, V. V. (2009). Homeostasis of polyamines and antioxidant systems in roots and leaves of Plantago major under salt stress. Russian Journal of Plant Physiology, 56(3), 323–331.
Shamsi, I. H., Wei, K., Zhang, G. P., Jilani, G. H., & Hassan, M. J. (2008). Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biologia Plantarum, 52(1), 165–169.
Sundar, D., Perianayaguy, B., & Ramachandra Reddy, A. (2004). Localization of antioxidant enzymes in the cellular compartments of sorghum leaves. Plant Growth Regulation, 44, 157–163.
Turan, S., & Tripathy, B. C. (2012). Salt and genotype impact on antioxidative enzymes and lipid peroxidation in two rice cultivars during de-etiolation. Protoplasma, 250, 209–222.
Vaseva, I., Akiscan, Y., Simova-Stoilova, L., Kostadinova, A., Nenkova, R., Anders, I., et al. (2012). Antioxidant response to drought in red and white clover. Acta Physiologiae Plantarum, 34, 1689–1699.
Weisany, W., Sohrabi, Y., Heidari, G. R., Siosemardeh, A., & Ghassemi-Golezani, K. (2011). Physiological responses of soybean [Glycine max L.] to zinc application under salinity stress. Australian Journal of Crop Science, 5, 1441–1447.
Zhang, M., Hu, C., Zhao, X., Tan, Q., Sun, X., Cao, A., et al. (2012). Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis). Plant and Soil, 355, 375–383.
Zhu, J. K. (2002). Salt and drought stress signal transduction in plants. Annual Review of Plant Biology, 53, 247–273.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sayyad-Amin, P., Borzouei, A. & Jahansooz, MR. Biochemical responses of sorghum cultivars under salinity at vegetative and reproductive stages. Ind J Plant Physiol. 20, 324–332 (2015). https://doi.org/10.1007/s40502-015-0180-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-015-0180-5