Abstract
Liquid dairy manure storages emit large amounts of methane (CH4), nitrous oxide (N2O) and ammonia (NH3). Gradually filling manure storages is a standard practice, however, most studies have batch filling approaches. Gradual manure filling may emit different GHGs when inoculum is present, as it changes the substrate/microorganism ratio, manure temperature, and distribution of solids. This study compared CH4, N2O and NH3 emissions from gradually-filled and batch-filled 11.9 m3 capacity liquid dairy manure tanks with 0%, 10% or 20% inoculum over 122 day of storage. On average, gradually-filled tanks had 1.8 °C higher manure temperature, which may have contributed to a 12% increase in total CH4 emissions to 6.26 kg m−3 and 28% increase in total NH3 emissions to 328 g m−3. The absence of inoculum reduced CH4 emissions by 25% and 23% compared to the 10% inoculum tanks (6.48 kg m−3) and 20% inoculum tanks (6.31 kg m−3), respectively. Absence of inoculum had no effect on N2O and NH3 emissions. Gradual filling of tanks containing inoculum increased CH4 emissions by 27% to 7.38 kg m−3, while in the absence of inoculum CH4 emissions were reduced by 29% to 4.03 kg m−3. Our results suggest that research using inoculant in batch-filled manure storage systems may underestimate GHG emissions. Future research should further characterize the effects of gradual filling on solids and temperature profiles, and substrate availability linked to production of GHGs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Canada, liquid dairy manure storage is a typical practice, with usually > 100 d of storage (Sheppard et al. 2011). During this time, significant greenhouse gases (GHG) such as methane (CH4) and nitrous oxide (N2O) are produced (VanderZaag et al. 2010a; Jayasundara et al. 2016). Additionally, ammonia (NH3) volatilizes from manure, which leads to N deposition in sensitive ecosystems causing eutrophication, acidification of water systems, and may be re-emitted as N2O following deposition (Krupa 2003; Kavanagh et al. 2019). Mitigating these emissions is important for both farmers and the environment, as loss of nutrients from manure depreciates its fertilizer value and GHGs contribute (CH4 and N2O) towards climate change. Better understanding of gaseous emissions from agricultural systems is important for finding reduction strategies and assessing predictive modelling tools.
On dairy farms, manure is constantly produced and gradually loaded into storage tanks before being applied to fields. Most experimental GHG emissions research, however, has utilized batch filling into meso-scale tanks or incubated jars (Massé et al. 2003; Møller et al. 2004; VanderZaag et al. 2010b, 2017; Wood et al. 2014; Ngwabie et al. 2016; Le Riche et al. 2017; Baral et al. 2018). In anaerobic digestion, the substrate to microorganism ratio is key to controlling digestion, as lower ratio means more of the substrate will be digested and converted to gas (Burke 2001). Additionally, high levels of substrate can unbalance the microbial reactions, and cause an accumulation of acidic compounds which are toxic to methanogens (Lyberatos and Skiadas 1999). As such, in the context of on-farm manure storage, gradual filling of smaller amounts of manure might enhance microbial activity and emissions due to the addition of fresh substrates and reduced chances of toxicity. This suggests that current research may be underestimating GHG emissions when utilizing batch filling. Understanding the effect that filling has on GHG production is important for building more accurate predictive models. Currently there has been no research comparing gradual and batch filling of liquid manure on subsequent GHG emissions.
Within manure storages the microbial activity is largely affected by the presence of inoculating cultures. Old manure remaining in storages is known to act as an inoculum and subsequently increases GHG emissions (Sommer et al. 2007; Jayasundara et al. 2016; Ngwabie et al. 2016; Habtewold et al. 2018). Sommer et al. (2007) noted that as little as 8% inoculant can reduce the lag phase (initial period of low emissions) in manure storage. Wood et al. (2014) found that complete removal of inoculum resulted in a 50% reduction of GHG emissions when the tanks were re-filled. Ngwabie et al. (2016) found a positive linear relationship of cumulative CH4 emissions and inoculum levels (0, 5, 10, 15, 20 and 25%).
Given that both the inoculum level and the filling type both have the potential to influence GHG emissions, there is a need to evaluate the compounding effect of these on emissions from stored liquid manure. This study assessed the effect of gradual and batch filling of tanks and different inoculum levels (0%, 10%, and 20%) on the production of CH4, N2O, and NH3, and the total CO2-equivalent GHG emissions over 122 day of warm-season storage.
Methods
Site description and tank filling
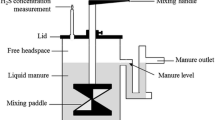
Dairy manure was stored in 6 pilot-scale, in-ground, concrete manure tanks (1.7 m deep, 6.6 m2) at Dalhousie University’s Bio-Environmental Engineering Centre in Bible Hill, NS, Canada (45°45′N, 62°50′W). Each tank was enclosed by a continuously flow-through steady-state chamber for monitoring emissions. The site has previously been described by VanderZaag et al. (2010a). This site provides a unique scale of research, because each tank contains ~ 11 m3 of manure, which is more realistic than laboratory bottles (< 1 L manure), while enabling treatments to be compared under the same conditions (unlike farm manure tanks). At the same time, the research site enables high temporal resolution. On the other hand, the cost of the site operation limits the number of tanks to six. Due to these constraints, there were no treatment replications, instead this preliminary study focused on continuous flux measurement for a full season of storage which will inform future research.
Three tanks were chosen at random to be batch-filled with 11.4 m3 of manure on 1 day (June 2, 2016). Three other tanks were gradually-filled, receiving 1/3 of the volume on three dates: day 1 (June 2), day 20 (June 22), and day 43 (July 15, 2016).
Each tank within the batch- and gradually-filled tanks was randomly assigned different inoculant level (0%, 10%, or 20%). Inoculant was prepared on May 24, by removing old manure (previously stored for about 6 months) from the tanks, mixing it and redistributing it into four cleaned tanks. The 10% inoculum tanks received 1.1 m3 of inoculant, while the 20% inoculum tanks received 2.3 m3 of inoculant (Table 1). All tanks were continuously monitored from June 1 to Oct 1, 2016 (122 day).
This study was performed in parallel with Habtewold et al. (2018), which focused on linking the CH4 emissions to microbial activity within the manure.
Chamber and tank set-up
To measure emissions, each tank was enclosed by a flow-through, steady-state chamber made of an aluminium frame covered by 6 mil greenhouse plastic (Livingston and Hutchinson 1995; Le Riche et al. 2017). Air was drawn into each chamber through three vents and exited through an exhaust venturi on the opposing side of the tank vents. Inflow air was sampled at two locations 1.7 m above ground on the inflow side of the tanks. Cup anemometers (7911, Davis Instruments, Hayward, CA) measured airspeed in the outflow venturi of each chamber and copper–constantan thermocouples (Omega Engineering Inc., Laval, QC) measured the air temperature 30 cm above the manure surface and manure temperature at 80 (mid-depth) and 150 cm (bottom) depth. All outputs were averaged by minute. The airspeed and surface temperature values were recorded by CR1000 datalogger (Campbell Scientific, Logan, UT) and the manure temperatures were recorded by a CR23X datalogger (Campbell Scientific, Logan, UT). Due to instrument failure there were a number of gaps in the manure temperature data at 150 cm depth. Temperature at 80 cm was near mid-depth of manure, and was considered to represent the manure temperature in each tank for comparison purposes. Due to depth changes the temperature in the gradually-filled tanks is not reported until all tanks reached the same manure volume on July 15 (43 day).
Methane and nitrous oxide
Air samples were automatically drawn (RC0021, Busch Vacuum Pumps and Systems, Boisbriand, QC, CA) from each sampling location (6 tanks and 2 ambient inflow location), through polyethylene tubing (3.2 mm i.d.; Rubberline Products Ltd., Kitchener, ON) into a 8 × 2 manifold (Campbell Scientific In., Logan, UT) containing 12 V DC valves (The Lee Co., Essex, CT). The valves were programmed to select two different air sample locations every 30 s whose air flow was directed into high-flow air dryers (Perma Pure LLC.; Toms River, NJ) before entering one of the two tunable diode laser trace gas analyzers (TDLTGA, Campbell Scientific, Logan, UT). A CR5000 datalogger (Campbell Scientific Inc., Logan, UT) recorded the data from each analyzer and a PC computer was continuously running with TDLTGA software to monitor the analyzer and download data from the CR5000.
The gas fluxes from each manure tank were calculated according to this equation (Livingston and Hutchinson 1995; Le Riche et al. 2017):
where F is the flux (e.g. mg m−2 s−1), Q is the flowrate of air out of the chamber (air speed measured in the venturi cross-sectional area of venturi (0.0645 m2), m3 s−1), A is the surface area of the manure tank (6.63 m2), and C (mg m−3) is the concentration of gas in the inlet air (Ci) and outlet air (Co). Due to technical issues, linear interpolation was used to fill CH4 and N2O flux data gaps on dates June 29–July 7, 2016 and Sep 15–19, 2016.
Ammonia
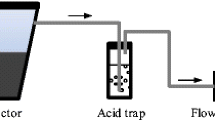
Ammonia gas was captured using 0.005 M H3PO4 acid traps. Air samples were pulled through 25 m of polyethylene tubing at a rate of 1.5 L min−1 using a vacuum pump (Model 2107CA20B; Thomas Pumps and Compressors, Sheboygan, WI) and bubbled through 125 ml of acid using dispersion tubes (id = 35 mm). Air flow for each sample was measured using inline flow meters (Gallus 2000; Actaris Metering Systems, Greenwood, SC). The system was deployed for 24 h 3 per week. During liquid collection, additional acid solution was added to standardize the volume to 125 ml to correct for evaporation. The liquid was analyzed for NH3–N at Agriculture and Agri-Food Canada (Ottawa, ON) using the QuikChem® Method 12–107-06–2-A modified for 0.005 mol L−1 H3PO4 matrix using a Lachat QuikChem FIA + Q8500 Series (Hofer 2003). The gas concentrations were calculated as:
where \(C_{{NH_{3} air}}\) is the NH3–N concentration in gas (mg m−3), \(C_{NH3 aq}\) is the NH3–N concentration in liquid (mg m−3), Vaq is the volume in the acid trap (m3), and Vair is the volume of air pumped through the acid (m3) (Hofer 2003).
Manure
Liquid dairy manure was obtained from a near-by dairy farm with ~ 100 lactating cows that used washed quarry sand for bedding (the same farm manure was used in Le Riche et al. 2017). Manure was obtained when fresh manure was being pumped into the outdoor storage.
Manure samples from the experimental tanks were taken monthly as a composite of 12 locations in each tank (6 locations in a grid and 2 depths at 80 and 160 cm from the surface). Samples were frozen until analyzed at the Nova Scotia Department of Agriculture’s Provincial Soils Lab in Bible Hill, NS. Samples were analyzed for dry matter (DM) and volatile solids (VS) according to American Public Health Association (APHA) method 2540 B, total nitrogen (TN) according to combustion method (AOAC 990.03–2002), ammonium–N (TAN) according to APHA 4500-NH3 B, and pH using an electrode according to APHA 4500-H+ (Clesceri et al. 1998). On Aug 5, Sep 24, and Oct 16, additional samples were collected for analysis of volatile fatty acids (VFAs). These samples were kept frozen until shipped to InnoTech Alberta Laboratory (Vegreville, AB). The VFA analysis was done through headspace gas chromatography using a DB-FFAP column on a Varian CP-3800 gas chromatograph with a flame ionization detector (Agilent Technologies, Santa Clara, CA) as described by Apelt et al. (2016). Individual VFA concentrations were calculated by comparing peak areas corresponding to calibrated standards of formic acid, acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, valeric acid, 4-methylvaleric acid, hexanoic acid, and heptanoic acid (Apelt et al. 2016).
Supplemental water was applied on a weekly basis to offset volume loss due to evaporation. Each tank, therefore received a unique volume of water based on evaporative loss. Water was added using a sprinkler to simulate rainfall and measured each watering day using a flow meter.
Data analysis
For a more direct comparison, cumulative gas fluxes were also scaled by the volume of manure as:
where Fv is the flux scaled by volume (g m−3), F is the flux scaled by area (g m−2), h is the depth of the manure within the tank. Tank volumes are shown in Table 1.
To account for variation between tanks of available N in the fresh manure, the cumulative N2O and NH3 flux was scaled by total TN and TAN (kg tank−1) in fresh manure. Similarly, the daily CH4 flux was scaled by total VS (kg tank−1) in fresh manure, which represents the available substrates for methane production. Additionally, the methane conversion factor (MCF), which is the ratio of CH4 produced compared to the maximum potential CH4 (Bo), was calculated following methods published by the Intergovernmental Panel on Climate Change (IPCC) (Dong et al. 2006). The Bo used to calculate the MCF was the IPCC default value of 0.24 m3 CH4 kg−1 VS (Dong et al. 2006).
Total GHG emissions for each tank were calculated as a sum of CH4, direct N2O, and indirect N2O on a 100-year CO2-eq basis to compare on the basis of their global warming potential. The global warming potentials used for conversions were 34 for CH4 and 298 for N2O (IPCC 2014). The conversion factor for calculating indirect N2O–N from NH3–N was 0.01 (Dong et al. 2006).
For all averaged data, the standard error of the mean (SEM) was calculated and expressed as mean ± SEM in tables or text and as error bars in figures.
Results
Environmental and manure parameters
The average ambient air temperature at the nearest Environment Canada weather station (Debert, NS, Station ID 8,201,380, ~ 15 km from the research site) from June to Oct was 15 °C. The monthly averages were within 1 °C of the 30 year normals. For July and Aug, when the CH4 emissions and ambient air temperature were the highest, the average manure temperature in the gradually-filled tanks (19.6 ± 0.081 °C) was 2.2 °C warmer than in the batch-filled tanks (17.4 ± 0.074 °C). Over the monitoring period (final filling date July 15–Oct 1), the gradually-filled tanks had on average 1.8 °C warmer manure compared to the batch-filled tanks (19.1 ± 0.11 °C vs. 17.3 ± 0.06 °C; Fig. 1). This difference was not seen at 150 cm manure depth, however, where the gradually and batch-filled tanks were consistently similar over the entire monitoring period (14.5 ± 0.11 °C and 14.7 ± 0.06 °C, respectively).
The VS of fresh manure was on average 49 ± 1.78% (dry basis), which decreased to 42 ± 0.89% at the end of the trial (Sep 24) (Table 2). During storage, VS content dropped in all tanks except the 0% gradually-filled tank (Tank 6) (Table 2). The DM of the fresh manure was on average 14.7 ± 1.36% (max = 19.9%; min = 10.7%; Table 2). The DM, including sand from the bedding, settled into a thick sludge layer deposited at the bottom of the tanks (~ 0.5 m thick).
The average N of fresh manure was 2.5 ± 0.14% (dry basis), while TAN was 0.6 ± 0.042% (Table 2). The average pH of the fresh manure was 7.0 ± 0.03, but increased to pH 7.8 ± 0.03 at the end of the study (Sep 24). There were no marked differences in N, TAN, or pH between tanks (Table 2).
Between the two sampling dates, Aug 5–Sep 24, there was an 88% drop in the total VFAs in all tanks (Fig. 2). On both dates, VFAs were highest in the 0% inoculum tanks, while the 10% inoculum tanks were 61% (Aug 5) and 97% (Sep 24) lower, and the 20% inoculum tanks were 85% (Sep 24) and 97% (Sep 24) lower (Fig. 2). On Aug 5, all tanks had elevated propionic acid relative to other VFAs, but by Sep 24 propionic acid was only elevated in the 0% inoculum tanks.
Methane emissions
The average daily CH4 emissions from all tanks were 79.5 ± 6.60 g m−2 day−1 for the entire 122 day monitoring period and the cumulative emissions were 5.88 ± 0.536 kg m−3 (Table 3). The CH4 emission curves of all tanks demonstrated a lag phase as microbes established in the manure, followed by a period of rapidly increasing emissions and a subsequent decrease as temperatures declined (Zeeman 1994; Le Riche et al. 2017; Habtewold et al. 2018). Most tanks had a similar length lag phase of ~ 30 day (Fig. 3), with the exception of 0% inoculum gradually-filled tank, where the lag phase was nearly twice as long (~ 60 day). Once the flux peaked, there was a period of elevated emissions lasting up to 5 weeks in July and Aug. The variability between tanks was higher during this phase (107 ± 17.0 g m−2 day−1), compared to the lag phase (17.3 ± 1.82 g m−2 day−1) and the post-peak phase (78.2 ± 3.55 g m−2 day−1) (Fig. 3). Additionally, of the total emissions from all the tanks, July made up 29% and Aug 44%, suggesting that differences in filling strategies were most important during the months of July and Aug when emissions were highest.
There was a small difference between the filling types, with batch-filled tanks producing 12% less CH4 than gradually-filled tanks when scaled by manure volume (5.49 ± 0.104 vs. 6.26 ± 1.13 kg m−3). This difference was also present when scaled by VS (83.8 ± 2.74 vs. 75.4 ± 16.2 g kg−1 VS) (Table 3; Fig. 3).
Emissions of CH4 differed between the tanks with no inoculum (0%) and those with inoculum (10% and 20%). When CH4 emissions were averaged by inoculum level, the 0% inoculum tanks had the least emissions (4.84 ± 0.811 kg m−3), producing 25% less than the 10% inoculum tanks (6.48 ± 1.19 kg m−3) and 23% less than the 20% inoculum tanks (6.31 ± 0.777 kg m−3).
There was no substantial difference between the 10% and 20% inoculum level tanks. Tanks containing inoculum (10% and 20%) had more CH4 emissions in gradually-filled (7.38 ± 0.288 kg m−3), than batch-filled tanks (5.42 ± 0.121 kg m−3) representing a 27% difference. This demonstrates that tanks with inoculum have higher CH4 emissions in continuously-filled tanks.
In the 0% inoculant tanks, CH4 emissions had opposite results; the gradually-filled tank had 29% fewer CH4 emissions compared to the batch-filled tank (5.65 vs. 4.03 kg m−3).
Nitrous oxide emissions
The average N2O emissions from all tanks was 30.5 ± 4.67 mg m−2 day−1 for the entire monitoring period and the cumulative emissions were 1.88 ± 0.27 g m−3 over 122 day (Table 3). There was no marked difference in the cumulative N2O emissions between batch-filled (1.92 ± 0.44 g m−3) and gradually-filled tanks (1.84 ± 0.43 g m−3) as shown in Fig. 4.
The amount of inoculum did not seem to have a discernible effect on the amount of N2O emissions. The largest emissions were from the tanks with 10% inoculum (2.63 ± 0.02 g m−3), which was nearly double both the 0% inoculum (1.46 ± 0.34 g m−3) and the 20% inoculum (1.56 ± 0.43 g m−3) tank emissions. This pattern remained similar when N2O emissions were scaled by TAN and TN (Fig. 4).
Ammonia
The average NH3 emissions from all tanks were 3.7 ± 0.36 g m−2 day−1 and cumulative emissions were 294 ± 24.4 g m−3 over the 122 day monitoring period (Table 3). The gradually-filled tanks produced consistently higher NH3 emissions throughout the study (Fig. 4). In total, they produced 28% more on a volume basis (259 ± 38.1 vs. 328 ± 7.16 g m−3). The largest difference between filling type was in June, where the batch filled tanks emitted 53% less NH3 (42.7 ± 5.36 vs. 80.3 ± 9.62 g m−3 month−1). From July to Sep batch filled tanks emitted on average 24% less NH3, with largest occurring throughout the month of Aug (102 ± 2.98 vs. 72.3 ± 17.1 g m−3 month−1). By the last two weeks of the monitoring period, emissions from gradual and batch-filled tanks were nearly identical (32.8 ± 1.86 vs. 31.0 ± 0.59 g m−3).
The amount of inoculum did not correlate linearly with NH3 emissions. On average, tanks with 10% inoculum emitted the most NH3 (331 ± 4.73 g m−3 or 524 ± 50.8 g m−2) and the 0% (281 ± 60.9 g m−3 or 422 ± 44.9 g m−2) and 20% (270 ± 47.4 g m−3 or 427 ± 46.1 g m−2) inoculum tanks differed from each other by < 5%.
CO 2 -equivalent emissions
For all tanks, the N2O emissions from direct (N2O) and indirect (NH3–N) sources contributed < 2% of the total CO2-eq GHG emissions (Table 4). The remaining 98% was from CH4 which was due to the anaerobic environment in liquid manure which agrees with what has previously been reported (Le Riche et al. 2017; VanderZaag et al. 2009; Wood et al. 2013). Overall, gradually-filled tanks emitted 12.4% more CO2-eq GHGs on a volume basis compared to batch-filled tanks. Considering only inoculated tanks, gradually-filled tanks emitted 26.5% more than batch-filled inoculated tanks. The 0% inoculum tanks emitted 24.2% fewer GHGs on a volumetric basis compared to the 10% and 20% inoculum tanks.
Discussion
Our results are comparable to those by Le Riche et al. (2017) who monitored manure mixed with sand bedding from the same farm for 207 day (Table 3). Our study produced > 50% more total CH4 (g m−2) compared to Le Riche et al. (2017). This was likely due to the higher VS content (26%) of the manure in this study which was double that of Le Riche et al. (2017). This is reflected by the MCF values which were slightly higher in our study (Table 3).
Gradually-filled tanks produced on average more (12.3%) CO2-eq GHGs compared to batch-filled tanks due to contributions of CH4 and NH3, while filling type had little effect on N2O emissions. It is important to note, that 100% of the manure in batch filled tanks was stored for 122 day, while in the gradually filled tanks 1/3 manure volume was stored for 122 day, another 1/3 volume for 102 day, and the last 1/3 volume for only 79 day. Therefore, if emissions were scaled by average storage length (101 day—gradual and 122 day—batch), the difference in emissions becomes larger (27%).
Increased emissions could be related to the higher manure temperature observed in gradually-filled tanks. Temperature is an important factor for both NH3 volatilization and CH4 production. NH3 volatilization is temperature dependent, where NH3 solubility in liquid decreases as temperature increases (Dewes 1996; Van der Stelt et al. 2007). Similarly, CH4 production is known to increase with rising temperature (Massé et al. 2003; VanderZaag et al. 2010b). In fact temperature differences, even at low ranges, have been shown to change CH4 emissions markedly. For example, Massé et al. (2003, 2008) measured CH4 emissions from various slurry types at temperatures between 10 and 20 °C and found consistently higher (50–65%) emissions at higher temperatures. The IPCC MCFs increase by 22% for a 2 °C increase in temperature, i.e. 0.32 at 17 °C and 0.39 at 19 °C for liquid slurry (Dong et al. 2006). Therefore, the 12% increase in CH4 emissions observed in this study is consistent with the gradually-filled tanks having 1.8 °C warmer manure compared to batch-filled tanks (19.1 ± 0.11 °C vs. 17.3 ± 0.06 °C).
The presence of inoculum increased overall CO2-eq GHG emissions, due to increased emissions of CH4. This is consistent with Wood et al. (2014), who found that tanks with inoculum had a shorter CH4 production lag phase, which indicates higher microbial growth compared to tanks with no inoculum. Microbial growth is reflected in the VFA results, where tanks with inoculum had consistently the lowest amounts. The breakdown of organic matter in the manure creates VFAs, which are further degraded by methanogens to produce CH4 (Lyberatos and Skiadas 1999; Mao et al. 2015). Therefore, lower VFAs reflect continued microbial activity as CH4 is produced. Indeed, Habtewold et al. (2018) reported a higher abundance of methanogens and bacteria in tanks with inoculum compared to tanks with no inoculum.
Ngwabie et al. (2016) reported a linear relationship between inoculum level and CH4 emissions over 163 day of liquid dairy manure storage. However, this study saw no difference between 10 and 20% inoculum. The reason for this difference is unclear, although it could be due to differences in fresh manure or in inoculum microbial abundance due to age, storage conditions, or manure characteristics (Habtewold et al. 2018).
The highest emissions were from inoculated, gradually-filled tanks. As already discussed, both inoculum presence and gradual-filling on average increased emissions, therefore it follows that these tanks would be the highest producing. Inoculum presence and gradual-filling also reduces the ratio of substrate to microorganisms, which leads to higher emissions. Higher concentrations of substrate will increase the rate of microbial degradation, creating an excess of VFAs and reducing the pH of manure. The observed pH varied little between tanks, though the pH of the 0% batch tank was slightly lower compared to the other tanks. On a farm scale, the effects of gradual filling may be greater, as fresh manure is added in comparatively smaller amounts, more frequently. On the other hand, laboratory research which uses batch filling with or without inoculum, may underestimate emissions compared to farm-scale emissions.
Conclusion
This study used 11.4 m3 tanks to study the effect of gradual versus batch filling on manure storage tanks with 0%, 10% and 20% inoculum. Our results show that tanks containing inoculum emit more total CO2-eq GHGs when filled gradually. Both CH4 and NH3 emissions were highest in gradually-filled tanks with inoculum, while N2O did not exhibit any clear relationship with fill type. Higher manure temperature and lower substrate/microbe ratio were key factors which might have contributed to these higher emissions in gradually-filled tanks. For both fill-types, tanks without inoculum produced the least CH4, N2O, and NH3. This resulted in 24% fewer total CO2-eq emissions when no inoculum was present. Our results suggest that batch-filling experiments underestimate emissions compared to gradual filling.
References
Apelt M, Benndorf R, Fischer PU, Hofmann J, Lorenz H, Muehlenberg J, Kim YS, Pröter J, Reinelt T, Schumacher B, Strach K, Weinrich S, Westerkamp T, Zechendorf M, Kube J, Jobst K, Lincke M, Lomtscher A, Bernhard S, Gómez JIS, Gerstner A, Jović A, Cuhls C, Kleyböcker A, Lienen T, Würdemann H, Möller LA, Goersch K, Köster Y, Zehnsdorf AD-I, Ramhold D, Zosel J, Junne S, Kielhorn E, Neubauer P, Dominik P, Zirkler D, Kraume M, Brehmer M, Sner CH, Kugelstadt O, Bahr T, Fricke K, Oechsner H, Engler N (2016) Collection of measurement methods for biogas—methods to determine parameters for analysis purposes and parameters that describe processes in the biogas sector. Deutsches Biomasseforschungszentrum gemeinnutzige GmbH, Leipzig
Baral KR, Jégo G, Amon B, Bol R, Chantigny MH, Olesen JE, Petersen SO (2018) Greenhouse gas emissions during storage of manure and digestates: key role of methane for prediction and mitigation. Agric Syst 166:26–35. https://doi.org/10.1016/j.agsy.2018.07.009
Burke D (2001) Dairy waste anaerobic digestion handbook. Environmental Energy Company, Olympia
Clesceri LS, Greenber AE, Eaton AD (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, American Water Works association, Water Environment Federation, Washington, DC
Dewes T (1996) Effect of pH, temperature, amount of litter and storage density on ammonia emissions from stable manure. J Agric Sci 127:501–509. https://doi.org/10.1017/S0021859600078722
Dong H, Mangino J, McAllister T, Hatfield JL, Johnson D, Lassey K, Aparecida de Lima M, Romanovskaya A (2006) Chapter 10 emissions from livestock and manure management. In: 2006 IPCC guidelines for national greenhouse gas inventories. IPCC, pp 10.1–10.87
Habtewold J, Gordon R, Sokolov V, VanderZaag A, Wagner-Riddle C, Dunfield K (2018) Targeting Bacteria and Methanogens To Understand the Role of Residual Slurry as an Inoculant in Stored Liquid Dairy Manure. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02830-17
Hofer S (2003) Determination of ammonia (Salicylate) in 2 M KCl soil extracts by flow injection analysis. Lachat Instruments, Loveland
IPCC (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva
Jayasundara S, Ranga Niroshan Appuhamy JAD, Kebreab E, Wagner-Riddle C (2016) Methane and nitrous oxide emissions from Canadian dairy farms and mitigation options: an updated review. Can J Anim Sci 96:306–331. https://doi.org/10.1139/cjas-2015-0111
Kavanagh I, Burchill W, Healy MG, Fenton O, Krol DJ, Lanigan GJ (2019) Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J Clean Prod 237:117822. https://doi.org/10.1016/j.jclepro.2019.117822
Krupa SV (2003) Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environ Pollut 124:179–221. https://doi.org/10.1016/S0269-7491(02)00434-7
Le Riche EL, Vanderzaag A, Wagner-Riddle C, Dunfield KE, Sokolov VK, Gordon R (2017) Do volatile solids from bedding materials increase greenhouse gas emissions for stored dairy manure? Can J Soil Sci 97:512–521
Livingston GP, Hutchinson GL (1995) Enclosure-based measurement of trace gas exchange: applications and sources of error. Biog Trace Gases Meas Emiss Soil Water 51:14–51
Lyberatos G, Skiadas IV (1999) Modelling of anaerobic digestion—a review. Glob Nest Int J 1:63–76
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sustain Energy Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Massé DI, Croteau F, Patni NK, Masse L (2003) Methane emissions from dairy cow and swine manure slurries stored at 10 °C and 15 °C. Can Biosyst Eng 45:1–6
Møller HB, Sommer SG, Ahring BK (2004) Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. Methane Product Nutr Recove Manure 74:496
Ngwabie NM, Gordon RJ, VanderZaag A, Dunfield K, Sissoko A, Wagner-Riddle C (2016) The extent of manure removal from storages and its impact on gaseous emissions. J Environ Qual 45:2023–2029. https://doi.org/10.2134/jeq2016.01.0004
Sheppard S, Bittman S, Swift M, Beaulieu M, Sheppard M (2011) Ecoregion and farm size differences in dairy feed and manure nitrogen management: a survey. Can J Anim Sci 91:459–473. https://doi.org/10.4141/cjas2010-004
Sommer SG, Petersen SO, Sorensen P, Poulsen HD, Moller HB (2007) Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr Cycl Agroecosystems 78:27–36. https://doi.org/10.1007/s10705-006-9072-4
Van der Stelt B, Temminghoff EJM, Van Vliet PCJ, Van Riemsdijk WH (2007) Volatilization of ammonia from manure as affected by manure additives, temperature and mixing. Bioresour Technol 98:3449–3455. https://doi.org/10.1016/j.biortech.2006.11.004
VanderZaag AC, Gordon RJ, Jamieson RC, Burton DL, Stratton GW (2009) Gas emissions from straw covered liquid dairy manure during summer storage and autumn agitation. Trans ASABE 52:599–608
VanderZaag AC, Gordon RJ, Jamieson RC, Burton DL, Stratton GW (2010a) Permeable synthetic covers for controlling emissions from liquid dairy manure. Appl Eng Agric 26:287–297
VanderZaag AC, Gordon RJ, Jamieson RC, Burton DL, Stratton GW (2010b) Effects of winter storage conditions and subsequent agitation on gaseous emissions from liquid dairy manure. Can J Soil Sci 90:229–239
VanderZaag AC, Baldé H, Crolla A, Gordon RJ, Ngwabie NM, Wagner-Riddle C, Desjardins R, MacDonald JD (2017) Potential methane emission reductions for two manure treatment technologies. Environ Technol. https://doi.org/10.1080/09593330.2017.1313317
Wood JD, Gordon RJ, Wagner-Riddle C (2013) Biases in discrete CH4 and N2O sampling protocols associated with temporal variation of gas fluxes from manure storage systems. Agric For Meteorol 171–172:295–305. https://doi.org/10.1016/j.agrformet.2012.12.014
Wood JD, VanderZaag AC, Wagner-Riddle C, Smith EL, Gordon RJ (2014) Gas emissions from liquid dairy manure: complete versus partial storage emptying. Nutr Cycl Agroecosyst 99:95–105. https://doi.org/10.1007/s10705-014-9620-2
Zeeman G (1994) Methane production/emission in storages for animal manure. Nutr Cycl Agroecosyst 37:207–211
Acknowledgements
Funding was provided by Natural Sciences and Engineering Research Council of Canada and Wilfrid Laurier University. We thank John McCabe, Donna MacLennan, Rod MacLennan, Ulrica McKim and Earl Jenson for their technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sokolov, V., VanderZaag, A., Habtewold, J. et al. Greenhouse gas emissions from gradually-filled liquid dairy manure storages with different levels of inoculant. Nutr Cycl Agroecosyst 115, 455–467 (2019). https://doi.org/10.1007/s10705-019-10023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-019-10023-2