Abstract
When manure slurry is removed from storages for land application, there is often ‘aged’ manure that remains because the storages are not completely emptied. Aged manure may act as an inoculum and alter subsequent methane (CH4), nitrous oxide (N2O) and ammonia (NH3) emissions when fresh manure is added to the system, compared to an empty storage that is filled with fresh manure. Completely emptying manure storages may be a practice to decrease gas emissions, however, little pilot-scale research has been conducted to directly quantify the inoculum effect. Therefore, we compared CH4, N2O, and NH3 emissions from three pilot-scale slurry tanks (~10.5 m3 each) filled with a mixture of fresh manure and an inoculum of previously stored manure (i.e., partial emptying) to three tanks that contained only fresh manure (i.e., complete emptying). Gas fluxes were continuously measured over 155 d of warm season storage using flow-through steady-state chambers. The absence of an inoculum significantly reduced CH4 emissions by 56 % compared to partially emptied (inoculated) tanks, while there was no difference in N2O emissions. There was a significant 49 % reduction in greenhouse gas (GHG) emissions because the overall budget (as CO2-eq) was dominated by CH4. Complete manure storage emptying could be an effective GHG mitigation strategy; however, NH3 emissions were significantly higher from un-inoculated tanks due to slower crust formation. Therefore additional NH3 abatement should be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many livestock production systems, manure is stored as a liquid or a slurry for extended periods in concrete tanks, earthen basins or lagoons prior to land application (Sheppard et al. 2011). These types of storage systems can be significant sources of greenhouse gases (GHG) including methane (CH4) and nitrous oxide (N2O); as well as ammonia (NH3) (Bjorneberg et al. 2009; Harper et al. 2000; Leytem et al. 2011, 2013; McGinn and Beauchemin 2012). Due to the environmental impacts of these gases, there is a need to mitigate emissions to reduce the environmental footprint of farming and improve agricultural sustainability.
Canadian dairy production largely occurs in 10 of the 12 ecoregions defined by the Soil Landscapes of Canada Working Group (Sheppard et al. 2011) that are mainly classified as warm summer continental or continental subarctic climates (Kottek et al. 2006). It is common practice for farmers to pump out storages and apply manure post-harvest (fall) and pre-plant (spring) (Sheppard et al. 2011), therefore there are typically two extended manure storage periods—the (1) warm summer and (2) cold winter. In Canadian cold climate conditions, winter-time emissions are typically much lower than during warmer periods (Park et al. 2006; VanderZaag et al. 2010a). Due to the temperature sensitivity of gas production reactions (Sommer et al. 2007), alternative warm season manure management practices are likely to offer the greatest opportunity for absolute emission reductions. This present research therefore focussed on investigating a possible mitigation strategy for warm season storage. Specifically, we examined the mitigation potential of completely emptying manure storages, in comparison to the common practice of partial emptying.
Managing the degree to which manure storages are emptied for land application may be a method to reduce gas emissions. Typically, when storage structures are emptied there is ‘aged’ manure that remains, which is subsequently mixed with fresh manure as the system is refilled. This aged manure may act as a biological and chemical inoculum, potentially affecting carbon (C) and nitrogen (N) turnover within the storage (Sommer et al. 2007), and ultimately the GHG and NH3 budgets.
Previous studies have noted lag phases of 1–9 months before the onset of increased CH4 emissions, when manure was loaded into clean storage vessels (Massé et al. 2008), or pilot-scale structures (VanderZaag et al. 2008, 2009, 2010b; Wood et al. 2012). This lag phase may be associated with the time required for methanogen and syntropic anaerobic microbial communities to establish. Lab experiments have demonstrated that when storages lack an inoculum (i.e., aged manure), it is possible that the absence of acclimated microbial communities will slow the anaerobic degradation of freshly added manure, and thus reduce CH4 emissions (Sommer et al. 2007). Rodhe et al. (2012) reported relatively rapid increases in CH4 fluxes when fresh pig manure was added to an inoculum, but could not quantify the inoculum effect because they did not have non-inoculated tanks for comparison. Massé et al. (2008) used modeling to extend the results of laboratory incubations, and recommended more frequent and complete emptying of manure storages as an effective management practice to reduce CH4 emissions.
The aforementioned studies investigating inoculum effects have not considered the impacts on N2O and NH3 emissions. If crusts form on the surface of slurries N2O may be produced (Sommer et al. 2000; Wood et al. 2012), while NH3 emissions may be reduced by up to ~50 % (Sommer et al. 1993; Misselbrook et al. 2005; Olesen and Sommer 1993). Previous research on crust dynamics and nitrogenous gas emissions have been conducted at the pilot-scale using fresh manure. There are currently no data available on how the presence of an inoculum influences the potential for crust formation and how this affects N2O and NH3 emissions. Several studies have noted that crust formation is induced by the onset of high CH4 fluxes (Wood et al. 2012; VanderZaag et al. 2009, 2010b). If the absence of an inoculum decreases CH4 production then crust formation may be inhibited. Without a crust, N2O production is low (Sommer et al. 2000; Wood et al. 2012), therefore inhibiting crust formation may decrease N2O emissions. However, inhibiting crust formation may extend the length of the time that the surface is open to the atmosphere, and thus increase NH3 emissions (Wood et al. 2012).
There is a need to quantify the inoculum effect under conditions that are more reflective of farming conditions, where surface crusts can form and temperature varies in both space and time; and to consider CH4, N2O and NH3 emissions simultaneously to identify possible GHG–NH3 emission trade-offs. Therefore, the objectives of this study were to examine the effect of a manure inoculum on subsequent CH4, N2O and NH3 emissions during summer/fall (June to Nov.) storage. Our hypothesis was that completely emptying tanks to remove the inoculum would reduce GHG emissions due to decreased CH4 fluxes, compared to inoculated tanks. We also hypothesized that the higher CH4 fluxes from inoculated tanks would promote crust formation, causing higher N2O emissions and lower NH3 emissions relative to non-inoculated tanks.
Methods
Site description
Liquid dairy manure (~10.5 m3) was stored in six concrete tanks at a previously described site (VanderZaag et al. 2010b; Wood et al. 2012) located at the Bio-Environmental Engineering Center (BEEC) at Dalhousie University in Truro, NS, Canada (45°45′N, 62°50′W) from June–Nov 2011 (155 d). Each tank was considered an experimental unit and randomly assigned one of the two treatments. Three tanks were completely emptied and filled with fresh manure, which hereafter will be referred to as the ‘non-inoculated’ treatment. Three other tanks were only partially emptied and hence had a volume of aged manure, to which fresh manure was added, which hereafter will be referred to as the ‘inoculated’ treatment.
The inoculum consisted of manure that was stored in the tanks during the previous 192 d (Nov–Jun). The surface crusts on three tanks were manually destroyed and mixed with the slurry, after which manure was removed until an arbitrary depth of 80 cm remained using a vacuum tanker. Fresh manure was then added to these tanks to bring the total depth to 160 cm. As a result, this provided an inoculum of 50 % by volume. This was considered to be the upper-end of typical ranges that might be experienced on commercial farms, as a possible worst-case scenario reflective of when conditions do not permit more complete emptying. The three tanks assigned the non-inoculated treatment were completely emptied using the vacuum tanker, and the walls sprayed down with a fire hose before fresh manure was added to a depth of 160 cm.
The dairy herd at the experimental farm consists of 40 lactating Holstein–Friesian cows. In the winter the cows were fed a ration at ~45–50 kg cow−1 d−1 and cows were in the barn 24 h d−1. The ration was 27 % corn silage, 47 % grass silage and 26 % concentrate. The concentrate consisted of barley (23.5 %), soyameal (20 %), corn (44.5 %), limestone (1.5 %), top soy (5 %) and micro-ingredients (5.5 %). In the warm months the cows were pastured for 19 h d−1, and fed 5 kg cow−1 d−1 of feed ration when in the barn. The main ingredients of the summer feed mix were corn (31 %), wheat (12 %), barley (15 %), soyameal (12 %), soyhulls (18 %) and top soy (2 %). The average feed N intake was 598 and 643 g cow−1 d−1 in the winter and summer, respectively; and the average milk yield for the year was 36.1 kg cow−1 d−1. Note that the aged manure that was used as the inoculum originated from cows on the winter diet, while the fresh manure originated from cows on the summer diet.
Manure characterization
At the start of the trial, one manure sample was collected from each full, homogenized tank. At the end of the trial, two samples were collected from each tank—one 5 cm below the crust, and a second 10 cm above the bottom. For each analyte, the mean of the two depths was taken and used when computing treatment means for the end of trial data. The TS and volatile solids (VS) were determined according to standard American Public Health Association (APHA) methods 2540 B: total solids dried at 103–105 °C, and 2450 E: fixed and VS ignited at 550 °C, respectively (Clesceri et al. 1998). Total ammoniacal N (TAN) was quantified by distillation (APHA 4500–NH3 B) and titration (APHA 4500–NH3 D). C and N were quantified by combustion using a Leco model 1000 CNS analyzer (LECO Corp., St. Joseph’s MI). The pH was measured using an electrode (APHA 4500–H+). Surface crusts were visually characterized on a weekly basis for % cover and whether the surface was moist or dry.
Environmental measurements
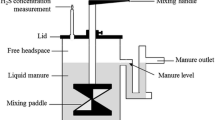
The air temperature (Ta) in each chamber was measured using copper/constantan (Type T) thermocouples with three measurement junctions wired to a common lead running to a CR3000 datalogger (Campbell Scientific Inc., Logan, UT). The shielded measurement junctions were suspended ~0.5 m above the slurry surface and regularly spaced along the length of the chamber. Manure temperatures (TM) were measured 10 cm above the bottom of each tank using a Type T thermocouple that had three measurement junctions wired to a common lead running to the data logger. One minute average data were recorded.
Flux measurements
Steady state chambers
Gas fluxes were measured using permanently fitted, flow-through steady state chambers (Livingston and Hutchinson 1995; Rochette and Hutchinson 2005) that completely enclosed each ~6.6 m2 tank (Fig. S1 in electronic supplementary information). The chamber frames were constructed from aluminum tubing and covered with 6-mil greenhouse plastic (refer to electronic supplementary information for photographs of a chamber). Air entered each chamber inlet through three louvered openings at the end opposite an exhaust venturi. Air was drawn through each chamber by a fan located in the venturi. The nominal airflow rate was set at 0.5 m3 s−1, which exchanged the chamber air volume 1–2 times per minute. The windspeed ~50 cm from the manure surface was typically 0.4–0.5 m s−1. Fluxes (mg m−2 s−1) were calculated according to:
where C out and C in are the concentrations (mg m−3) of target gas in outlet and inlet air, respectively; A is the surface area (m2) and Q is the airflow rate (m3 s−1). The C out was measured in each exhaust venturi, while the C in was measured at two locations at a height of 1.7 m and 50 cm upstream of the inlets of chambers 2 and 5. The air velocity in each venturi was continuously measured with a cup anemometer (Davis Instruments, Hayward, CA), and the airflow rate calculated as the product of the air velocity and the cross sectional area of the venturi. One minute average air velocities were recorded with a CR1000 datalogger (Campbell Scientific Inc.).
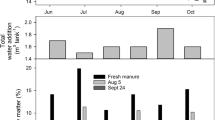
When using chambers to measure gas fluxes, modifications of the enclosed microclimate compared to ambient conditions are unavoidable. We took steps to minimize these effects. Rainfall was simulated inside each chamber using lawn sprinklers to maintain a near neutral water balance. Each week from June through 17 Oct., the weekly normal rainfall for Truro NS was added over two irrigations. The airflow rates were set to minimize chamber-ambient temperature differences, however, the problem could not be eliminated entirely on the warmest days because the plastic transmitted solar radiation. 65 % of hourly chamber-ambient air temperature differences were within ±2 °C, 85 % within ±4 °C. On a daily basis, which corresponds to the shortest time-average fluxes that we report here, the mean temperature differences was 1.8 ± 0.05 °C (mean ± SD). It is important to note that the chamber-ambient temperature differences were the same for all chambers, and thus did not affect treatment comparisons.
Methane and nitrous oxide concentrations
The CH4 and N2O monitoring system has been previously described in greater detail (Wood et al. 2012), thus only a brief description will be provided here. Filtered (Acro 50, 0.2 μm, Pall Canada Ltd., Mississauga, ON) sample air was continuously drawn from all eight intakes through polyethylene tubing to a trailer which housed the sampling system and gas analyzers. The sample tubing was plumbed into an 8 × 2 valve manifold inside the trailer, which at any time directed sample air from two sites to the analyzers (in parallel), and exhausted sample air from the other six. Water vapor was removed from sample air between the manifold and the analyzers by driers (Perma Pure LLC, Toms River, NJ). There were two tunable diode laser trace gas analyzers (TGA100A; Campbell Scientific Inc.), one for measuring CH4 and the other for N2O. A CR5000 datalogger (Campbell Scientific Inc.) recorded TGA data and controlled the sampling system. The valves were switched every 30 s to continuously cycle through the sample intakes. A complete cycle through all eight sample sites took 4 min. To permit flushing of the sample cell, the data from the first 10 s after a valve switch were discarded. Each TGA had certified reference gas (Air Liquide Inc., Stellarton, NS) continuously flowing through a reference cell to provide the template of the absorption feature. The measured dry air mixing ratios were converted to concentrations (mg m−3) assuming constant pressure (101.3 kPa) and temperature (293 K). Every 4 min, an average flux was computed for each chamber according to Eq. (1), from which daily means were calculated. Flux units were converted to g m−2 d−1. CH4 and N2O flux calculations and data filtering were performed using MATLAB (R2008b; The Mathworks Inc., Natick, MA).
Ammonia concentrations
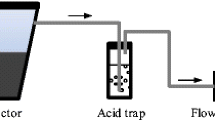
The NH3 concentrations in sample air drawn from each of eight intakes was measured using a gas washing technique (VanderZaag et al. 2010b; Wood et al. 2012) three times per week. During each 24 h deployment, unfiltered sample air was drawn through pre-conditioned tubing into a sampling shed which housed acid traps. Sample air was diffused into each acid trap by a dispersion tube with a fritted cylindrical end (Ace Glass, Vineland, NJ). The air flow through each sample line was measured with an in-line flow meter (Actaris Metering Systems, Greenwood, SC) that was located between the acid traps and pumps. There were four sample pumps (Thomas Pumps and Compressors, Sheboygan, WI), each drawing sample air from two sites. The sample airflow rate for each sampling line was set at 1.5 L min−1 by a critical orifice (O’Keefe Controls, Trumbull, CT) attached to the pump.
Acid traps were filled with phosphoric acid (0.005 M), and at the end of each deployment the volume was standardized to 0.125 L. The entire trap volume was then transferred to a sample bottle with subsequent quantification of NH3 by the standard phenate method, 4500-NH3 F (Clesceri et al. 1998). The time-averaged NH3 concentration in the sample air, CNH3,air (mg m−3), was calculated according to:
where CNH3,aq is the aqueous NH3 concentration (mg L−1), Vl is the standardized volume of the acid trap (0.125 L) and Vair is the volume of air that passed through the trap during the deployment (m3). The 24 h time-averaged NH3 concentrations from Eq. (2) were used in Eq. (1) to calculate daily average fluxes, and units were then converted to g m−2 d−1.
Calculations and statistical analyses
The total GHG budget (as CO2-eq) was calculated including contributions from CH4, N2O and NH3. Global warming potentials (100 y) of 25 and 298 were used for CH4 and N2O, respectively (Forster et al. 2007). The contribution of NH3 to the GHG budget was accounted for as indirect N2O emissions by assuming 1 % of emitted NH3–N was converted to N2O–N (Dong et al. 2006). Indirect N2O–N emissions were converted to a mass of N2O, which was then converted to CO2-eq as described previously.
Two-tailed t tests were used to test for differences in treatment means. If the variances were equal (according to an F test) Student’s t tests were used, otherwise the unequal variance t test was used. Statistics were computed using MS Excel. Means were considered significantly different when p < 0.05.
Results and discussion
Environmental and slurry surface conditions
Ambient daily Ta ranged from 1 to 26 °C with an average of 15 °C for the monitoring period. For context, the annual average Ta over the entire year at the site was 8.0 °C. For comparison, the 30-year climate normal (1971–2000) at the closest weather station is 5.8 °C (Truro, NS; Environment Canada 2012). The research site therefore falls into the “cool” category (≤10 °C) for IPCC (2006) manure management emission factors (Dong et al. 2006). Mean TM measured 10 cm above the bottom of the tanks were quite stable throughout the study (Fig. 1d), and averaged 12.1 °C.
Daily a methane (CH4), b nitrous oxide (N2O) and c ammonia (NH3) fluxes for inoculated and non-inoculated treatments (each symbol represents the mean of three tanks and error bars represent standard deviations); and d the daily air temperature outside the chambers and the mean manure temperature (10 cm above the bottom) of all six tanks
Crusts formed on the surfaces of all slurries. Within 3 weeks (by July 1), the inoculated tanks were mostly covered by a dry crust, with ~10 % of the surface remaining open. This was faster crust development than has been observed in prior studies at this site (VanderZaag et al. 2010b; Wood et al. 2012). Over the same time period, the non-inoculated tanks had largely open surfaces with some moist floating solids present. Although the non-inoculated tanks were eventually totally covered by crusts, the surfaces were visibly wet and contained cracks where liquid was visible until 17 July, when all tanks had crusts and no liquid was visible on either treatment.
Manure characteristics
The manure pH ranged from 7 to 8. The TS ranged from 10 to 14 % (Table 1). At the start of the trial the inoculated tanks had significantly higher TS, C, and P levels, and significantly lower TAN and K levels. These differences reflect the relatively large quantity of manure that remained as inoculum. Moreover, the inoculum which originated from cows on the winter diet, contained more solids and was difficult to pump, which was possibly due to a larger bedding fraction in this material due to 24 h confinement in the barn and slower VS degradation rates during winter storage.
Fluxes
Methane
Methane (CH4) fluxes were consistently higher from inoculated tanks from July through Oct (Fig. 1a). These fluxes from inoculated tanks increased consistently over the first ~2 months of storage and peaked in Sep. During Aug, and particularly in Sep, there was substantial variability in daily fluxes from inoculated tanks as indicated by the wide error bars associated with treatment means (Fig. 1a). This highlights the heterogeneity of manure storage systems, and underscores the value of high frequency flux measurements for accurately quantifying emission differences between treatments. Fluxes from non-inoculated tanks were typically lower than from inoculated slurries, and consistently ranged from 5–15 g m−2 d−1, with the highest variability in daily treatment means occurring in Sep (Fig. 1a). Monthly mean CH4 fluxes are provided in Table 2, along with Ta and TM data to highlight the importance of temperature as a regulator of emissions. Although monthly fluxes were not different in June (Table 2), mean daily fluxes from inoculated tanks were increasing through the end of the month, unlike the non-inoculated treatment, which remained consistent (Fig. 1a). This suggests that there was more rapid microbial growth in the inoculated tanks that supported higher rates of CH4 production. Monthly CH4 fluxes were significantly different until Nov, when presumably the low TM slowed production reactions in both inoculated and non-inoculated tanks. When fluxes were integrated over the entire storage period, significant (p < 0.05) differences in cumulative CH4 emissions (g m−2) were observed, regardless of the scaling that was used (Table 3). The average cumulative CH4 emissions (g m−2) from non-inoculated tanks were 56 % lower than inoculated tanks. Thus, completely removing the stored ‘aged’ manure resulted in a significant reduction in subsequent CH4 emissions.
A complicating factor in this analysis was that inoculated tanks contained, on average, ~22 % more TS and VS than non-inoculated tanks. It is therefore unclear whether the elevated CH4 emissions from inoculated tanks were due to the presence of established microbial communities, due to the retention of substrates (i.e., VS in the aged manure within the system, or a combination of both (Steed and Hashimoto 1994). When total emissions were scaled by the initial VS, they were also significantly lower (~45 %) for the non-inoculated treatment (Table 3).
The fact that VS scaled emissions were 11 % points lower that when scaling by area, suggests that the presence of an adapted consortium of microorganisms was perhaps the more important factor than substrate availability (Sommer et al. 2007). Further research employing molecular techniques to profile the microbial communities and their activities in the inoculum compared to fresh manure is needed to conclusively identify the processes responsible for increasing CH4 emissions from manure storages that are not completely emptied. It is, however, important to consider that the confounding effects of established microbial communities and increased quantity of substrate will also be present on farms, so from an emission inventory standpoint it matters little whether an inoculum increases CH4 emissions because of greater substrate retention within the system, a microbial inoculation effect, or both. That is, provided that the inventory method is able to account for an inoculum effect.
A final point of note is that additional research investigating simple methods to decrease the manure inoculum effect for times when complete emptying is not possible is warranted. For example, attempting to inhibit the microorganisms in the inoculum by treatment with acid treatment (Petersen et al. 2012) or other additives (Shah and Kolar 2012) may be able to achieve an effect that is similar to completely emptying the storage.
Nitrous oxide
The magnitudes of peak daily N2O fluxes were similar for inoculated and non-inoculated slurries but were offset in time by ~50 d (Fig. 1b). The rapid rise in N2O fluxes from inoculated tanks was associated with faster crust establishment on these slurries, compared to non-inoculated ones. In both treatments, N2O fluxes declined from peak values over a ~1 month period. Monthly mean N2O fluxes were higher from inoculated slurries in June and July; while they were higher from non-inoculated tanks in Aug and Sep (Table 2). There was no difference in emissions from inoculated and non-inoculated tanks when fluxes were integrated over the entire storage period (Table 3).
Crust characteristics controlled the temporal dynamics of N2O emissions (Fig. 1b; Table 2) in this experiment as the TN content and thermal environment of both treatments was similar. Large increases in N2O fluxes were not observed until there was appreciable crust formation. The month long decline from peak fluxes was possibly due to progressively thicker crust increasing resistance to NH3 diffusion or moving the level of the bulk slurry, which contains ammonium, further form the atmosphere—both of which could limit N availability for nitrification–denitrification near the crust-atmosphere interface (Petersen et al. 2005; Petersen and Miller 2006). Declining fluxes from inoculated slurries occurred during the warmest months, suggesting that temperature was not limiting N2O production reactions, further supporting that N availability may have been constraining emissions. The inoculum clearly has no impact on total N2O emissions when storage time is longer than 2 months during the summer. It is interesting that N2O contributed a greater fraction of the GHG budget for non-inoculated tanks (up to 30 %, Fig. 2), mainly due to the significantly lower CH4 emissions from these slurries. Therefore, if storages are completely emptied, methods to mitigate N2O emissions will have a greater impact on the GHG budget.
Overall greenhouse gas budgets for the inoculated and non-inoculated treatments including cumulative emissions of methane (CH4), nitrous oxide (N2O), and ammonia (NH3) where the percentages within stacked bars represent the relative contribution of the different gases to the overall GHG budget, and total emissions from the non-inoculated tanks were significantly (p < 0.05) lower than the inoculated treatment
Ammonia
Ammonia (NH3) fluxes were highest during the first 1.5–2 months for both treatments and declined as storage time increased (Fig. 1c; Table 2). During this time, fluxes from non-inoculated slurries were higher, more variable, and declined more slowly compared to inoculated tanks. The temporal dynamics of NH3 fluxes constrained the overall budget, with total emissions being significantly higher for non-inoculated slurries (Table 3), largely due to significantly higher monthly fluxes in July and Aug (Table 2).
The more rapid decline in NH3 fluxes from inoculated slurries was associated with faster crust formation on these tanks. Previous research has shown that complete cover by crusts can reduce NH3 emissions (Misselbrook et al. 2005; Sommer et al. 1993). However, it has been pointed out that if it takes several months for crusts to establish, their effectiveness for reducing total NH3 emissions over long-term (5–6 months) storage may be limited (Wood et al. 2012). Therefore, management practices that promote more rapid crust formation, such as partial tank emptying did in this study, should reduce total NH3 emissions. Changes in manure N concentrations were consistent with higher NH3 emissions from non-inoculated tanks (Tables 1 and 2), reflecting the lower N-retention and higher NH3 losses associated with complete emptying. The TN concentration decreased for both treatments, and the extent of the decrease was slightly greater in the non-inoculated tanks. NH3 emissions were responsible for substantially higher losses of slurry N compared to N2O (Table 3). This is further evidence that there is greater opportunity for N conservation to maintain fertilizer value through managing NH3 emissions.
Overall effect of complete tank emptying
The GHG budget (in CO2 equivalents, CO2-eq) including all three gases is provided in Fig. 2. Completely emptying the tanks reduced total GHG emissions by 49 % (p = 0.013) compared to partial emptying. This reduction was due to significant reductions of CH4, since both NH3 and N2O emissions either increased or were not significantly different (Table 3; Fig. 2). These results confirm the laboratory studies of Sommer et al. (2007) and simulation results of Massé et al. (2008), who recommended more frequent and complete emptying of storages, particularly during the summer, to decrease CH4 emissions. Furthermore, this study shows that when other GHGs are considered, completely emptying storage tanks still offers significant mitigation potential.
An important consideration is that there was a trade-off between GHGs and NH3. The CH4 emission reduction associated with complete emptying resulted in less favorable conditions for crust formation in comparison to inoculated tanks. There was thus a less effective barrier to slurry-atmosphere NH3 exchange on non-inoculated tanks, resulting in significantly higher NH3 emissions (more than triple). Therefore investigating NH3 abatement strategies to be used in combination with complete storage emptying should be considered to develop a more comprehensive gas mitigation strategy.
Future assessments should also attempt to determine the practicality and cost of implementing complete storage emptying at the farm-scale. Determining what constitutes a “completely empty” storage system is another important avenue of research. That is, is there a level of inoculum below which the effect on emissions is negligible? This could have particular significance on farms with earthen storages that are more difficult to completely empty, compared to concrete tanks. Alternatively, developing methods to treat residual manure to control the inoculum effect would perhaps be an important avenue of research to promote more widespread adoption. Another practical impediment to the effectiveness of complete storage emptying for reducing GHG emissions could be encountered on farms that store large quantities of manure beneath the barns for extended periods. With these types of systems controlling the inoculum effect in the outdoor storage may be a moot point, because the manure being transferred from the barn is in a sense an inoculum.
Conclusions
This research shows that completely emptying manure storages can reduce overall GHG and more specifically CH4 emissions during subsequent storage. There was a 49 % reduction in the total GHG budget associated with complete tank emptying, which was largely controlled by differences in CH4 emissions. There was, however, a trade-off between GHGs and NH3, which bears consideration. Further research is needed to ascertain whether the inoculum effect is due to an optimized consortium of syntrophic anaerobic bacteria and methanogens, or increased VS and/or methanogenic substrate availability. From a practical perspective there is a need to identify what constitutes a “completely empty” storage system, which could be accomplished by investigating a wider range of inocula (e.g., 0–25 %), and the practicality of complete emptying at the operational level on farms.
Abbreviations
- CO2-eq:
-
Carbon dioxide equivalents
- GHG:
-
Greenhouse gas
- TAN:
-
Total ammoniacal nitrogen
- TN:
-
Total nitrogen
- TS:
-
Total solids
- VS:
-
Volatile solids
References
Bjorneberg DL, Leytem AB, Westerman DT, Griffiths PR, Shao L, Pollard MJ (2009) Measurements of atmospheric ammonia, methane and nitrous oxide at a concentrated dairy production facility in Southern Idaho using open-path FTIR spectrometry. Trans ASABE 52(2):1749–1756
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington
Dong H, Mangino J, MacAllister T, Hatfield JL, Johnson DE, Lassey KR, de Lima MA, Romanovskaya A, Bartman D, Gibb D, Martin JH Jr (2006) Emissions from livestock and manure management. In: Eggleston S, Buendia L, Miwa K, Ngara T, Tanabe K (eds) 2006 IPCC guidelines for national greenhouse gas inventories, prepared by the national greenhouse gas inventories programme, vol 4. IGES, Japan, pp 10.11–10.87
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga G, Schulz M, Van Dorland R (2007) Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin D, Manning M et al (eds) Climate change 2007: the physical science basis. contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Harper LA, Sharpe RR, Parkin TB (2000) Gaseous nitrogen emissions from anaerobic swine lagoons: ammonia, nitrous oxide, and dinitrogen gas. J Environ Qual 29(4):1356–1365
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen–Geiger classification updated. Meteorol Z 15(3):259–263
Leytem AB, Dungan RS, Bjorneberg DL, Koehn AC (2011) Emissions of ammonia, methane, carbon dioxide, and nitrous oxide from dairy cattle housing and manure management systems. J Environ Qual 40(5):1383–1394. doi:10.2134/jeq2009.0515
Leytem AB, Dungan RS, Bjorneberg DL, Koehn AC (2013) Greenhouse gas and ammonia emissions from an open-freestall dairy in Southern Idaho. J Environ Qual 42(1):10–20. doi:10.2134/jeq2012.0106
Livingston GP, Hutchinson GL (1995) Enclosure-based measurement of trace gas exchange: applications and sources of error. In: Matson P, Harriss R (eds) Biogenic trace gases: measuring emissions from soil and water. Blackwell Science Ltd., Oxford, pp 14–51
Massé DI, Masse L, Claveau S, Benchaar C, Thomas O (2008) Methane emissions from manure storages. Trans ASABE 51(5):1775–1781
McGinn S, Beauchemin KA (2012) Dairy farm emissions using a dispersion model. J Environ Qual 41:73–79
Misselbrook TH, Brookman SKE, Smith KA, Cumby T, Williams AG, McCrory DF (2005) Crusting of stored dairy slurry to abate ammonia emissions: pilot-scale studies. J Environ Qual 34(2):411–419
Olesen JE, Sommer SG (1993) Modelling effects of wind speed and surface cover on ammonia volatilization from stored pig slurry. Atmos Environ 27A(16):2567–2574
Park K-H, Thompson AG, Marinier M, Clark K, Wagner-Riddle C (2006) Greenhouse gas emissions from stored liquid swine manure in a cold climate. Atmos Environ 40(4):618–627
Petersen SO, Miller D (2006) Greenhouse gas mitigation by covers on livestock slurry tanks and lagoons? J Sci Food Agric 86:1407–1411
Petersen SO, Amon B, Gattinger A (2005) Methane oxidation in slurry storage surface crusts. J Environ Qual 34(2):455–461
Petersen SO, Anderson AJ, Eriksen J (2012) Effects of cattle slurry acidification on ammonia and methane evolution during storage. J Environ Qual 41:88–94
Rochette P, Hutchinson GL (2005) Measurement of soil respiration in situ: chamber techniques. In: Hatfield J, Baker JM, Viney M (eds) Micrometeorology in agricultural systems. ASA-CSSA-SSSA Publishers Inc., Madison, pp 247–286
Rodhe LKK, Abubaker J, Ascue J, Pell M, Nordberg A (2012) Greenhouse gas emissions from pig slurry during storage and after field application in northern European conditions. Biores Technol 113:379–394
Shah SB, Kolar P (2012) Evaluation of additive for reducing gaseous emissions from swine waste. Agric Eng Int: CIGR J 14(2):10–20
Sheppard S, Bittman S, Swift M, Beaulieu M, Sheppard M (2011) Ecoregion and farm size differences in dairy feed and manure nitrogen management: a survey. Can J Anim Sci 91(3):459–473
Sommer SG, Christensen BT, Nielsen NE, Schjorring JK (1993) Ammonia volatilization during storage of cattle and pig slurry—effect of surface cover. J Agric Sci 121:63–71
Sommer SG, Petersen SO, Sogaard HT (2000) Greenhouse gas emissions from stored livestock slurry. J Environ Qual 29(3):744–751
Sommer SG, Petersen SO, Sorensen P, Poulsen HD, Moller HB (2007) Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr Cycling Agroecosyst 78(1):27–36
Steed J, Hashimoto A (1994) Methane emissions from typical manure management systems. Biores Technol 50:123–130
VanderZaag AC, Gordon RJ, Glass VM, Jamieson RC (2008) Floating covers to reduce gas emissions from liquid manure storages: a review. Appl Eng Agric 24(5):657–671
VanderZaag AC, Gordon RJ, Jamieson RC, Burton DL, Stratton GW (2009) Gas emissions from straw covered liquid dairy manure during summer storage and autumn agitation. Trans ASABE 52(2):599–608
VanderZaag AC, Gordon RJ, Jamieson RC, Burton DL, Stratton GW (2010a) Effects of winter storage conditions and subsequent agitation on gaseous emissions from liquid dairy manure. Can J Soil Sci 90:229–239
VanderZaag AC, Gordon RJ, Jamieson RC, Burton DL, Stratton GW (2010b) Permeable synthetic covers for controlling emissions from liquid dairy manure. Appl Eng Agric 26(2):287–297
Wood JD, Gordon RJ, Wagner-Riddle C, Dunfield KE, Madani A (2012) Relationships between dairy slurry total solids, gas emissions, and surface crusts. J Environ Qual 41(3):694–704. doi:10.2134/jeq2011.0333
Acknowledgments
We wish to acknowledge the technical support provided by Donna MacLennan, John McCabe, Paul MacNeil, Devon Pires, and the staff of the Dalhousie University Agriculture Faculty Experimental Farm. The funding support provided by the Natural Sciences and Engineering Research Council (NSERC), the Dairy Farmers of Canada, the Ontario Agricultural College, the Ontario Ministry of Agriculture and Food, and Agriculture and Agri-Food Canada under the Agricultural Greenhouse Gases Program is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wood, J.D., VanderZaag, A.C., Wagner-Riddle, C. et al. Gas emissions from liquid dairy manure: complete versus partial storage emptying. Nutr Cycl Agroecosyst 99, 95–105 (2014). https://doi.org/10.1007/s10705-014-9620-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-014-9620-2