Abstract
Animal slurry stored in-house and outside is a significant source of atmospheric methane (CH4). The CH4 source strength of stored slurry is greatly affected by temperature. To improve emission calculations on a global scale there is a need for knowledge about the relationship between production of CH4 in slurry and temperature. In this study, the filling of slurry channels was reproduced in the laboratory by gradually filling 1 m-high PVC vessels during 9 days followed by incubation for 100–200 days. A preliminary test showed that little CH4 was produced from animal slurry during 10 days of incubation at 20°C, if no inoculum (slurry incubated anaerobically at the test temperature for 1.5–2 months) was present. However, the addition of 7.6% inoculum supported an immediate production of CH4. Vessels amended with inoculum and gradually filled with cattle or pig slurry were then incubated at 10, 15 and 20°C. Methane production from stored pig and cattle slurry was not significant at temperatures below 15°C, where CO2 was the main product of decomposition processes. In contrast, the anaerobic production of CH4 was high and significant relative to the production of CO2 at 20°C. Peak emissions of CH4 averaging 0.012 and 0.02 g C h−1 kg−1 volatile solids (VS) were reached within about 10 days at 10 and 15°C, respectively. At 20°C, the emission of CH4 from pig slurry was about 0.01 g C h−1 kg−1 for 10 days, and thereafter emissions increased to about 0.10 g C h−1 kg−1 VS. For cattle slurry a peak emission of 0.08 g C h−1 kg−1 VS was measured after 180 days. Degradation of organic nitrogen (N) in cattle slurry was related to the reduction of organic material as reflected in CO2 and CH4 emission. The mineralization of organic N during storage represented 10–80% of organic N in cattle slurry, and 40–80% of the organic N in pig slurry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic emissions of the greenhouse gas (GHG) methane (CH4) have increased significantly during the twentieth century (IPCC 2001). Compared to carbon dioxide (CO2), the amounts of CH4 are low in the atmosphere, but the global warming potential is 23 times higher than that of CO2 on a molecular basis and within a 100-year time horizon (IPCC 2001).
During storage of slurry, CH4 and CO2 are formed by anaerobic bacterial degradation of organic matter (Steed and Hashimoto 1994). Significant amounts of CO2 are also produced at the slurry–air interface by aerobic microbial degradation processes (Møller et al. 2004). It has been suggested that there is a need for information about effects of temperature and residence time on CH4 and CO2 emissions from stored slurry (Sommer et al. 2004). This information should contribute to develop methods for calculating GHG emissions from manure which are more dynamic than the existing approach based on region-specific emission factors (IPCC 1997). Reviews have also uncovered a need for better prediction of nitrogen (N) turnover in stored animal manure (Sommer et al. 2005), which is needed for the development of models for predicting emissions of reduced and oxidized N to the atmosphere, and also for estimating nitrate leaching.
This study compiles data from storage experiments conducted over a period of 18 months. The aim was to examine the degradation of organic components and the production of CH4 and CO2 in slurry channels (pits) inside animal houses as influenced by temperature. In the study, faeces + urine and water were gradually added to open incubation vessels from day 1 to day 9 of the experiment, and thereafter the slurry was incubated for up to 200 days. Emissions from the slurry were measured using a dynamic chamber technique, and slurry was sampled during the study to determine the concentration of N components. The intention was to simulate the degradation of organic components and gas emissions during the initial storage in slurry channels. The study was also designed to provide information about the degradation processes during subsequent outside storage corresponding to a summer temperature regime, which is in the temperature interval 10°C till 20°C (Hansen et al. 2006).
Materials and methods
The pigs producing urine and faeces for the storage experiment were fed a diet containing 24% soybean meal, 49.8% barley, 20% wheat, 1% molasses, 2% fat and 3.2% minerals and vitamins. Faeces and urine was collected separately and stored at −18°C until needed. Faeces and urine portions were thawed and mixed at a ratio of 1:3.5 to produce the pig slurry used in the storage experiment. The dairy cows from which urine and faeces were collected were fed a diet based on [on a dry matter (DM) basis] 21.6% concentrates, 55.2% roughage, 22.8% barley and 0.4% minerals. Cattle urine and faeces were collected in unseparated form (maximum time between deposition and collection 1–2 h), and stored at −18°C until the day of use. Some characteristics of the batches of pig and cattle slurry used for the experiments are presented in Table 1.
Incubation
For the storage experiments, slurry was incubated in PVC vessels (height 100 cm; diameter 15 cm). A preliminary experiment was conducted in which pig slurry with and without 7.6% inoculum was incubated in duplicate at 20 ± 0.5°C. In the main experiment, three vessels containing cattle slurry and three containing pig slurry were incubated with 7.6% inoculum at each of the temperatures 10 ± 0.5, 15 ± 0.5 and 20 ± 0.5°C.
The vessels were gradually filled to approximately 5 cm from the upper rim by initially adding 0.5 kg inoculum, 0.55 kg slurry and 0.10 kg water (simulating water leak), and then from day 1 until day 9 with 0.55 kg slurry and 0.05 kg water each day. Thus, in total 0.5 kg inoculum (7.6%), 5.5 kg slurry (84.8%) and 0.55 kg water (7.6%) were added. The inoculum consisted of pig or cattle slurry which had been stored for 1.5–2 months at 10, 15 or 20°C, allowing a population of anaerobic bacteria to adapt to the storage environment. The vessels were incubated in temperature-controlled chambers (10°C) or in a water bath at constant temperature (15 or 20°C). The incubation at 10, 15 and 20°C lasted 138, 114 and 216 days, respectively.
Slurry sampling and analysis
Slurry samples were retrieved for analysis during and after the storage period. The samples were stored at −18°C until analysis. At 15°C, representative samples of stirred slurry were taken after 21 and 114 days, and at 20°C after 15, 147 and 216 days. At 10°C a different procedure was used to enable more frequent sampling without excessive disturbance: 12 smaller vessels (height 1 m; diameter 2.8 cm) for each slurry type were filled using the same procedure as for the large vessels. Three of these small vessels with cattle slurry and three with pig slurry were removed after 12, 20, 41 and 63 days. After 138 days the incubation was stopped and slurry samples were taken for analysis from each of the large vessels. Gaseous emissions from stored slurry will be a function of the slurry volume and the air–slurry interface area. The interface-to-volume ratio was identical in large and small diameter vessels, and so it was assumed that C and N turnover and emissions were comparable.
Slurry samples were analysed for DM, volatile solids (VS), chemical oxygen demand (COD), total N (TN), total ammoniacal N (TAN) and total P (TP). Pig urine composition was determined on fresh samples collected from six pigs weighing 50–80 kg.
Gas emissions
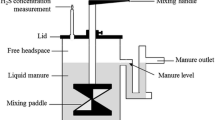
Throughout filling and storage of vessels with cattle and pig slurry, gas emissions were measured continuously using the vessels as dynamic chambers. These dynamic chambers were established by capping the vessels with gas-impermeable lids, leaving an open headspace above the slurry surface; input and output ports on opposite sides of each lid were used to direct an airstream across the slurry surface. The air was sucked through each headspace by pumping an airflow of 1.1 l min−1. The concentration of CH4 and CO2 in incoming and exhaust air were analysed with an on-line multi-gas monitor (photoacoustic monitor; Brüel & Kjaer, Nærum, Denmark).
Analytical techniques
Dry matter was determined after a 24-h drying period at 105°C. After dry ashing and solubilization in acid, the content of TP was measured colorimetrically (Spectronic 1001; Bausch & Lomb, UK) after a colouring reaction with ammonium molybdate vanadate. The TAN content of slurry samples was analysed by means of a QuickChem 4200 flow injection analyser (Lachat Instruments, Wisconsin, USA). Slurry pH was determined with a pH-meter (Radiometer A/S, Copenhagen, Denmark). The COD was analysed colorimetrically (Spektroquant Nova 60; Merck, Germany) in accordance with the method described by APHA (1995). TN was analysed by the Kjeldahl method using a Kjellfoss 16200 (Copenhagen, Denmark). Urea was determined by the method of Mulvaney and Bremner (1979).
Results and discussion
The pig faeces and urine could be collected separately and slurry produced by mixing the samples at initiation of an experiment. The housing of cattle did not allow us to use the same procedure for collection of cattle slurry. Instead the cattle slurry was most carefully collected within 1–2 h after excretion of faeces and urination and then frozen. Having taken these precautions at sampling, we are of the opinion that the pattern of microbial transformation of the C and N in the pig and cattle slurries can be compared. Compositions of the cattle and of the pig slurries prepared by mixing faeces and urine are presented in Table 1. The DM content of the slurries was higher than is normally observed in slurry collected on farms but N and P content was in the same range as shown in previous studies (Sommer and Husted 1995; Birkmose and Knudsen 2005). Indicating that the slurries produced and collected was representative for slurries produced in traditional animal houses.
During periods with high CH4 production, a 10–15-cm-thick surface crust of fibrous organic material developed at the surface of the pig slurry, whereas little or no surface crust was observed at the surface of the cattle slurry during incubation. The density of water-saturated fibres is greater than 1; therefore a surface crust will only establish during periods with CH4 production and ebullition, because gas bubbles captured by the solids will enhance the buoyancy of the organic material. Emissions of CH4 were lower from cattle slurry than from pig slurry, which is the probable reason for the absence of crust formation on cattle slurry. It should be noticed that in practice bedding material, faeces and animal hairs contribute to crust formation, whereas in this experiment the crust material was derived entirely from faecal matter.
Effect of inoculum on CH4 emissions
Sommer et al. (2004) assumed that slurry left behind after emptying of slurry channels will act as a methanogenic inoculum, because microorganisms in the slurry will adapt to the ambient storage temperature (Kotsyurbenko et al. 1993). The effect of inoculum on CH4 production was examined in a preliminary experiment, where one treatment was initially amended with 7.6% slurry which had been stored at an incubation temperature of 20°C (Fig. 1). At first, CH4 emissions from the vessel without inoculum were low, and 12 days of incubation passed before significant emissions were observed. The temporal dynamics in methane emissions may reflect microbial succession or use of different pools of organic matter in the slurry. In contrast, CH4 emissions from the vessel with inoculum were significant from day 1 onwards. Zeeman et al. (1988) found that no CH4 was produced for 5.5 months when fresh dairy cattle manure was stored in sealed incubators (batch) at 5–15°C, whereas CH4 production was significant from day 1 when 50% inoculum pre-cultured at 18°C was added at the beginning. The present study indicates that, for the initiation of CH4 production, the amount of inoculum needed may be <10% of the amount of slurry stored, i.e. a fraction of slurry that is often left in slurry channels when they are emptied. These findings suggest that more efficient flushing of the slurry channels to remove slurry that can act as inoculum may significantly reduce in-house emissions of CH4, as proposed by Sommer et al. (2004).
Effect of storage temperature on CH4 emissions
At 10 and 15°C, CH4 emissions from pig slurry were low and varied considerably (Fig. 2), probably due to a discontinuous release of CH4 via ebullition (Husted 1994). Peak emissions averaging 0.012 and 0.02 g C h−1 kg−1 VS were reached within about 10 days at 10 and 15°C, respectively. Thereafter emissions declined gradually to below 0.01 g C h−1 kg−1 after 50–60 days of incubation (Fig. 2). A lag period of 50 days before peak emission from pig slurry was observed at 20°C. The reason may be that the mixing or condition of the inoculum used in the 20°C experiment differed from the other two experiments, or that new pools of less degradable substrates became available for methanogens upon prolonged storage as a result of microbial community changes.
Average CH4 emissions from incubated cattle slurry did not exceed 0.01 g C h−1 kg−1 VS in the temperature interval 10–15°C. The maximum emission rates were twice as high as those presented by Hill et al. (2001), but the modest increase in emissions with an increase in temperature from 10 to 15°C was in accordance with previous studies (Hill et al. 2001; Husted 1994). The CH4 emission at 15°C was variable, this may be a consequence of that in periods with low emission the measurement with the instrument was near the detection limit. Methane emissions from pig slurry stored at 10°C were similar to the daily emission (0.011 g C h−1 kg−1 VS) during 15 days of storage in pig housing calculated using the algorithms by Sommer et al. (2004). At 20°C, the emission of CH4 from pig slurry was about 0.01 g C h−1 kg−1 for 10 days, and thereafter emissions increased to about 0.10 g C h−1 kg−1 VS. For cattle slurry the highest emission of 0.08 g C h−1 kg−1 VS was measured after 180 days. This rate was significantly higher than that observed by Hill et al. (2001), but only about one-tenth of the emission calculated using the algorithms of Sommer et al. (2004).
These studies indicate that below 15–20°C emissions of CH4 from stored slurry are low, whereas emissions from animal slurry at and above 20°C can be a significant source of GHG in the atmosphere. However, there is a need to improve the models in order to calculate CH4 emissions from slurry channels by parameterizing the effect of temperature and storage time, and the effect of gradual filling, which may have contributed to the lag phase observed before CH4 production became significant.
Methane to carbon dioxide ratios
Fractions of CH4-C emissions to total carbon emissions, CH4-C/(CO2-C + CH4-C), are presented in Fig. 3. At 10°C, the ratio of both pig and cattle slurry was 0.1–0.3 during the initial phase with high emissions, declining to an average of around 0.01 after 50 days. At 15°C, the CH4-C/(CO2-C + CH4-C) ratio was variable and no clear pattern was observed. At 20°C, the CH4-C/(CO2-C + CH4-C) ratio during peak CH4 production increased from 0.2 to 0.65 and 0.5 in pig and cattle slurry, respectively. In anaerobic digesters with a stable and high production of biogas, the ratio is about 0.60 (Hansen et al. 1998; Ianotti et al. 1979). This indicates that at low storage temperatures, methanogenesis was slow compared with other degradation pathways producing CO2. This includes fermentation processes, but also aerobic microbial activity at the slurry–air interface, where oxygen from the atmosphere enters the slurry (Møller et al. 2004). It was only during peak CH4 production at 20°C that decomposition of organic material appeared to be dominated by a methanogenic microbial community.
Models developed to predict the transformation of organic matter aiming at predicting GHG emissions from slurry channels should include submodels for both anaerobic subsurface processes and aerobic surface processes. The latter may also be important for the prediction of pH in the slurry surface layer, which is much needed when estimating ammonia (NH3) emissions from animal housing (Canh et al. 1998; Olesen and Sommer 1993; Sommer and Husted 1995). Degradation of organic acids to CO2 will increase surface pH, as seen in the study of Canh et al. (1998).
Transformations of organic N
Under Danish manure management conditions, the proportions of TAN in fresh cattle and pig slurry are typically around 50–60 and 70–80% of TN, respectively (Petersen et al. 1998). The level of TAN in the cattle slurries used in this study agreed well with this range in the 10 and 15°C storage experiments, but was relatively low at 20°C. With pig slurry the initial proportions of TAN were always much lower than expected. The preparation of cattle and pig slurries differed in that pig faeces and urine batches were thawed and mixed only a few hours before incubations started, whereas cattle faeces and urine had been mixed prior to freezing. Whitehead and Raistick (1993) reported that urea hydrolysis in cattle excreta was complete within 7 days of storage at 20°C. Due to the prolonged contact between faeces and urine in the present study, urea hydrolysis in cattle slurry was therefore expected to be complete. In contrast, a significant amount of urea could have been present in pig slurry when the experiments were initiated due to the slow hydrolysis of urea at low temperatures (Braam et al. 1997). In urine samples from six growing pigs representing the stock from which faeces and urine was collected for this study, urea-N constituted 50 ± 13% of TN (data not shown), corresponding to a relatively low-protein diet (Misselbrook et al. 2005; Pfeiffer et al. 1995).
Figure 4 shows reductions in organic N (i.e. net N mineralization) during incubation at 10, 15 and 20°C. At all temperatures there was a large initial reduction in organic N in pig slurry, whereas the reduction was more gradual in cattle slurry. As indicated in the previous paragraph, urea hydrolysis may have contributed to the reductions in organic N of 350–681 g N kg−1 in pig slurry vs. 96–202 g N kg−1 in cattle slurry during the first 10–22 days of incubation (Fig. 4, upper panel). Zhang and Day (1996) also observed a large accumulation of TAN during the first 1–2 days after preparation of slurry by mixing of faeces and urine.
Rates of net N mineralization are presented in Fig. 4 (lower panel). At all three temperatures the highest rates were observed during the initial storage period in both cattle and pig slurry. Cattle slurry showed organic N reduction rates of 12 g N kg−1 organic N day−1 at 10°C (0–12 days) and 18.4 g N kg−1 organic N day−1 at 20°C (0–14 days). In contrast, net N mineralization at 15°C was much lower. It is not known to what extent urea could have also been present in the cattle slurry, but the fact that reduction of organic N in cattle slurry continued at similar rates beyond the first sampling indicates that other sources of organic N predominated. Between 15 and 147 days, organic N reduction in cattle slurry averaged 5.0 g N kg−1 organic N day−1 at 20°C, and at 10°C the rate between 21 and 114 days incubation was 0.3 g N kg−1 organic N day−1. There was no significant reduction in organic N in the cattle slurry incubated at 15°C after 10 days of incubation. In pig slurry, the reduction of organic N after 10–15 days was small, irrespective of temperature.
The peak rate of net N mineralization, as reflected in the initial organic N reduction, declined with a reduction in temperature in pig slurry. In cattle slurry, this was also the case when comparing 10 and 20°C incubation, whereas organic N reduction remained low at 15°C. Unfortunately, the initial composition of the cattle slurry stored at different temperatures varied somewhat, as reflected in the proportions of TAN (Table 1). Therefore the temperature effects on net N mineralization may have been confounded by differences in chemical composition.
Net N mineralization in the different treatments varied from 5 to 85%. Sørensen et al. (2003) found that the mineralization of organic N in cattle slurry from a dairy cattle feeding experiment varied between 9 and 50% during storage at 8–15°C, but they did not identify the dietary parameters that influenced N mineralization. A relatively high mineralization of organic N in anaerobically stored manure has also been observed by Kirchmann and Witter (1992), who found that 51% of total faecal N from both dairy cattle and pigs was present as TAN after 7 months storage at 25°C as compared to only 1% (cattle) and 8% (pig) at the start of faeces storage. In Fig. 5, the accumulated reduction of organic N is plotted against accumulated emissions of CH4 + CO2 for all 11 replicate incubations included in this study (2 replicates in the preliminary study and 3 replicates per temperature in the main experiment). Net N mineralization was related to net C mineralization, as reflected in gaseous emissions, up to a level of 75 g C kg−1 VS, where net N mineralization reached a maximum level of 80–85%.
The organic N reductions recorded in these storage experiments may also be compared with organic N reductions observed during anaerobic digestion of pre-stored slurry. In an experiment with anaerobic digestion of pig slurry (pre-stored for approximately 20 days) at 35°C for 15 days, 47% of the organic N was transformed to TAN, while approximately 180 g C kg−1 VS was collected in gaseous products, assuming that C constitutes 30% of VS in manure (Ianotti et al. 1979). Similarly, recent experiments with anaerobic digestion of slurry, pre-stored for 1 month, showed that 51 and 48% of the organic N in pig and cattle slurry, respectively, was transformed to TAN, while 190 and 120 g C kg−1 VS was released in CH4 and CO2 (H.B. Møller, unpublished data). The reduction in organic N relative to C gas production was much lower in these digestion experiments than observed during the storage of freshly excreted slurry in the present study, which partly included the degradation of urea and amides. This is reflected in the steep curve of Fig. 5.
Conclusions
Without inoculum, little CH4 was produced during the initial 10 days of incubation of slurry at 20°C, whereas addition of 7.6% inoculum resulted in an immediate production of CH4. Below 15–20°C, methane production was insignificant compared with CO2 production, including aerobic production of CO2 in the surface layer. Only at 20°C was the anaerobic production of CH4 significant relative to the production of CO2. Little organic N was transformed to NH +4 at 10°C (cattle and pig slurry) and 15°C (cattle slurry), whereas about 80% of organic N was degraded at 15°C (pig slurry) and 20°C (cattle and pig slurry). These observations clearly show that N transformations and C emissions from cattle and pig slurry are dynamic over time and are strongly influenced by storage conditions such as temperature and the presence of an adapted microbial community in pre-stored slurry. Additional data are much needed for the development of models to describe N and C transformations, NH3 and CH4 emissions, and N availability in stored slurry.
References
APHA (1995) Standard methods for the examination of water and wastewater. 19th edn. American Public Health Association, Washington, DC
Birkmose TS, Knudsen L (2005) Content of nutrients in slurry. Planteavlsorientering nr. 07-540. The Danish Agricultural Advisory Service, Skejby (in Danish)
Braam CR, Ketelaars JJMH, Smits MCJ (1997) Effects of floor design and floor cleaning on ammonia emission from cubicle houses for dairy cows. Neth J Agric Sci 45:49–64
Canh TT, Sutton AL, Aarnink AJA, Verstegen MWA, Schrama JW, Bakker GCM (1998) Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J Anim Sci 76:1887–1895
Hansen KH, Angelidaki I, Ahring BK (1998) Anaerobic digestion of swine manure: inhibition by ammonia. Water Res 32:5–12
Hansen TL, Sommer SG, Gabriel S, Christensen TH (2006) Methane production during storage of anaerobically digested municipal organic waste. J Environ Qual 35:830–836
Hill DT, Taylor SE, Grift TE (2001) Simulation of low temperature anaerobic digestion of dairy and swine manure. Bioresour Technol 78:127–131
Husted S (1994) Seasonal variation in methane emission from stored slurry and solid manures. J Environ Qual 23:585–592
Ianotti EL, Porter JH, Fischer JR, Sievers DM (1979) Changes in swine manure during anaerobic digestion. Dev Ind Microbiol 20:519–529
IPCC (1997) Greenhouse gas inventories: reference manual. Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories, vol 3. http://www.ipcc-nggip.iges.or.jp/public/gl/invs6.htm
IPCC (2001) Climate change 2001: the scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK
Kirchmann H, Witter E (1992) Composition of fresh, aerobic and anaerobic farm animal dungs. Bioresour Technol 40:137–142
Kotsyurbenko OR, Nozhevnikova AN, Kalyuzhnyi SV, Zavarzin GA (1993) Methane digestion of cattle manure at low temperatures. Microbiology 62:462–467
Misselbrook T, Powell JM, Broderick GA, Grabber JH (2005) Dietary manipulation in dairy cattle: laboratory experiments to assess the influence on ammonia emissions. J Dairy Sci 88:1765–1777
Mulvaney RL, Bremner JM (1979) A modified diacetyl monoxime method for colorimetric determination of urea in soil extracts. Commun Soil Sci Plant Anal 10:1163–1170
Møller HB, Sommer SG, Ahring BK (2004) Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. J Environ Qual 33:27–36
Olesen JE, Sommer SG (1993) Modelling effects of wind speed and surface cover on ammonia volatilization from stored pig slurry. Atmos Environ 27A:2567–2574
Petersen SO, Sommer SO, Aaes O, Søgård K (1998) Ammonia losses from urine and dung of grazing cattle: effect of N intake. Atmos Environ 32:295–300
Pfeiffer A, Henkel H, Verstegen MWA, Philipczyk I (1995) The influence of protein intake on water balance, flow rate and apparent digestibility of nutrients at the distal ileum in growing pigs. Livest Prod Sci 44:179–187
Sommer SG, Husted S (1995) The chemical buffer system in raw and digested animal slurry. J Agric Sci Camb 124:45–53
Sommer SG, Petersen SO, Møller HB (2004) Algorithms for calculating methane and nitrous oxide emissions from manure management. Nutrient Cycling Agroecosyst 69:143–154
Sommer SG, Bannink A, Chadwick D, Hutchings NJ, Misselbrook T, Menzi H, Ni J-Q, Oenema O, Webb J, Monteny G-J (2005) Algorithms determining ammonia emission from livestock houses and manure stores. Adv Agron 89:261–335
Sørensen P, Weisbjerg MR, Lund P (2003) Dietary effects on the composition and plant utilization of nitrogen in dairy cattle manure. J Agric Sci Camb 141:79–91
Steed J, Hashimoto AG (1994) Methane emissions from typical manure management systems. Bioresour Technol 50:123–130
Whitehead DC, Raistick N (1993) Nitrogen in the excreta of dairy cattle: changes during short-term storage. J Agric Sci Camb 121:73–81
Zeeman G, Sutter K, Vens T, Koster M, Wellinger A (1988) Psychrophilic digestion of dairy cattle and pig manure: start procedures of batch, fed-batch and CSTR-type digesters. Biol Waste 26:15–31
Zhang RH, Day DL (1996) Anaerobic decomposition of swine manure and ammonia generation in a deep pit. Trans ASAE 39:1811–1815
Acknowledgements
This study was supported by grants from the Danish Ministry of Environment under the work programme ‘Greenhouse Gas Emission’ and the Danish Ministry of Food, Agriculture and Fisheries under the work programme ‘Water Environment Plan III (2004–2007)’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sommer, S.G., Petersen, S.O., Sørensen, P. et al. Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr Cycl Agroecosyst 78, 27–36 (2007). https://doi.org/10.1007/s10705-006-9072-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-006-9072-4