Abstract
In this study, striped catfish larvae were gradually exposed to the increase of different salinities, and then they reached the levels of 0, 5, 10, 15, and 20 psu after 10 days, followed by heat shock at 39 °C to determine stress tolerance. After the 10-day experiment, the survival rate of fish exposed to the 20 psu treatment was only 28.6 ± 4%, significantly lower than that of the other treatments. The results showed that the osmolality of the whole-body (WB) homogenate was gradually and significantly increased with salinity elevation, except in fish exposed to freshwater and 5 psu treatments, while there were no significant changes in WB Na+/K+-ATPase activity. Digestive enzymatic activities, i.e., pepsin, α-amylase, alkaline phosphatase, and leucine alanine peptidase (leu-ala) generally increased with salinity, but not aminopeptidase and trypsin. Lysozyme and peroxidase activities increased in fish larvae exposed to 15 and 20 psu. These increases proportionally improved growth performance, with the lowest and the highest final weights observed in fish reared at 0 psu (0.08 ± 0.03 g/larvae) and 20 psu (0.11 ± 0.02 g/larvae), respectively, although the average growth recorded at 20 psu could be biased by the high mortality in this group. Occurrence of skeleton deformities, such as in caudal vertebrae and branchiostegal rays, was significantly higher in fish exposed to the higher osmotic conditions (15.0 ± 1.2% and 10.3 ± 2.1% respectively at 0 psu vs. 31.0 ± 2.9% and 49.0 ± 5.6%, respectively at 15 psu). After the 12.5-h heat shock, survival rates significantly differed between treatments with the highest survival observed in fish submitted to 5 psu (68.9%), followed by those exposed to 0 (27%) and 10 (20%) while all fish died at 15 psu. These findings suggest that the striped catfish larvae could be reared in salinity up to 5 to 10 psu with a higher survival and tolerance to thermal stress when compared to fish maintained in freshwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Striped catfish Pangasianodon hypophthalmus is the most important aquaculture species in Vietnam, with export turnover reaching US$2.3 billion in 2019 (FAO 2020). In recent years, the sector has been negatively affected by climate changes, especially salinity increase (Nguyen et al. 2014). It is predicted that the environmental water in these regions will further suffer from dam construction in upstream countries along the Mekong River (Binh et al. 2020). In response to these factors, the striped catfish industry is compelled to adapt its culture methods to a brackish water environment to maintain its sustainability.
In teleosts, one of the most significant responses to saline environment exposure is the osmoregulatory modification that directly affect their tolerances and living habits (Varsamos et al. 2005). Fish species maintain osmolality in extracellular body fluids at values close to 300 mOsmol kg−1 while the fish isosmotic salinity point is at 9 psu (Kültz 2015). Some marine and euryhaline fish are able to slightly regulate the osmolality of body fluids under hyper- and/or hypoosmotic stress at embryonic and larval stages (Caberoy and Quinitio 2000; Haddy and Pankhurst 2000; Fridman et al. 2012a). Before gills are fully formed, osmoregulation in the larval stage mainly happens through the integument where a high density of ionocytes are located (Alderdice 1988; Varsamos et al. 2005). In some fish species, gills are present from early developmental stages such as hatching or the yolk sac period (Coughlan and Gloss 1984; Li et al. 1995; Katoh et al. 2000). The development of gills coincides with functional osmoregulatory shift from skin to gills due to changes in chloride cell distributions (van der Heijden et al. 1999; Katoh et al. 2000; Varsamos et al. 2005). Many studies previously showed that osmoregulation in freshwater fish was significantly changed with the elevation of osmotic environment through the increase of osmolality and Na + /K + -ATPase activity (Woo and Tong 1982; Schmitz et al. 2016; Phuc et al. 2017). Particularly, it was reported in striped catfish that plasma osmolality and Na + /K + -ATPase activity were significantly increased in the fish subjected to 10 and 20 psu compared to that in freshwater after 20 experimental days (Schmitz et al. 2016). At the early stage, whole-body osmolality in Nile Tilapia eggs and larvae increased with salinity rise (Fridman et al. 2012a). Furthermore, energy utilization during osmoregulation processes may directly affect growth and survival in larvae (Varsamos et al. 2005). Beside the energy cost, environmental salinity possibly influences the growth rate by altering food intake, food conversion, and hormone stimulation (Bœuf and Payan 2001). It is worth noting that, despite being not systematic, growth rate in marine and euryhaline fish is improved in fish cultured in an environment between 8 and 20 psu (Ostrowski et al. 2011; Fridman et al. 2012b; Kim et al. 2017). However, freshwater fish often seem to have better growth rates in water salinity of less than 5 psu (Altinokand and Grizzle 2001; Fashina Bombata and Busari 2003; Overton et al. 2008).
Environmental salinity may directly or indirectly affect the growth performance and enzymatic activities in teleost fish (Tsuzuki et al. 2007; Ronkin et al. 2015; Hamed et al. 2016). The former is due to modifications in pH and ion strength in the gut lumen through water ingestion or excretion during hyper- or hypoosmoregulation processes, respectively. The latter is related to homeostasis maintainability, requiring higher food intake and then increased enzymatic activity for digestion (Usher et al. 1990; Bœuf and Payan 2001; Barman et al. 2005; Pujante et al. 2018).
As far as immunity is concerned, several studies have investigated the interactions between salinity and immunomodulatory responses (Yada et al. 2001; Jiang et al. 2008; Schmitz et al. 2016). The modifications in the immune system during osmotic changes are possibly related to the regulation of different hormones. Prolactin is important in freshwater adaption, while growth hormone (GH) and cortisol are mainly responsible for seawater adaption (Eckert et al. 2001; Yada et al. 2004). The key of innate immunity as lysozyme and complement molecules was reported to be affected with the change of osmotic environment. Particularly, lysozyme activities increased in rainbow trout Oncorhynchus mykiss when fish were exposed to higher salinity at 1 h and 24 h after transfer to 25 psu condition while alternative complement activity increased 8 h after transfer (Jiang et al. 2008). Similarly, Schmitz et al. (2016) showed that striped catfish fingerlings up-regulated lysozyme activity according to the increase of salinity at 10 and 20 psu. Additionally, the key of adaptive system being immunoglobulins (Ig) was affected by environmental salinity in gilthead seabream through total Ig evaluation after 14 days exposed to 55 psu (Cuesta et al. 2005). Moreover, antioxidant responses as peroxidase activities were reduced in gilthead seabream plasma after exposure to lower salinity (Cuesta et al. 2005).
Although the mechanisms are not clearly understood, the effects of the changes of salinity on skeletal deformity have been investigated in early developmental stages of both marine and freshwater fish (Borode et al. 2002; Okamoto et al. 2009). Lingcod Ophiodon elongatus Girard larvae exposed to different salinities during the hatching phase displayed an increase of spinal deformity but a decrease of jaw deformity (Cook et al. 2005). Specifically, the percentage of abnormalities in spine and jaw was gradually elevated and decreased from 7.1 to 31.3% and from 34.9 to 7.2%, respectively when embryos were exposed to salinity from 15 to 35 psu. In Japanese eel Anguilla japonica larvae, the rate of total deformity was observed at the lowest level for 36 psu conditions at around 30% compared to the corresponding larvae either in 24 or 42 psu environments, at 98.2% and 68.4%, respectively (Okamoto et al. 2009). In a freshwater fish, African catfish Clarias gariepinus, saline exposure in larvae during embryonic stages induced an increase of deformity rate when exposed to 4 and 8 psu (52% and 71%) compared to freshwater and 2 psu (10.4 and 16.1%) (Borode et al. 2002).
Beside salinity intrusion, climate changes have induced an increase of temperature in the Mekong Delta, with potential interactions of these two stressors on striped catfish. A previous study on striped catfish showed that a temperature of 35 °C increased the survival rate in fish exposed to 6 and 12 psu in comparison with those exposed to freshwater while there was no significant differences among salinities at 25 °C (Phuc et al. 2017). Additionally, the survival rate of Nile tilapia exposed to different salinities was affected by the temperature, with the highest survival observed in the treatments of 0 and 8 psu at 32 °C and the lowest in the treatment of 16 psu at 24 °C (Likongwe et al. 1996), suggesting that salinity exposure may change the temperature tolerance in fish.
The present study aimed to provide a general insight on the responses of striped catfish larvae to a salinity gradient, in terms of osmoregulation capacity, digestive enzyme activities, innate immunity, skeleton malformations, and temperature tolerance.
Materials and methods
Fish and experimental conditions
One day, post-hatching striped catfish larvae were obtained from Viet-Uc hatchery, An Giang province. The fish were acclimated for 10 days in freshwater conditions and reached the average weight of 1 ± 0.1 mg/larvae. The experiment was conducted in composite tanks (water volume 300L) and closed water. Then, the larvae were distributed into 5 triplicate treatments with 300 larvae/tank, with final salinity of 0, 5, 10, 15, and 20 psu. Salinity increase was 0, 0.5, 1, 1.5, and 2 psu per day, respectively. The increase was done twice daily to reduce environmental shock in larvae. Throughout the rearing period with aeration, the larvae were fed with approximately 10 Moina brachiata/mL four times per day. The cultured temperature fluctuated from 27 to 29 °C during the experiment. After 10 experimental days, all treatments reached the targeted salinity and then fish were sampled (Fig. 1).
Sampling and output variables
Different parameters were evaluated in this study, such as husbandry variables, osmolality and Na+/K+-ATPase activities, digestive enzyme activities, as well as immunological responses and skeleton deformities. The larvae were rinsed with distilled water before pooling 4–6 fish in Eppendorf tube and immediately snap-frozen in liquid nitrogen. Although only one tube was collected from each tank, the samples including 4–6 fish were representative of the tank. All samples were then kept at − 80 °C for further analysis, except for 50 fish/tank that were fixed in 10% buffered formalin for deformity evaluation.
After the 10-day experiment, survival rate was calculated by individually counting all living larvae and was expressed as a percentage of the initial fish numbers. Growth of larvae was calculated by the wet body weight of all surviving larvae in a pool of 5 larvae at each time. Average weight was used for weight gain (mg/larvae).
Osmolality and Na+/K+-ATPase activity
Because of the small size of larvae, blood was inefficiently collected; therefore, although a real internal osmolality comes from animal’s blood, we homogenized the whole-body (WB) of the larvae and considered the measure of homogenate osmolality as the most efficient method to estimate internal osmolality. The protocol followed the method of Fridman et al. (2012a) with modifications. Pooled samples including 4–6 fish from each tank thawed on ice were rinsed with distilled water and then wiped by tissue paper in order to remove the water present on the fish surface. The samples were homogenized without buffer and then centrifuged at 6000 g for 10 min. Supernatant was collected and measured using a Gonotec Osmomat 3000 (Gonotec, Germany) for WB osmolality.
WB homogenates were obtained by homogenizing tissue (1:10) in ice-cold SEI buffer (sucrose 0.25 M, EDTA 1 mM, imidazole 50 mM, pH 7.4). Main debris were removed by centrifugation at 10 000 g for 5 min at 4 °C. Na+/K+-ATPase activity was measured according to the method of McCormick (1993). Before the assay, a standard curve of 0–20 nmol ADP/well was obtained. The assay was measured at 340 nm for 5 min and the linear regression was used to calculate Na+/K+-ATPase activity in WB larvae. Protein concentration was estimated by the method of Bradford (1976). The specific activities are expressed as U mg protein−1 h−1.
Digestive enzyme activities
The head and tail of striped catfish larvae were removed to separate the digestive segment including stomach and intestinal tract on a glass maintained on ice. Pooled samples from each tank were homogenized with the ratio of 1:10 cold distilled water. The centrifugation was done at 4200 g and 4 °C for 30 min and the supernatant was collected in aliquots and stored at − 80 °C for further analysis. Alkaline phosphatase activities were performed following the method of Bessey et al. (1946). Ten microliters of homogenate was added to a 1-ml substrate of p-nitrophenyl phosphate at 37 °C and measured a spectrophotometer at 407 nm within 5 min. Aminopeptidase activity was estimated according to the method of Maroux et al. (1973). Twenty microliters of homogenate was mixed with 20 µL of substrate of L-leucine-p-nitroanilide and 1 ml buffer of Na2HPO4 80 mM, pH 7. The mixture was measured with a spectrophotometer at 410 nm in 5 min. Leucine alanine peptidase (leu-ala) was assayed according to Nicholson and Kim (1975) using leucine alanine as substrate. Pepsin was assayed by the method of Worthington (1982) modified by Cuvier-Péres and Kestemont (2001). One hundred microliters of homogenate were mixed with 500 µL of hemoglobin substrate (2% w/v hemoglobin in 0.06 HCl).The reaction was stopped by trichloroacetic acid (TCA) 5% after 10 min. The blank test was only added homogenate after TCA. The mixtures were centrifuged at 4000 g for 6 min and then the supernatants were measured at the optical density of 280 nm. Trypsin activity was measured following the method of Holm et al. (1988) that used benzoyl-DLarginine- p-nitroanilide (BAPNA) as substrate and absorbance was read at 410 nm. The activity of alpha amylase was estimated using starch as the substrate at 25 °C and absorbance was read at 540 nm (Metais and Bieth 1968). Protein concentration of each sample was estimated by the method of Bradford (1976). Specific activities are expressed as U mg protein−1 min−1.
Immunological response
Homogenates were obtained by homogenizing tissue (1:3) in ice PBS buffer, pH 6.2. Then, centrifugation was performed at 6000 g for 10 min and the supernatant was collected in aliquots for further analysis.
Lysozyme, complement, and total Ig were measured by the method of Milla et al. (2010). For the lysozyme assay, 10 µL of the WB homogenate was placed in a 96-well microplate with 10 µL of buffer (0.05 M of disodium hydrogenophosphate isodecahydrate) and 130 µL of the Micrococcus luteus solution (0.6 g/L), in triplicate. The positive control contained 20 µL of buffer and 130 µL of the M. luteus solution, and the negative control was only buffer (150 µL) (in triplicate). The absorbance wells of the microplate were read with a spectrophotometer at 450 nm every 5 min for 60 min. The 0.001 factor characterizes the lysozyme activity: 0.001 unit of absorbance represents 1 unit of lysozyme activity. The complement assay was performed by diluting WB homogenate at a ratio of 1:1.5, 1:3, 1:6, and 1:12 with veronal buffer in order to reach a volume of 60 µl. Ten microliters of diluted rabbit erythrocytes (3% erythrocyte in veronal buffer) were then added before mixing the solution for 2 h at 27 °C. The mix was centrifuged at 3000 g for 5 min at 4 °C. Moreover, two negative controls (60 µL of veronal buffer and 10 µL of rabbit erythrocytes) and four positive controls (60 µL of water and 10 µL of rabbit erythrocytes) were used. For each sample, 35 µL of the obtained supernatant were placed in duplicate in a 96-well microplate and read once at 405 nm. Regarding total Ig, WB homogenate was mixed with 12% PEG solution (1:1) and incubated for 2 h at room temperature under constant shaking. The supernatant was collected after centrifugation at 1000 g for 10 min and assayed for its protein concentration. The concentration of total Ig is calculated as the difference between the concentration before and after precipitation with PEG and expressed as mg prot−1.
In terms of peroxidase activity, 10 µL of WB homogenate were mixed with 65 µL of Hanks’ balanced salt solution (HBSS) no calcium, no magnesium, and 25 µL of reactional solution (62.65 mg of tetramethylbenzidine dihydrochloride and 5.15 µL of H2O2 in 10 mL of distilled water). This mix was incubated for exactly 2 min at room temperature before adding 25 µL of sulfuric acid (38.88 mL of distilled water and 11.11 mL of H2SO4 95–97%) and directly read at 450 nm. These immune responses are expressed as U mg prot − 1.
Whole mount staining for skeleton deformities
Fifty larvae per tank were fixed and stored in 10% phosphate-buffered formalin. The whole larval skeleton was stained with alizarin red according to a modified method from previous studies (Izquierdo et al. 2013). Briefly, the larvae depigmentation was performed by the solution of KOH 0.05% and H2O2 3% and then washed using absolute ethanol. The samples were dehydrated by ethanol 50% during 24 h followed by ethanol 95% during 12 h. After neutralization with KOH 5%, the larvae were stained with alizarin red during 24 h. Trypsin porcine was applied before the larvae were fixed in solution of KOH and glycerin. Deformity classification was made following the protocol of Boglione et al. (2001). Specifically, each deformity was recorded by type and position and then clustered into groups as serious spinal skeleton deformities (i.e., kyphosis, lordosis, and scoliosis), caudal vertebrae, and brachiostegal rays. The percentage of total deformities was compared statistically.
Heat shock challenge
A pre-test was conducted to estimate the upper temperature threshold that should be applied during the challenge test to study how salinity affects the stress tolerance in larvae. The result showed that 39 °C was considered as the upper threshold when mortality of larvae appeared. During the heat shock, the ambient temperature fluctuated from 27 to 29 °C. After sampling, 30 larvae from each tank were transferred to 10-L glass tanks and acutely exposed to a 39 °C water environment. All tanks were equipped with an aeration system and kept in a big composite tank of 2 m3 to ensure the same temperature in the small glass tanks. Dead fish were recorded and removed from each tank during the heat shock in 24 h.
Statistical analyses
To determine the differences between treatments, the data were firstly tested for homogeneity of variance and normal distribution and were log10 transformed if necessary. Then, one-way ANOVAs using SPSS 20.0 were applied. Comparisons among treatment means were made using a Tukey test applying a 95% confidence interval. The pooled survival rate of each treatment after heat shock challenge was statistically analyzed by the Kaplan–Meier model. Comparisons between the curves were made using the log-rank test (Goel et al. 2010).
Results
Husbandry variables
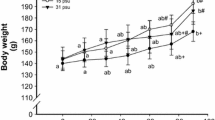
After the 10-day experiment, survival rates were not significantly different between the treatments of 0, 5, and 10 psu (from 86.2 ± 2.7 to 87.3 ± 4.6%); however, survival decreased in the 15 psu treatment and the lowest value was observed at 20 psu (76.3 ± 2.2% and 28.7 ± 2.4%, respectively) (p < 0.001) (Fig. 2). In contrast, weight gain showed the opposite trend with the biggest and smallest larvae in 20 psu and freshwater, respectively (p = 0.001).
Survival rate and weight gain in striped catfish larvae exposed to different experimental salinities. Results are expressed as the mean ± SEM (n = 3). Different lower and uppercase letters denote statistically significant differences between treatments in survival rate and weight gain, respectively (p < 0.05)
Whole-body osmolality and Na+/K+-ATPase
A sharp rise of WB homogenate osmolality was found in relation to the salinity increase, with the highest level in 20 psu (510 ± 33.7 mOsmol kg−1) and lowest level in 0 psu (216 ± 9.3 mOsmol kg−1) (p < 0.001). The difference between the treatments of 0 psu and 5 psu was not significant (Fig. 3A). Water osmolality was gradually increased with 0, 142, 273.5, 390.5, and 540 mOsmol kg−1 in freshwater, 5, 10, 15, and 20 psu, respectively. However, Na+/K+-ATPase activity did not show any significant difference between treatments (Fig. 3B). The protein concentrations were shown in the Table 1.
Enzymatic activities
In general, digestive enzyme activities showed a similar pattern which increased with salinity level, except for aminopeptidase and trypsin activities (Fig. 4). The activities of aminopeptidase and trypsin did not show any significant difference between treatments. In particular, the larvae exposed to 15 psu and 20 psu exhibited a significantly higher specific activity of alkaline phosphatase than that of 0 psu and 5 psu (p = 0.011). The peak of leu-ala enzyme activity was 2.8 ± 0.3 (U mg−1 protein−1 min−1) for treatment 20 psu and was significantly higher than those of 0, 5, and 10 psu (p = 0.013). Interestingly, stronger activities of α-amylase were found in treatments 15 psu and 20 psu compared to treatments 0 psu and 5 psu (p = 0.006). For pepsin, significant differences were observed between treatments; the highest activity was observed in larvae reared in hyperosmotic conditions, i.e., 10, 15, and 20 psu, compared to that of lower salinity (p < 0.001). The protein concentrations were shown in the Table 1.
Immunological responses
Regarding immunological aspects, lysozyme and peroxidase activities were considered as the most sensitive responses during the experiment. Specifically, WB homogenate lysozyme activity increased proportionally with salinity, with significant differences in groups exposed to 15 and 20 psu (28.3 ± 3.5 and 30.6 ± 3.6 U mg protein−1, respectively) (Fig. 5A) (p = 0.002). Similarly, the activity of WB homogenate peroxidase was significantly higher in fish reared in 20 psu, and this value significantly differed between 10 and 15 psu reared fish (Fig. 5C) (p = 0.004). ACH50 and total Ig in larval WB homogenate did not show any significant difference between treatments (Fig. 5B and 5D). The protein concentrations were shown in the Table 1.
Deformities
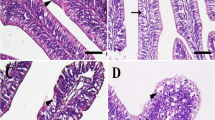
Abnormalities occurred in all treatments. In treatment 20 psu, the number of surviving larvae was too low (28.7 ± 2.4%) to determine the percentage of skeletal deformities. Figure 6 shows some of the main deformities in striped catfish under experimental conditions. Serious spinal abnormalities (i.e., kyphosis, lordosis, and scoliosis) were displayed with very low frequency, with a peak of 6% in larvae subject to 10 psu, but there were no significant differences between treatments. Interestingly, caudal vertebrae and branchiostegal ray abnormalities had a similar increasing pattern when the larvae were exposed to salinity increase. The highest level of abnormalities happened in caudal vertebrate found in the 15 psu treatment while the lowest was seen for 0 and 5 psu treatments (p = 0.002). The frequency of branchiostegal ray deformity was significantly lower in fish reared in freshwater (10.3 ± 2.0%) than in those under saline treatments (from 37.3 ± 5.4% to 49.0 ± 5.8%) (p = 0.001). We also found other skeletal abnormalities in the caudal fin and numbers of epural; however, the frequencies were very low and not significantly different between treatments (data not shown). Figure 7 describes some typical skeletal deformities observed in the present study.
Survival rate after heat shock at 39 °C
Survival rate differed significantly between all treatments (p < 0.001), except in treatments 0 psu and 10 psu (p = 0.051) (Fig. 8). The treatment of 5 psu had the highest survival rate (68.9%) while the lowest one was obtained in the treatment of 15 psu (0%) after 12.5 h of heat shock. Interestingly, after 6 h heat shock the survival rates were around 0 and 30% in treatments 15 and 0 psu, respectively. Subsequently, all treatments showed a stable pattern in survival rate, except for the group exposed to 10 psu that exhibited a significant decrease from 49 to 23% at 7.5 h.
Discussion
In this study, striped catfish larvae (20 days post-hatching) displayed high survival rates in all treatments, with more than 75% at the end of the salinity increase, except for the treatment of 20 psu with a survival rate of 28.7% only. This was similar to the findings of Nguyen et al. (2014) showing that the survival of striped catfish juveniles submitted to 18 psu was significantly lower than that of fish exposed to lower salinities. This suggests that striped catfish larvae can experimentally tolerate up to 15 psu, and a 10 psu environment does not affect its survival. However, rearing conditions over 15 psu seem to be outside the adaptive capacity of striped catfish larvae. Interestingly, Kumar et al. (2017) also found that striped catfish with an average weight of 0.40 ± 0.04 g only survived in 20 psu and 25 psu for 48 h and 18 h, respectively.
Besides the survival rate, the larvae showed an increase in weight gain when exposed to a hyperosmotic environment. This result is consistent with the reports on Nile tilapia larvae exposed to different salinities, which had the lowest weight in 0 psu salinity in comparison with treatments of 7.5, 15, 20, and 25 psu (Fridman et al. 2012b). While some freshwater fish such as channel catfish Ictalurus punctatus or goldfish Carassius auratus display the highest weight gain under salinity below 5 psu (Altinokand and Grizzle 2001). The increase of growth in euryhaline and marine fish is very often, but not systematically, found at intermediate salinities (from 8 to 20 psu). This could be due to a lower standard metabolic rate at the isosmotic salinity point (Bœuf and Payan 2001). Moreover, there is an interesting observation that better growth was exhibited in juvenile striped catfish exposed to a 10 psu environment (Nguyen et al. 2014). This result was possibly related to the isosmotic point of 9 psu in the species (Huong and Quyen 2012). However, the higher weight gain in fish exposed to 20 psu might be related to the low density is probably due to high mortality observed in this salinity treatment. It was in line with results obtained by other researchers that showed better growth performances at low densities in comparison with that of high densities because of less competition of food and better living environment (Dou et al. 2003; Bolasina et al. 2006). Additionally, drinking water in fish occurred when transferring to hyperosmotic environment (Fuentes and Eddy 1997) probably also explaining this result because growth was calculated on the basis of wet weight gain. Moreover, the mortality probably affected differentially the larvae according to their body weight, the biggest larvae being more resistant to a salinity of 20 psu, thus inducing a higher mean body weight of the few survivors in this group.
In teleosts, osmoregulatory capacity is one of the most important functions for adapting to different salinities, directly affecting salinity tolerance and, consequently, their survival in various saline environments (Varsamos et al. 2005). Although the osmolality measurement of WB homogenate or “body fluid” were affected by their composition including the mixture of blood, yolk, extra, and perhaps intracellular fluids (Davenport et al. 1981), some research have applied this technique as relative estimation of internal osmolality, due to the limitation of larval fish size (Henne and Watanabe 2003; Ostrowski et al. 2011; Fridman et al. 2012b). In the current study, the WB homogenate osmolality exhibited a significant increase with salinity in all treatments, except between the treatments of 0 and 5 psu. Similar results were found in striped catfish juveniles reared at 0 and 5 psu, while plasma osmolality remarkably rose in fish reared at 10 and 15 psu (Kumar et al. 2017). The results support the reports on two other catfish species, channel catfish and Indian catfish Heteropneustes fossilis, which displayed higher plasma osmolality in hyperosmotic environment (Goswami et al. 1983; Eckert et al. 2001; Schmitz et al. 2016) in comparison with the environmental osmolality. However, in the present study, the consistency of Na+/K+-ATPase activity through different salinities indicates that striped catfish larvae may not shift the function of the gill epithelium from ion uptake in freshwater to excretion in saltwater, leading to a plasma osmolality higher than that of the environment (Eckert et al. 2001). In addition to the osmoregulation through gill Na+/K+-ATPase activity, it was found that catfish reduced free water clearance, urine flow rate, and glomeruli when plasma osmolality increased (Eckert et al. 2001). This suggests that catfish may change the kidney function when exposed to a hyperosmotic environment. However, the elevation in electrolyte clearance and the decrease in free water clearance are ineffective for coping with the increased salt load (Eckert et al. 2001; Goswami et al. 1983). This was also reported in striped catfish juveniles. Specifically, plasma osmolality significantly increased but Na+/K+-ATPase activity was not significantly different when the fish were exposed to 0 psu and 10 psu water. Nevertheless, Na+/K+-ATPase activity sharply increased when the fish were exposed to 20 psu (Schmitz et al. 2016). Although gill cavities starts to appear in 2 days post-hatching in the larvae, it is required up to 2 weeks post-hatching for the complete metamorphosis (Islam 2005). The functional transition from skin to gill has not been deeply investigated yet in striped catfish so far; however, rainbow trout gills become the primary sites for Na+ uptake and O2 uptake (50–80% longer) at 8–12 and 23–28 days post-hatching, respectively (Fu et al. 2010). In the present study, therefore, the osmoregulation ability was slightly weaker in 20 days post-hatching larvae than in juvenile stage.
Freshwater fish are required to drink water in a hyperosmotic environment to compensate for water loss by the osmosis process, and this may affect enzymatic activities in teleosts through intestinal morphological changes especially in larval stages due to their immature digestive system and lower enzyme capacity (Usher et al. 1990; Moutou et al. 2004). In the present study, α-amylase, pepsin, and leu-ala activities showed a significant increase with salinity. Our results are in line with those of the euryhaline American shad Alosa sapidissima incubated and reared in freshwater that showed higher activities in some digestive enzymes in fish submitted to 21 psu compared to the corresponding fish in freshwater and lower salinity conditions (Hamed et al. 2016; Tsuzuki et al. 2007). In addition, the pepsin and α-amylase in snakehead (Channa striata) exhibited the increasing pattern with salinity gradient from freshwater to 9 psu in 28 °C (Lan et al. 2020). This may be affected by the activity of acid proteolysis (generated mostly by pepsin-like enzymatic activity) that displays a weaker activity in a low salinity environment (Vargas-Chacoff et al. 2015). The mechanisms of the end product absorption can partially explain the increase of digestive enzyme activity under a hyperosmotic environment (Harpaz et al. 2005). Specifically, the absorption of the end products such as glucose and amino acids depends on the Na+/K+-ATPase pump and, as a result, the increase of Na + concentration in the intestinal tract can cause a better absorption of carbohydrates and amino acids. Regarding morphological aspects, Nile tilapia juveniles submitted to a salinity challenge test led to an increasing number of goblet cells secreting mucin in the proximal intestine (Tran-Ngoc et al. 2017). Because mucin is a glycosylated protein, this morphological change might be considered to be one reason for improved α-amylase activity, since glycolytic chains, glycogen, and starch can stimulate amylase activity in fish larvae (Torrecillas et al. 2015; El Kertaoui et al. 2019). Regarding brush border enzymes, it has been established that alkaline phosphatase is expressed by active and mature mucosal enterocytes and is therefore indicative of enterocyte functional activity (Traber et al. 1991; Uni et al. 1998). In our study, the activities of alkaline phosphatase in the larvae exposed to a hyperosmotic environment were significantly higher than that of fish in hypoosmotic water. Caspian kutum (Rutilus kutum) larvae also showed a stronger activity of alkaline phosphatase when both acutely and chronically exposed to 10 psu saline compared to those exposed to a freshwater control treatment (Gheisvandi et al. 2015). As already mentioned, salinity can significantly alter intestinal morphology through increased goblet cell numbers as well as the thickness of the submucosa and lamina propria (Tran-Ngoc et al. 2017). The changes are involved in the improvement of brush border enzyme activities, which indicates the process of enterocyte maturation in larvae (Kvåle et al. 2007; El Kertaoui et al. 2019). However, another brush border enzyme, aminopeptidase, showed no significant differences in activity between treatments, although there was a slight increasing pattern with salinity. Summarily, the increases of enzymatic activities in striped catfish larvae might be highly related to the rise of water drinking that regulates activities of acid proteolysis, Na + concentration in intestine tract and/or morphology.
The link between osmoregulation and the immune system has been investigated in some species; however, the specific mechanism remains largely unknown. In the current study, the innate immune responses under hyperosmotic exposure in the larvae were assessed by measuring lysozyme, complement, peroxidase activities, and total Ig. An enhancement of lysozyme activity was also confirmed on some euryhaline and freshwater fish that were acutely and chronically exposed to hyperosmotic environments (Jiang et al. 2008; Marc et al. 1995; Schmitz et al. 2016; Yada et al. 2001). Peroxidase activity in the marine species gilthead seabream Sparus aurata did not change significantly under 12, 38, and 55 psu conditions after a 14-day acclimation period, but this response sharply increased in 55 psu after 100 days of acclimation (Cuesta et al. 2005). Also, spleen respiratory burst activity in striped catfish exposed to 20 psu was significantly higher than that of the corresponding fish reared at 0 psu (Schmitz et al. 2016). It was supposed that the hormones involved in osmoregulation, especially GH and cortisol, increased under the effects of hyperosmotic stressor and this is probably responsible for the modifications of immunological responses (Marc et al. 1995; Eckert et al. 2001; Yada et al. 2004). GH is involved in hypoosmoregulatory actions in teleosts and its elevation according to salinity has been reported in number of studies in a variety of species, including Atlantic salmon Salmo salar, rainbow trout, channel catfish, and Nile tilapia. These increases are not only found in plasma concentration (Arnesen et al. 2003; Drennon et al. 2003) but also on pituitary gene expression levels (Riley et al. 2002; Ágústsson et al. 2003). One possible mechanism of GH acting on the immune system is related to the phagocytic activity. Production of superoxide anions as a killing mechanism following phagocytosis was increased by GH administration in vitro and in vivo in rainbow trout and channel catfish (Sakai et al. 1995). In gene expression levels in the species, mRNA of superoxide dismutase catalyzing the dismutation of superoxide into oxygen and hydrogen peroxide was improved with GH (Yada et al. 2006). Furthermore, another study on rainbow trout showed that lysozyme secretion in neutrophils, the main source of plasma lysozyme, increased with in vitro GH concentration (Yada et al. 2004). Although the responses of lysozyme and peroxidase activities tended to increase at high salinities, especially at 20 psu, it may be related to short-term stressful effect due to hyperosmotic exposure. This is in agreement with the findings of Tort (2011) showing that the acute stressors stimulated some immune responses. In the present study, plasma glucose concentrations, one of the secondary stress indicators, increased significantly in fish submitted to 20 psu in comparison with fish kept in freshwater conditions (data in Appendix Fig. 9). The present study showed that ACH50 and total Ig in larvae did not significantly change under salinity exposure. In striped catfish juveniles, complement activity did not significantly change between treatments of 0, 10, and 20 psu after a 20-day salinity increase (Schmitz et al. 2016). Cuesta et al (2005) found that the complement activities and Ig in gilthead seabream may increase or decrease with salinity, depending on acclimation time.
In terms of deformity frequency, the larvae exposed to a freshwater environment generally exhibited the lowest rate of deformity of the caudal vertebrae and branchiostegal rays. These results agree with those on Acipenser persicus larvae indicating that salinity over 4 psu significantly affects cumulative abnormalities (Mardaneh Khatooni et al. 2012). Moreover, African catfish larvae exposed from 0 to 8 psu also displayed an increase of deformity rate with salinity increase (Borode et al. 2002). To some extent, a possible reason is that unfavorable salinity affects the deformity rate in larval stages (Ottesen and Bolla 1998; Cook et al. 2005). In our study, branchiostegal rays were significantly affected by the salinity increase. Operculum abnormalities, including branchiostegal rays, clearly decrease the quality of fish and thus the market value, but are not life-threatening (Beraldo et al. 2003). Also, caudal fin abnormalities did not lead to lethality in gilthead seabream larvae and the fish continued their development; however, the deformity might more seriously affect the fish quality in the latter stages (Koumoundouros et al. 1997). We found that the serious deformities kyphosis, lordosis, and scoliosis directly affecting larval survival and life activities had no remarkable differences in frequency between treatments. Skeletal deformity in teleost species may be related to many factors such as a lack of vitamins, contamination by heavy metals, and temperature (Berillis 2015). However, the mode of action of salinity on skeleton deformities is still poorly understood. One possible mechanism is related to calcium and phosphorus contents, which are the two main components in bone formation. In a hyperosmotic environment, the ratio of Ca/P increases because of the abundant level of calcium. The dietary Ca/P increase induces the elevation of skeletal deformity in pikeperch Sander lucioperca (El Kertaoui et al. 2019). Calcium transportation is closely associated to chloride cells and their number and maturation may be significantly affected by salinity (Ishihara and Mugiya 1987; Hwang 1990). Although chloride cells were not measured in the present study, the Na+/K+-ATPase activity mainly localized in chloride cells did not significantly change with salinity increase. The correlation between salinity, chloride cells, and deformity should be further investigated. In combination with the survival and weight gain results, it is suggested that the saline environment up to 15 psu may proportionally increase abnormality in striped catfish larvae; however, it does not significantly affect their survival and growth performance.
Interaction effects of salinity and temperature have been investigated in some fish species but the use of temperature tolerance as a stressor challenge test is still limited. It reported that the survival of striped catfish juveniles reared in freshwater was significantly lower than the corresponding survival at 6 and 12 psu and 35 °C (Phuc et al. 2017). On the other hand, the combination of salinity and temperature in euryhaline tilapia did not affect the survival rate (Qiang et al. 2013). In our study, larvae exposed to 5 psu showed the highest level of survival in the heat shock test, with a significant difference to that of freshwater and 15 psu. This is difficult to explain according to available data but is likely related to energy cost in stress adaptation that may be lower in larvae subject to salinity of 5 and 10 psu. Although there was a gradual decrease of survival in fish submitted to 10 psu after 7.5 h of heat shock, fish proportionally increased their tolerance to high temperature as shown by the higher survival in early period compared to that of 0 and 15 psu. Besides, the challenge temperature of 39 °C is significantly higher than the average temperatures of Mekong Delta, with a prediction of 33 °C during the twenty-first century (Change 2014). To some extent, exposure to 10 psu positively affected the tolerance of striped catfish larvae to heat stress, at least under aquaculture conditions.
Conclusions
Generally, we observed significant increases of enzymatic activities and immune responses in fish exposed to 15 and 20 psu compared to that in the lower salinities. Additionally, these effects may proportionally support the performance of weight gain. However, when combining the results of survival rate and tolerance to thermal stress, the intermediate salinities of 5 and 10 psu were considered as the better environment in striped catfish larvae. Regarding deformities, although the increased levels of salinity induced some skeletal abnormalities, the malformations were not lethal at this stage. Besides, the results suggest that 20 psu condition represents the upper salinity threshold due to the low survival rate. The results were observed within a short-time experiment; therefore, it is required in further studies to estimate how the fish acclimate to salinity in the long run.
References
Ágústsson T, Sundell K, Sakamoto T et al (2003) Pituitary gene expression of somatolactin, prolactin, and growth hormone during Atlantic salmon parr–smolt transformation. Aquaculture 222:229–238. https://doi.org/10.1016/S0044-8486(03)00124-8
Alderdice DF (1988) 3 Osmotic and ionic regulation in teleost eggs and larvae. Fish Physiol 11:163–251. https://doi.org/10.1016/S1546-5098(08)60200-9
Altinokand I, Grizzle JM (2001) Effects of brackish water on growth, feed conversion and energy absorption efficiency by juvenile euryhaline and freshwater stenohaline fishes. J Fish Biol 59:1142–1152. https://doi.org/10.1111/j.1095-8649.2001.tb00181.x
Arnesen AM, Toften H, Agustsson T et al (2003) Osmoregulation, feed intake, growth and growth hormone levels in 0+ Atlantic salmon (Salmo salar L.) transferred to seawater at different stages of smolt development. Aquaculture 222:167–187. https://doi.org/10.1016/S0044-8486(03)00109-1
Barman UK, Jana SN, Garg SK et al (2005) Effect of inland water salinity on growth, feed conversion efficiency and intestinal enzyme activity in growing grey mullet, Mugil cephalus (Linn.): field and laboratory studies. Aquac Int 13:241–256. https://doi.org/10.1007/s10499-004-2479-5
Beraldo P, Pinosa M, Tibaldi E, Canavese B (2003) Abnormalities of the operculum in gilthead sea bream (Sparus aurata): morphological description. Aquaculture 220:89–99. https://doi.org/10.1016/S0044-8486(02)00416-7
Berillis P (2015) Factors that can lead to the development of skeletal deformities in fishes: a review. J Fish Sci 9:17–23
Bessey OA, Lowry OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem 164:321–329
Van BD, Kantoush S, Sumi T (2020) Changes to long-term discharge and sediment loads in the Vietnamese Mekong Delta caused by upstream dams. Geomorphology 353:107011. https://doi.org/10.1016/j.geomorph.2019.107011
Bœuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol Part C Toxicol Pharmacol 130:411–423. https://doi.org/10.1016/S1532-0456(01)00268-X
Boglione C, Gagliardi F, Scardi M, Cataudella S (2001) Skeletal descriptors and quality assessment in larvae and post-larvae of wild-caught and hatchery-reared gilthead sea bream (Sparus aurata L. 1758). Aquaculture 192:1–22. https://doi.org/10.1016/S0044-8486(00)00446-4
Bolasina S, Tagawa M, Yamashita Y, Tanaka M (2006) Effect of stocking density on growth, digestive enzyme activity and cortisol level in larvae and juveniles of Japanese flounder, Paralichthys olivaceus. Aquaculture 259:432–443. https://doi.org/10.1016/j.aquaculture.2006.05.021
Borode A, Balogun A, Omoyeni B (2002) Effect of salinity on embryonic development, hatchability, and growth of African Catfish, Clarias gariepinus, eggs and larvae. J Appl Aquac 12:89–93. https://doi.org/10.1300/J028v12n04_08
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Caberoy NB, Quinitio GF (2000) Changes in Na+, K+-ATPase activity and gill chloride cell morphology in the grouper Epinephelus coioides larvae and juveniles in response to salinity and temperature. Fish Physiol Biochem 23:83–94. https://doi.org/10.1023/A:1007827331137
Change, IC (2014) Synthesis report summary chapter for policymakers. IPCC: Geneva, Switzerland, 31.
Cook MA, Guthrie KM, Rust MB, Plesha PD (2005) Effects of salinity and temperature during incubation on hatching and development of lingcod Ophiodon elongatus Girard, embryos. Aquac Res 36:1298–1303. https://doi.org/10.1111/j.1365-2109.2005.01346.x
Coughlan DJ, Gloss SP (1984) Early morphological development of gills in smallmouth bass (Micropterus dolomieui). Can J Zool 62:951–958. https://doi.org/10.1139/z84-134
Cuesta A, Laiz-Carrion R, Del Rio MPM et al (2005) Salinity influences the humoral immune parameters of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 18:255–261. https://doi.org/10.1016/j.fsi.2004.07.009
Cuvier-Péres A, Kestemont P (2001) Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol Biochem 24:279–285. https://doi.org/10.1023/A:1015033300526
Davenport J, Lønning S, Kjørsvik E (1981) Osmotic and structural changes during early development of eggs and larvae of the cod, Gadus morhua L. J Fish Biol 19:317–331. https://doi.org/10.1111/j.1095-8649.1981.tb05835.x
Dou S, Masuda R, Tanaka M, Tsukamoto K (2003) Identification of factors affecting the growth and survival of the settling Japanese flounder larvae, Paralichthys olivaceus. Aquaculture 218:309–327. https://doi.org/10.1016/S0044-8486(02)00531-8
Drennon K, Moriyama S, Kawauchi H et al (2003) Development of an enzyme-linked immunosorbent assay for the measurement of plasma growth hormone (GH) levels in channel catfish (Ictalurus punctatus): assessment of environmental salinity and GH secretogogues on plasma GH levels. Gen Comp Endocrinol 133:314–322. https://doi.org/10.1016/S0016-6480(03)00194-1
Eckert SM, Yada T, Shepherd BS et al (2001) Hormonal control of osmoregulation in the channel catfish Ictalurus punctatus. Gen Comp Endocrinol 122:270–286. https://doi.org/10.1006/GCEN.2001.7633
El Kertaoui N, Lund I, Assogba H et al (2019) Key nutritional factors and interactions during larval development of pikeperch (Sander lucioperca). Sci Rep 9:7074. https://doi.org/10.1038/s41598-019-43491-1
FAO (2020) GLOBEFISH Highlights January 2020 ISSUE, with Jan. – Sep. 2019 Statistics – a quarterly update on world seafood markets. Globefish Highlights no. 1–2020. Rome
Fashina Bombata HA, Busari AN (2003) Influence of salinity on the developmental stages of African catfish Heterobranchus longifilis (Valenciennes, 1840). Aquaculture 224:213–222. https://doi.org/10.1016/S0044-8486(03)00273-4
Fridman S, Bron J, Rana K (2012a) Ontogenic changes in the osmoregulatory capacity of the Nile tilapia Oreochromis niloticus and implications for aquaculture. Aquaculture 356–357:243–249. https://doi.org/10.1016/j.aquaculture.2012.05.010
Fridman S, Bron J, Rana K (2012b) Influence of salinity on embryogenesis, survival, growth and oxygen consumption in embryos and yolk-sac larvae of the Nile tilapia. Aquaculture 334–337:182–190. https://doi.org/10.1016/j.aquaculture.2011.12.034
Fu C, Wilson JM, Rombough PJ, Brauner CJ (2010) Ions first: Na+ uptake shifts from the skin to the gills before O2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proceedings Biol Sci 277:1553–1560. https://doi.org/10.1098/rspb.2009.1545
Fuentes J, Eddy FB (1997) Drinking in Atlantic salmon presmolts and smolts in response to growth hormone and salinity. Comp Biochem Physiol Part A Physiol 117:487–491. https://doi.org/10.1016/S0300-9629(96)00397-0
Gheisvandi N, Hajimoradloo A, Ghorbani R, Hoseinifar SH (2015) The effects of gradual or abrupt changes of salinity on digestive enzymes activity of Caspian kutum, Rutilus kutum (Kamensky, 1901) larvae. J Appl Ichthyol 31https://doi.org/10.1111/jai.12891
Goel MK, Khanna P, Kishore J (2010) Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res 1:274–278. https://doi.org/10.4103/0974-7788.76794
Goswami SV, Parwez I, Sundararaj BI (1983) Some aspects of osmoregulation in a stenohaline freshwater catfish, Heteropneustes fossilis (Bloch), in different salinities. J Fish Biol 23:475–487. https://doi.org/10.1111/j.1095-8649.1983.tb02928.x
Haddy JA, Pankhurst NW (2000) The effects of salinity on reproductive development, plasma steroid levels, fertilisation and egg survival in black bream Acanthopagrus butcheri. Aquaculture 188:115–131. https://doi.org/10.1016/S0044-8486(00)00326-4
Hamed SS, Jiddawi NS, Poj B (2016) Effect of salinity levels on growth, feed utilization, body composition and digestive enzymes activities of juvenile silver pompano Trachinotus blochii. Int Fish Aquat Stud 4:279–283
Harpaz S, Hakim Y, Slosman T, Eroldogan OT (2005) Effects of adding salt to the diet of Asian sea bass Lates calcarifer reared in fresh or salt water recirculating tanks, on growth and brush border enzyme activity. Aquaculture 248:315–324. https://doi.org/10.1016/J.AQUACULTURE.2005.03.007
Henne JP, Watanabe WO (2003) Effects of light intensity and salinity on growth, survival, and whole-body osmolality of larval southern flounder Paralichthys lethostigma. J World Aquac Soc 34:450–465. https://doi.org/10.1111/j.1749-7345.2003.tb00084.x
Holm H, Hanssen LE, Krogdahl A, Florholmen J (1988) High and low inhibitor soybean meals affect human duodenal proteinase activity differently: in vivo comparison with bovine serum albumin. J Nutr 118:515–520. https://doi.org/10.1093/jn/118.4.515
Huong D, Quyen N (2012) The effect of salinity on the embryonic development and osmoregulatory of the striped catfish (Pangasianodon hypophthalmus) larvae and fingerling stages. J Sci Tho Univ 29–37
Hwang PP (1990) Salinity effects on development of chloride cells in the larvae of ayu (Plecoglossus altivelis). Mar Biol 107:1–7. https://doi.org/10.1007/BF01313236
Ishihara A, Mugiya Y (1987) Ultrastructural evidence of calcium uptake by chloride cells in the gills of goldfish, Carassius auratus. J Exp Zool 242:121–129. https://doi.org/10.1002/jez.1402420202
Islam A (2005) Embryonic and larval development of Thai Pangas (Pangasius sutchi Fowler, 1937). Dev Growth Differ 47:1–6. https://doi.org/10.1111/j.1440-169x.2004.00773.x
Izquierdo MS, Scolamacchia M, Betancor M et al (2013) Effects of dietary DHA and alpha-tocopherol on bone development, early mineralisation and oxidative stress in Sparus aurata (Linnaeus, 1758) larvae. Br J Nutr 109:1796–1805. https://doi.org/10.1017/S0007114512003935
Jiang I-F, Bharath Kumar V, Lee D-N, Weng C-F (2008) Acute osmotic stress affects Tilapia (Oreochromis mossambicus) innate immune responses. Fish Shellfish Immunol 25:841–846. https://doi.org/10.1016/J.FSI.2008.09.006
Katoh F, Shimizu A, Uchida K, Kaneko T (2000) Shift of chloride cell distribution during early life stages in seawater-adapted killifish, Fundulus heteroclitus. Zoolog Sci 17:11–18. https://doi.org/10.2108/zsj.17.11
Kim J-H, Park H-J, Kim K-W et al (2017) Growth performance, oxidative stress, and non-specific immune responses in juvenile sablefish, Anoplopoma fimbria, by changes of water temperature and salinity. Fish Physiol Biochem 43:1421–1431. https://doi.org/10.1007/s10695-017-0382-z
Koumoundouros G, Gagliardi F, Divanach P et al (1997) Normal and abnormal osteological development of caudal fin in Sparus aurata L. fry. Aquaculture 149:215–226. https://doi.org/10.1016/S0044-8486(96)01443-3
Kültz D (2015) Physiological mechanisms used by fish to cope with salinity stress. J Exp Biol 218:1907–1914. https://doi.org/10.1242/jeb.118695
Kumar A, Harikrishna V, Krishna Reddy A et al (2017) Salinity tolerance of Pangasianodon hypophthalmus in inland saline water: effect on growth, survival and haematological parameters. Eco Env Cons 23:475–482
Kvåle A, Mangor-Jensen A, Moren M et al (2007) Development and characterisation of some intestinal enzymes in Atlantic cod (Gadus morhua L.) and Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Aquaculture 264:457–468. https://doi.org/10.1016/J.AQUACULTURE.2006.12.024
Lan TTP, Hien TTT, Le Cam TuT et al (2020) Salinization intensifies the effects of elevated temperatures on Channa striata, a common tropical freshwater aquaculture fish in the Mekong Delta. Vietnam Fish Sci 86:1029–1036. https://doi.org/10.1007/s12562-020-01463-9
Li J, Eygensteyn J, Lock R et al (1995) Branchial chloride cells in larvae and juveniles of freshwater tilapia Oreochromis mossambicus. J Exp Biol 198:2177–2184
Likongwe JS, Stecko TD, Stauffer JR, Carline RF (1996) Combined effects of water temperature and salinity on growth and feed utilization of juvenile Nile tilapia Oreochromis niloticus (Linneaus). Aquaculture 146:37–46. https://doi.org/10.1016/S0044-8486(96)01360-9
Marc AM, Quentel C, Severe A et al (1995) Changes in some endocrinological and non-specific immunological parameters during seawater exposure in the brown trout. J Fish Biol 46:1065–1081. https://doi.org/10.1111/j.1095-8649.1995.tb01410.x
Mardaneh Khatooni M, Mojazi Amiri B, Mirvaghefi A et al (2012) The effects of salinity on the fertilization rate and rearing of the Persian sturgeon (Acipenser persicus) larvae. Aquac Int 20:1097–1105. https://doi.org/10.1007/s10499-012-9560-2
Maroux S, Louvard D, Baratti J (1973) The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta 321:282–295. https://doi.org/10.1016/0005-2744(73)90083-1
McCormick SD (1993) Methods for nonlethal gill biopsy and measurement of Na+, K+ and -ATPase activity. Can J Fish Aquat Sci 50:656–658
Metais P, Bieth J (1968) Determination of alpha-amylase by a microtechnic. Ann Biol Clin (paris) 26:133–142
Milla S, Mathieu C, Wang N et al (2010) Spleen immune status is affected after acute handling stress but not regulated by cortisol in Eurasian perch, Perca fluviatilis. Fish Shellfish Immunol 28:931–941. https://doi.org/10.1016/j.fsi.2010.02.012
Moutou K, Panagiota P, Mamuris Z (2004) Effects of salinity on digestive protease activity in the euryhaline sparid Sparus aurata L.: a preliminary study. Aquac Res - AQUAC RES 35:912–914. https://doi.org/10.1111/j.1365-2109.2004.01068.x
Nguyen PTH, Do HTT, Mather PB, Hurwood DA (2014) Experimental assessment of the effects of sublethal salinities on growth performance and stress in cultured tra catfish (Pangasianodon hypophthalmus). Fish Physiol Biochem 40:1839–1848. https://doi.org/10.1007/s10695-014-9972-1
Nicholson JA, Kim YS (1975) A one-step l-amino acid oxidase assay for intestinal peptide hydrolase activity. Anal Biochem 63:110–117. https://doi.org/10.1016/0003-2697(75)90194-3
Okamoto T, Kurokawa T, Gen K et al (2009) Influence of salinity on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. Aquaculture 293:113–118. https://doi.org/10.1016/j.aquaculture.2009.04.005
Ostrowski AD, Watanabe WO, Montgomery FP et al (2011) Effects of salinity and temperature on the growth, survival, whole body osmolality, and expression of Na+/K+ ATPase mRNA in red porgy (Pagrus pagrus) larvae. Aquaculture 314:193–201. https://doi.org/10.1016/J.AQUACULTURE.2011.02.010
Ottesen OH, Bolla S (1998) Combined effects of temperature and salinity on development and survival of Atlantic halibut larvae. Aquac Int 6:103–120. https://doi.org/10.1023/A:1009234122861
Overton J, Bayley M, Paulsen H, Wang T (2008) Salinity tolerance of cultured Eurasian perch, Perca fluviatilis L.: effects on growth and on survival as a function of temperature. Aquaculture 277:282–286. https://doi.org/10.1016/j.aquaculture.2008.02.029
Phuc NTH, Mather PB, Hurwood DA (2017) Effects of sublethal salinity and temperature levels and their interaction on growth performance and hematological and hormonal levels in tra catfish (Pangasianodon hypophthalmus). Aquac Int 25:1057–1071. https://doi.org/10.1007/s10499-016-0097-7
Pujante IM, Moyano FJ, Martos-Sitcha JA et al (2018) Effect of different salinities on gene expression and activity of digestive enzymes in the thick-lipped grey mullet (Chelon labrosus). Fish Physiol Biochem 44:349–373. https://doi.org/10.1007/s10695-017-0440-6
Qiang J, Wang H, Kpundeh MD et al (2013) Effect of water temperature, salinity, and their interaction on growth, plasma osmolality, and gill Na+, K+-ATPase activity in juvenile GIFT tilapia Oreochromis niloticus (L.). J Therm Biol 38:331–338. https://doi.org/10.1016/J.JTHERBIO.2013.04.002
Riley LG, Richman NH 3rd, Hirano T, Gordon GE (2002) Activation of the growth hormone/insulin-like growth factor axis by treatment with 17 alpha-methyltestosterone and seawater rearing in the tilapia. Oreochromis Mossambicus Gen Comp Endocrinol 127:285–292. https://doi.org/10.1016/S0016-6480(02)00051-5
Ronkin D, Seroussi E, Nitzan T et al (2015) Intestinal transcriptome analysis revealed differential salinity adaptation between two tilapiine species. Comp Biochem Physiol Part D Genomics Proteomics 13:35–43. https://doi.org/10.1016/j.cbd.2015.01.003
Sakai M, Kobayashi M, Kawauchi H (1995) Enhancement of chemiluminescent responses of phagocytic cells from rainbow trout, Oncorhynchus mykiss, by injection of growth hormone. Fish Shellfish Immunol 5:375–379. https://doi.org/10.1006/FSIM.1995.0035
Schmitz M, Douxfils J, Mandiki SNM et al (2016) Chronic hyperosmotic stress interferes with immune homeostasis in striped catfish (Pangasianodon hypophthalmus, S.) and leads to excessive inflammatory response during bacterial infection. Fish Shellfish Immunol 55:550–558. https://doi.org/10.1016/j.fsi.2016.06.031
Torrecillas S, Montero D, Caballero MJ et al (2015) Effects of dietary concentrated mannan oligosaccharides supplementation on growth, gut mucosal immune system and liver lipid metabolism of European sea bass (Dicentrarchus labrax) juveniles. Fish Shellfish Immunol 42:508–516. https://doi.org/10.1016/J.FSI.2014.11.033
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35:1366–1375. https://doi.org/10.1016/j.dci.2011.07.002
Traber PG, Gumucio DL, Wang W (1991) Isolation of intestinal epithelial cells for the study of differential gene expression along the crypt-villus axis. Am J Physiol - Gastrointest Liver Physiol 260:G895–G903. https://doi.org/10.1152/ajpgi.1991.260.6.G895
Tran-Ngoc KT, Schrama JW, Le MTT et al (2017) Salinity and diet composition affect digestibility and intestinal morphology in Nile tilapia (Oreochromis niloticus). Aquaculture 469:36–43. https://doi.org/10.1016/J.AQUACULTURE.2016.11.037
Tsuzuki MY, Sugai JK, Maciel JC et al (2007) Survival, growth and digestive enzyme activity of juveniles of the fat snook (Centropomus parallelus) reared at different salinities. Aquaculture 271:319–325. https://doi.org/10.1016/J.AQUACULTURE.2007.05.002
Uni Z, Ganot S, Sklan D (1998) Posthatch development of mucosal function in the broiler small intestine. Poult Sci 77:75–82. https://doi.org/10.1093/ps/77.1.75
Usher ML, Talbot C, Eddy FB (1990) Effects of transfer to seawater on digestion and gut function in Atlantic salmon smolts (Salmo salar L.). Aquaculture 90:85–96. https://doi.org/10.1016/0044-8486(90)90285-U
van der Heijden AJ, van der Meij JC, Flik G, Wendelaar Bonga SE (1999) Ultrastructure and distribution dynamics of chloride cells in tilapia larvae in fresh water and sea water. Cell Tissue Res 297:119–130. https://doi.org/10.1007/s004410051339
Vargas-Chacoff L, Saavedra E, Oyarzún R et al (2015) Effects on the metabolism, growth, digestive capacity and osmoregulation of juvenile of Sub-Antarctic Notothenioid fish Eleginops maclovinus acclimated at different salinities. Fish Physiol Biochem 41:1369–1381. https://doi.org/10.1007/s10695-015-0092-3
Varsamos S, Nebel C, Charmantier G (2005) Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol A Mol Integr Physiol 141:401–429. https://doi.org/10.1016/j.cbpb.2005.01.013
Woo NYS, Tong WCM (1982) Salinity adaptation in the snakehead, Ophiocephalus maculatus Lacépède: changes in oxygen consumption, branchial Na+-K+-ATPase and body composition. J Fish Biol 20:11–19. https://doi.org/10.1111/j.1095-8649.1982.tb03890.x
Worthington TM (1982) Enzymes and related biochemicals. Biochemical Products Division. Worthington Diagnostic System Freehold, NJ
Yada T, Azuma T, Takagi Y (2001) Stimulation of non-specific immune functions in seawater-acclimated rainbow trout, Oncorhynchus mykiss, with reference to the role of growth hormone. Comp Biochem Physiol Part B Biochem Mol Biol 129:695–701. https://doi.org/10.1016/S1096-4959(01)00370-0
Yada T, Kaiya H, Mutoh K et al (2006) Ghrelin stimulates phagocytosis and superoxide production in fish leukocytes. J Endocrinol 189:57–65. https://doi.org/10.1677/joe.1.06187
Yada T, Muto K, Azuma T, Ikuta K (2004) Effects of prolactin and growth hormone on plasma levels of lysozyme and ceruloplasmin in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 139:57–63. https://doi.org/10.1016/j.cca.2004.09.003
Acknowledgements
The authors thank to the Académie de Recherche et d’Enseignement Supérieur (ARES) and the General Directorate and Development for Cooperation (DGD) in Belgium through the Research for Development Project (RDP) “Towards a sustainable pangasius breeding, a selection approach (PANGAGEN).”
Funding
The research was supported by the Académie de Recherche et d’Enseignement Supérieur (ARES) and the General Directorate and Development for Cooperation (DGD) in Belgium.
Author information
Authors and Affiliations
Contributions
Patrick Kestemont, Frédéric Farnir, and Nguyen Thanh Phuong designed and coordinated the project. Dang Quang Hieu carried out the experiment and data analysis. Bui Thi Bich Hang, Do Thi Thanh Huong, and Najlae El Kertaoui supported to carry out and analyzing tools. Dang Quang Hieu and Patrick Kestemont drafted the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Completing interests
The authors declare no completing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dang Quang Hieu and Bui Thi Bich Hang are co-first authors
Rights and permissions
About this article
Cite this article
Hieu, D.Q., Hang, B.T.B., Huong, D.T.T. et al. Salinity affects growth performance, physiology, immune responses and temperature resistance in striped catfish (Pangasianodon hypophthalmus) during its early life stages. Fish Physiol Biochem 47, 1995–2013 (2021). https://doi.org/10.1007/s10695-021-01021-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-01021-9