Abstract
In this study we assessed the influence of three different environmental salinities (5, 15 and 31 psu during 90 days) on growth, osmoregulation, energy metabolism and digestive capacity in juveniles of the Notothenioid fish Eleginops maclovinus. At the end of experimental time samples of plasma, liver, gill, intestine, kidney, skeletal muscle, stomach and pyloric caeca were obtained. Growth, weight gain, hepatosomatic index and specific growth rate increased at 15 and 31 psu and were lower at 5 psu salinity. Gill Na+, K+-ATPase (NKA) activity presented a “U-shaped” relationship respect to salinity, with its minimum rates at 15 psu, while this activity correlated negatively with salinity at both anterior and posterior intestinal portions. No significant changes in NKA activity were observed in kidney or mid intestine. Large changes in plasma, metabolite levels and enzymatic activities related to energy metabolism in liver, gill, intestine, kidney and muscle were generally found in the groups exposed to 5 and 31 psu compared to the 15 psu group. Only the pepsin activity (digestive enzymes) assessed enhanced with environmental salinity, while pyloric caeca trypsin/chymotrypsin ratio decreased. This study suggests that juvenile of E. maclovinus presents greater growth near its iso-osmotic point (15 psu) and hyperosmotic environment (31 psu). Acclimation to low salinity increased the osmoregulatory expenditure as seen by the gill and anterior intestine results, while at high salinity, branchial osmoregulatory activity was also enhanced. This requires the mobilization of lipid stores and amino acids, thereby holding the growth of fish back. The subsequent reallocation of energy sources was not sufficient to maintain the growth rate of fish exposed to 5 psu. Thus, E. maclovinus juveniles present better growth efficiencies in salinities above the iso-osmotic point and hyperosmotic environment of this species, showing their best performance at 15 psu as seen by the main osmoregulatory and energy metabolism enzymatic activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The acclimation of euryhaline teleosts to different environmental salinities induces osmoregulatory changes in order to maintain their internal milieu within “safe” physiological ranges. These changes involve structural and functional modifications in several osmoregulatory organs (such as gill, kidney or intestine) as well as changes in several physiological processes (tegumental permeability and/or drinking rate) associated to osmoregulation (McCormick 1995; Marshall 2002; Mancera and McCormick 2007). These studies also demonstrate that salinity acclimation induces metabolic reorganization to meet the energetic demands of osmoregulation (Boeuf and Payan 2001; Soengas et al. 2007). Thus, in several fish species, growth rates were higher at environmental salinities close to the isoosmotic point of their internal fluids (see Boeuf and Payan 2001). Abetting this, several studies have examined intermediate metabolism changes in euryhaline fishes after short- and long-term acclimation to environmental salinity (Sangiao-Alvarellos et al. 2003, 2005; Arjona et al. 2007; Herrera et al. 2009; Vargas-Chacoff et al. 2009a), and long-term periods (Laiz-Carrión et al. 2005; Arjona et al. 2009; Nikolopoulou et al. 2011). Moreover, changes in the digestive capacity due to modifications in environmental salinity have been also reported (Moutou et al. 2004; Psochiou et al. 2007; Nikolopoulou et al. 2011).
Eleginops maclovinus (Valenciennes 1830) is a monotypic species of the Family Eleginopidae (Osteichthyes), suborder Notothenioidei. This species is distributed mainly in marine and estuarine environments in southern South America, but also appears in limnetic environments (Pequeño et al. 2010). In addition, this species is considered to be a good option for the aquaculture industry in Chile (Sá et al. 2014). Our group previously assessed, in a time-course survey of 14 days, the effects of acclimation to four different environmental salinities (5, 15, 31 and 45 psu). According to our results, E. maclovinus is able to adjust its osmotic and metabolic systems, through allostatic modifications, across a wide range of environmental salinities (Vargas-Chacoff et al. 2014a). The objective of the present study is to examine the influence of three environmental salinities (5, 15, and 31 psu) on the growth, osmoregulation, energy metabolism, and digestive capacity of E. maclovinus juveniles. Insights into the effects of environmental salinity on E. maclovinus physiology will provide a valuable tool to improve the potential culture of this species.
Materials and methods
Fish
Immature individuals of Sub-Antarctic Patagonian blennie (E. maclovinus) (143 ± 5 g body mass) were captured in the Valdivia river estuary and transported to the Calfuco coastal laboratory of the Sciences Faculty, Universidad Austral de Chile, Chile. Fish were acclimated for 5 weeks to seawater (SW, 31 psu, 1085 mOsm kg−1), maintained at a density of 2 kg m−3 in a flow-through system in 500 L tanks with natural photoperiod and temperature (12.0 ± 0.5 °C). Fish were fed daily with commercial dry pellets (Skretting Nutrece Defense 100™, containing 48 % protein, 22 % fat, 13 % carbohydrate, 8 % moisture and 8.5 % ash, energy 22.3 MJ kg−1) using a feeding rate of 1 % of body weight. Lack of appetite was not observed in fish at any time.
Experimental design
The juvenile fish (n = 48) acclimated at SW were randomly divided into 3 groups and transferred directly to 500 L tanks containing one of three salinity conditions: (1) 5 psu (140 mOsm kg−1), (2) 15 psu (364 mOsm kg−1) and (3) 31 psu (SW, control, 1085 mOsm kg−1) with duplicate tanks (n = 8 animals per tank, 2.4 kg m−3 of density). Experimental salinities were achieved by mixing full-strength SW with dechlorinated fresh water and maintained in a recirculation system (using SunSun Outside Filter Hw-304b). Then, according to the protocol described previously by our research group fish were kept in those salinities for 1 week before the start of the experiment (Vargas-Chacoff et al. 2009a, 2011, 2014a). The experiment was performed during a period of 90 days; water quality parameters (hardness, levels of oxygen, carbon dioxide, hydrogen sulphide, nitrite, nitrate, ammonia, calcium, chlorine and suspended solids) were assayed weekly. Salinity was measured daily in the middle of the water column with a refractometer (ATAGO S/MILL), and corrected when necessary. Tanks were cleaned and siphoned daily. Fish were not fed for 24 h before sampling. No mortality was recorded during this period. The experiments complied with the guidelines of the “Comisión Nacional de Ciencia y Tecnología de Chile (CONICYT)” and the “Universidad Austral de Chile” for the use of laboratory animals.
All fish were captured, slightly anaesthetized with 2-phenoxyethanol (0.5 mL L−1 water) and measured for body weight and total length at 0, 15, 30, 45, 60, 75 and 90 days. At these times feeding doses were recalculated. At the end of the experiment (day 90), fish were collected and sampled for analytical procedures. In order to evaluate the effects of salinity on growth of E. maclovius, the following parameters were calculated: Weight gain (%) and specific growth rate SGR, were calculated as Weight gain (%) = [(W f − W i)/W i] × 100 and SGR = [(ln W f − ln W i)/T] × 100, where W f is the final weight (g), W i is the initial weight (g) and T is the number of days (90) from the start until the end of the experiment. The fish were weighted individually but they were not marked (i.e. pit tag), therefore the weight is means of tank.
Sampling procedure
At the end of the experiment, fish were netted and sacrificed with a lethal dose of 2-phenoxyethanol (1 mL L−1 water). Blood was collected from the caudal peduncle into 1 mL heparinized syringes (25,000 units ammonium heparin 3 mL−1 saline solution 0.6 % NaCl). Plasma was separated from cells by centrifugation of whole blood (5 min, 2000 g, 4 °C), snap frozen in liquid N2 and stored at −80 °C until analysis. From each fish the left second gill arch was taken, dried with absorbent paper and 3–5 branchial filaments were obtained using fine-point scissors. A small section of the posterior portion of the kidney was taken. Samples of the anterior, mid and posterior intestine were also taken. The intestinal epithelium was obtained by scraping with a scalpel blade. These tissue portions were placed in 100 µL ice-cold sucrose-EDTA-imidazole (SEI) buffer (150 mM sucrose, 10 mM EDTA, 50 mM imidazole, pH 7.3) and frozen at −80 °C. From each fish a gill arch (dried with absorbent paper), the remaining kidney, stomach, pyloric caeca, muscle and liver were collected, frozen in liquid N2 and stored at −80 °C until assay. The liver was weighed to calculate the hepatosomatic index (HSI), where HSI = (liver wet weight/body weight) × 100.
Plasma parameters
Plasma osmolality was measured with a vapour pressure osmometer (Advanced Instruments, INC, model 3320) and expressed as mOsm kg−1. Plasma glucose, lactate and triglycerides (TAG) were measured using commercial kits from Spinreact (Glucose-HK Ref. 1001200; Lactate Ref. 1001330 and Triglycerides Ref. 1001311) adapted to 96-well microplates. Plasma protein was determined using the Pierce BCA Protein Assay Kit (#23225). Total α-amino acid levels were assayed colorimetrically using the ninhydrin method of Moore (1968) adapted to a microplate assay. All assays were performed with a Microplate Reader (Multizcan GO, Thermo Scientific), using Software Scan 3.2 to Multizcan GO. Plasma cortisol was measured by ELISA using a commercial kit from DIA Source Immuno Assays S.A. (Cortisol Ref. KAPDB270): the inter-assay coefficient of variation at 50 % binding was 5.6 % (n = 4), while the mean intra-assay coefficient of variation (calculated from the sample duplicates) was 5.6 %; the mean percentage of recovery was 95 % (n = 4); main cross-reactivity 100 %, given by cortisol, was detected with prednisolone (13.6 %), deoxycorticosterone (7.2 %), cortisone (6.2 %) and corticosterone (7.6 %).
Gill, Kidney and Intestine NA+, K+-ATPase (NKA) Activity
Gill, kidney and intestine (anterior, mid and posterior portions) NKA activities were determined using the micro-assay method of McCormick (1993) with the modification for non-salmonid fish described by Mancera et al. (2002). Sample total protein was measured in triplicate in undiluted samples (Pierce BCA Protein kit, #23225) using bovine serum albumin (provided by the kit) as standard. Both assays were run on a Microplate Reader (Multiscango, Thermo Scientific), using Software Scan 3.2 to Multizcan GO.
Tissue metabolites and enzymatic activities
Frozen liver, gills, kidney, and muscle were finely minced in an ice-cooled Petri dish and divided into two aliquots to assess enzymatic activities and metabolite levels. The frozen tissue used for the assessment of metabolite concentration was homogenised by ultrasonic disruption with 7.5 vols of ice-cooled 0.6 N perchloric acid, neutralized (using 1 M potassium bicarbonate), centrifuged (30 min at 13,000 g, Eppendorf 5415R), and the supernatant used to assay tissue metabolite levels. Tissue lactate and triglyceride levels were determined spectrophotometrically using commercial kits (Spinreact, see before). Tissue glycogen concentrations were assessed using the method of Keppler and Decker (1974). Glucose obtained after glycogen breakdown (after subtracting free glucose levels) was determined with a commercial kit (Spinreact, see above). Total α-amino acid levels were assessed colorimetrically using the ninhydrin method of Moore (1968) adapted to a microplate assay. The water content in the muscle was measured according to Tipsmark et al. (2002). The aliquots of tissues used for the assessment of enzymatic activities were homogenized by ultrasonic disruption with 10 vol of ice-cold stopping-buffer containing 50 mM HCl (pH 7.5), 1 mM 2-mercaptoethanol, 50 mM NaF, 4 mM 165 EDTA, 250 mM sucrose and 0.5 mM p-methyl-sulphonylfluoride (Sigma Chemical Co., St. Louis, MO, USA), the last added as dry crystals immediately before homogenization. The homogenate was centrifuged (30 min at 13,000 g) and the supernatant used in enzyme assays of FBPase (Fructose 1,6-bisphosphatase, EC 3.1.3.11), G3PDH (Glycerol-3-phosphate dehydrogenase, EC 1.1.1.8), G6PDH (Glucose-6-phosphate dehydrogenase, EC 1.1.1.49), GDH (Glutamate dehydrogenase, EC 1.4.1.2), HK (Hexokinase, EC 2.7.1.1), LDH-O (Lactate dehydrogenase-oxidase, EC 1.1.1.27), GPT (Alanine aminotransferase, EC 2.6.1.2) and GOT (Aspartate aminotransferase, EC 2.6.1.1). Enzymatic activities were determined using a Microplate Reader (Multiscango, Thermo Scientific), using Software Scan 3.2 to Multizcan GO. Reaction rates of enzymes were determined by changes in absorbance of NAD(P)H at 340 nm. The reactions were started by addition of homogenates (15 μL) at a pre-established protein concentration, omitting the substrate in control wells (final volume of 275–295 μL) and allowing the reactions to proceed at 37 °C for pre-established times (5–15 min). Protein levels were assayed in triplicate. Enzyme assays were carried out at initial velocity conditions. The specific conditions for enzyme assays have been described elsewhere (Sangiao-Alvarellos et al. 2005; Vargas-Chacoff et al. 2009a, b, 2014b, c).
Digestive enzymatic activity
For digestive enzyme extracts, stomach and pyloric caeca tissues were finely minced in an ice-cooled Petri dish. Tissue homogenization was performed using an ultraturrax (model: HG-15A, Analog Homogenizer). Pyloric caeca were homogenized using a buffer solution of 50 mM Tris–HCl pH 7.5 (Alarcón et al. 1998). In the case of stomach tissue, the homogenization was performed using only cold distilled water. Homogenization of both tissues was performed at a temperature below 4 °C. Subsequently, the homogenates were centrifuged at 16,000 g for 30 min at 4 °C and the supernatant recovered and stored at −80 °C until further use. For measurements of leucine-aminopeptidase activity, aliquots of homogenates (not centrifuged to avoid underestimating enzymes) were stored. Determinations of the soluble protein in the enzyme extract were tested in triplicate, using the Quick Start Kit (Bio Rad, Hercules, CA), using bovine serum albumin as a standard protein. In the stomach, the pepsin-like or total acid proteolytic activity was quantified with the method of Sarath et al. (1989) using 2 % haemoglobin as substrate. For pyloric caeca, total alkaline proteolytic activity was determined using 5 % casein as substrate according to the method of Walter (1984); trypsin-like activity was quantified using 560 µL of 1 mM BAPNA (N-α-benzoyl-dl-arginine-p-nitroanilide) as substrate and 80 µL of enzyme extract according Erlanger et al. (1961); chymotrypsin activity was quantified by the method of Hummel (1959) using 0.56 mM BTEE (N-benzoyl-l-tyrosine ethyl ester) as substrate. Leucine aminopeptidase activity was quantified using 1.2 mM l-leucine-p-nitroanilide as substrate according to the method of Appel (1974). The α-amylase-like activity was quantified using 0.1 % soluble starch as substrate (Worthington and Worthington 2011). Finally, the activity of bile salt-dependent lipase was quantified using 1 mL of a solution of 4-nitrophenyl caproate (4-NPC, 100 mM ethanol to give a final concentration of 0.35 mM in the reaction mixture), 6 mM sodium taurocholate (Na-TC), 0.1 M NaCl and 0.5 M Tris–HCl at pH 7.4 as substrate according to Gjellesvik et al. (1992).
Statistics
Assumptions of both normality and homogeneity of the variances were tested. For each physiological variable, a nested ANOVA from General Linear Model (GLM) was performed. This model was chosen assuming the duplicate tanks as nested factors (Quinn and Keough 2002), while the factor of variance is the salinity. Tanks were considered as a random effect and treatment groups (salinities) as fixed effects. A Tukey post hoc test was used to identify significantly different groups. Differences in the experiment were considered to be significant at a level of P < 0.05.
Results
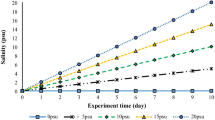
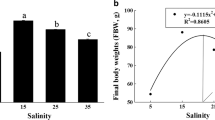
No mortality or pathologies were observed in any group of fish throughout the experimental time. The effect of exposure to different environmental salinities during a period of 90 days on growth of E. maclovinus juveniles is presented in Fig. 1, and several growth performance indices are summarized in Table 1. At the end of the experimental period, 15 and 31 (control group) psu-acclimated fish exhibited significantly greater body weight than 5 psu-acclimated fish (Fig. 1).
Changes in body weight in E. maclovinus juveniles exposed to three different environmental salinities (5, 15 and 31 psu) during 90 days. Data are expressed as mean ± SEM (n = 16 per group). Different letters indicate significant differences between groups for the same salinity. Different symbols indicate significant differences between groups for the same time (P < 0.05, two-way Nested ANOVA followed by Tukey’s test)
Plasma parameter values are shown in Table 2. Osmolality, glucose, and lactate levels did not differ among fish exposed to different environmental salinities. Triglycerides were significantly greater in fish exposed to low environmental salinity (5 psu, P < 0.05). Proteins and total α-amino acids had a similar pattern of change, decreasing in 5 psu-exposed fish to respect at 31 and 15 psu groups. Plasma cortisol values were not statistically different between any groups tested.
Gill NKA activity showed a “U-shaped” relationship with respect to environmental salinity, while no differences was observed in renal NKA activity. Intestinal NKA activity presented a different pattern of change depending on portion assessed: anterior and posterior portions showed an inverse linear relationship with respect to environmental salinity, while no changes were observed in the mid-portion were observed (Table 3).
Hepatic metabolite concentrations and enzymatic activities are shown in Table 4 and Fig. 2a, respectively. Glucose levels were not significantly different between groups, while glycogen levels were significantly lower in 5 and 15 psu-exposed fish as compared to 31 psu-exposed fish, with the 15 psu group exhibiting the lowest values. Total α-amino acids exhibited the highest values at 15 psu, while triglycerides showed the lowest values in 31 psu acclimated fish (Table 4). Environmental salinity did not influence HK, G6PDH and GOT activities (data not shown). FBPase activity enhanced in 5 psu-exposed fish and decreased in 15 psu-accclimated fish. GPT and G3PDH activities significantly increased in 31 psu group. Finally, GDH activity significantly enhanced in 5 and 15 psu-exposed fish (P < 0.05) (Fig. 2a).
Enzymatic activities (a liver; b gills; c kidney; d muscle) in E. maclovinus juveniles exposed to three different environmental salinities (5, 15 and 31 psu) during 90 days. Data are expressed as mean ± SEM (n = 16 per group). Different letters indicate significant differences (P < 0.05, nested one-way ANOVA followed by Tukey’s test) between groups for the same enzyme
Branchial metabolite concentrations and enzyme activities are presented in Table 5 and Fig. 2b, respectively. Glucose, total amino acids, and lactate levels were not significantly different between groups. Glycogen levels increased in salinities lower than 31 psu, but triglyceride values were lower at 5 and 31 psu (Table 5). No significant differences due to environmental salinity were observed in FBP, G3PDH and LDH-O activities (data not shown). HK activity exhibited an inverse relationship with respect to environmental salinity (Fig. 2b), while G6PDH and GDH activities enhanced at 5 psu (P < 0.05).
Renal metabolite concentrations and enzyme activities are presented in Table 6 and Fig. 2c, respectively. Glycogen and triglycerides were not significantly different between groups. Glucose levels had the lowest values in the salinity of 5 psu, while total α-amino acid and lactate levels showed the highest values at the lowest salinity (Table 6). The enzymatic activities HK, FBP and LDH-O were not altered by environmental salinity (data not shown). G6PDH and GDH activities significantly decreased at 31 psu compared to animals exposed to 5 and 15 psu (P < 0.05), while G3PDH activity presented the lowest values at 15 psu and the highest at 5 psu (Fig. 2c).
Muscle metabolite concentrations and enzyme activities are presented in Table 7 and Fig. 2d, respectively. Glucose, glycogen, lactate, and percent water content were not significantly different between groups. Moreover, total α-amino acid and triglyceride levels enhanced when environmental salinity decreased (Table 7). Some enzymatic activities such as G6PDH, HK, and FBP showed scarce activity and significant differences among animals exposed to different salinities were not observed (data not shown). Similarly, GDH and LDH-O activities were not significantly different between groups (data not shown). G3PDH and GOT activities significantly increased in those animals exposed to 15 psu compared to those exposed to 5 and 31 psu (P < 0.05), while GPT activity exhibited highest values at 31 psu, with no significant differences between those animals acclimated to 5 and 15 psu.
Stomach and pyloric caeca enzymes are presented in Fig. 3. Stomach pepsin activity increased with environmental salinity, exhibiting significant differences (P < 0.05) between animals maintained at 5 and 31 psu (5 ≤ 15 ≤ 31 psu) (Fig. 3a). Pyloric caeca enzyme activities of assessed proteases (trypsin, chymotrypsin, leucine-and aminopeptidase) and other hydrolases (α-amylases and lipases) were not altered by changes in environmental salinity (Table 8); while, trypsin/chymotrypsin ratio (T/C ratio) enhanced significantly in those fish exposed to 5 and 15 psu (5 ≥ 15 > 31 psu) (Fig. 3b).
Digestive enzyme activities in E. maclovinus juveniles exposed to three different environmental salinities (5, 15 and 31 psu) during 90 days: a stomach pepsin-like activity, b Trypsin/Chymotrypsin ratio registered in pyloric caeca. Data are expressed as mean ± SEM (n = 16 per group). Different letters indicate significant differences (P < 0.05, nested one-way ANOVA followed by Tukey’s test) between groups for the same enzyme
Discussion
In the present study the influence of environmental salinity on metabolism, growth, digestive capacity and osmoregulation of E. maclovinus was assessed. The results indicated that acclimation of E. maclovinnus to isosmotic conditions (around 15 psu) enhanced growth and other biometric factors (weight gain and SGR). However, and because the low number of specimens used in our study, it is necessary to treat our results with caution and further studies using large number of specimens will be necessary. Individuals acclimated to a hyperosmotic environment (31 psu) also exhibited good growth, indicating that E. maclovinus juveniles exhibit a wide optimum salinity range (at least between 15 and 31 psu). The highest growth rates observed in 15 and 31 psu-acclimated fish may be related to less energy spent on osmoregulatory strategies as well as better utilization of energy and nutrients from the food, investing it in physiological processes such as growth and tissue accretion (Kirschner 1995; Boeuf and Payan 2001; Vargas-Chacoff et al. 2011). Our results agree with those reported for other species, where environmental salinity close to the isosmotic point (between 10 and 15 psu) induced the greatest growth (Scopththalmus maximus, Imsland et al. 2001; Sparus aurata, Laiz-Carrión et al. 2005; Pagrus pagrus, Vargas-Chacoff et al. 2011). However, in other model species (i.e. Solea senegalensis, Arjona et al. 2009) the standard growth rate of the juveniles was not higher at intermediate salinities but at hyperosmotic environments (39 psu), while larval stages show no differences in growth in a salinity range from 10 to 30 psu (Salas-Leitón et al. 2012). These results indicated that optimal growth salinity depends on the species and developmental stage of the fish. Thus, there is a need for future studies to examine the effect of environmental salinity on the growth of E. maclovinus at different stages of development.
Here it was shown that E. maclovinus can handle variations within a wide range of environmental salinities without significant changes in plasma cortisol. Considering that cortisol levels are interpreted as an indicator of stress level experienced by fish (Wendelaar-Bonga 1997), E. maclovinus juveniles exposed to 5, 15 or 31 psu did not appear to exhibit a chronic stress situation. No differences in cortisol levels indicate the maintenance of the homeostatic balance throughout the salinities assessed as well as a balance between energy metabolism expenditures and osmoregulatory costs. Thus in environments with high or low salinity, where the animal has to cope with an additional expense due to the need to incorporate ions or eject them to the medium, extra energy expenditure occurs.
Osmoregulatory balance was achieved through efficient regulation of the Na+, K+-ATPase (NKA) activity in different osmoregulatory tissues (gill, kidney and intestine), and consequently E. maclovinus was able to ionoregulate and maintain its plasma osmolality constant throughout the salinity range tested (Table 2). These results disagree with that reported previously for E. maclovinus submitted to a similar salinity range but only during 14 days (Vargas-Chacoff et al. 2014a). In this study gill NKA activity exhibited a direct and positive relationship with respect to environmental salinity, while kidney NKA activity showed a “U shaped” relationship. These discrepancies suggest that more than 14 days is required for this Sub-Antarctic species to reach the chronic regulatory period, where osmoregulatory and metabolic parameters reach a new homeostatic point (see Bath and Eddy 1979; Jensen et al. 1998; Laiz-Carrión et al. 2005). According to NKA activity changes, a hyposmotic environment of 5 psu forced the gills and intestine (anterior and posterior portions) to increase their energy expenditure in osmoregulation. However, a hyperosmotic environment of 31 psu seems to rely most of its osmoregulatory investment mainly through the gills, while the intestine regions decrease their NKA activity rates. The NKA enhancement observed in gill and intestine of 5 psu-acclimated fish could be responsible for the low growth observed at this salinity due to a higher energetic cost. This means that the energy expenditure relevance in osmoregulation at 5 psu salinity compromises the energy available for other functions such as growth. Similar results were reported in euryhaline species maintained far from their optimum environmental salinity for growth (S. aurata: Laiz-Carrión et al. 2005; S. senegalensis: Arjona et al. 2009).
The metabolic balance in low salinity (5 psu)-acclimated fish showed increased hepatic gluconeogenesis, demonstrated by the rise in FBP activity. This indicates a metabolic cost associated with high energy expenditure in osmoregulation at this salinity. This differs from the results of others who reported high HK activity and glycolysis, as another way to obtain energy for high energy expenditure in osmoregulation at this salinity (Laiz-Carrión et al. 2005; Vargas-Chacoff et al. 2009a). Also, an enhancement in synthesis of fatty acids in the gill and kidney is suggested by the increased G6PDH and GDH enzyme activities, which could be fuelled by total α-amino acids from other tissues. G3PDH activity increased in the kidney and TAG levels increased in plasma, indicating a peripheral mobilization of lipids (Soengas et al. 2007). Likewise, the decrease in total plasma α-amino acids may suggest their use to obtain energy or reductive power in peripheral tissues such as gills, kidney and intestine. Branchial HK activity increased in this salinity, suggesting that in addition to TAG and amino acids, carbohydrates are also utilized as substrates for energy production (Soengas et al. 2007).
In an isosmotic environment (15 psu), where the growth rates of individuals are greater, there is an increase in the biosynthesis and deposition of amino acids to form structural proteins in muscle, as suggested by the increase of GOT activity in this tissue. Also, reserves of TAG are reduced in this tissue (as seen by the increased G3PDH activity) serving for the production of substrates that enter glycolytic routes forming pyruvate, allowing de novo amino acids synthesis. These results suggest this species will induce amino acid mobilization, through the transamination route from food intake. These results differ from previous results reporting that S. senegalensis juveniles exposed to 15 psu (Arjona et al. 2009) showed increased GDH activity, supporting the idea that they are metabolizing amino acids that could be derived from muscle proteolysis (Vijayan et al. 1993, 1994).
Metabolism in 31 psu-acclimated fish we demonstrate an increase in liver and muscle GPT activity, indicating amino acid consumption in these tissues for fuel production. Also hepatic G3PDH activity enhanced, suggesting an increases in TAG consumption, due to its use as energy source. In this study E. maclovinus did not show differences for hepatic G6PDH activity, similar results have been reportedin S. aurata (Laiz-Carrión et al., 2005) and S. senegalensis (Arjona et al., 2009) kept at different environmental salinities, indicating that the pentose shunt not presented variations in this action rates. Laiz-Carrión et al. (2005) did not show significant differences in FBP activity in S. aurata kept at different salinities, which confirms our suggestion.
The digestive tract of marine teleosts has a dual role as a food processing organ and as an osmoregulatory organ (Taylor and Grosell 2006). Analysis of digestive enzyme activity is a methodology that can be used as an indicator of digestive processes and nutritional condition of fish in different life stages and environmental conditions (Moutou et al. 2004; Barman et al. 2005; Bolasina et al. 2006; Nikolopoulou et al. 2011).
In teleosts, several studies have reported changes in digestive capacity induced by environmental salinity (Asha-Devi and Aravindan 1997; Moutou et al. 2004; Barman et al. 2005; Tsuzuki et al. 2007). In the stomach of E. maclovinus juveniles environmental salinity affected the acid proteolytic activity (generated mainly by the action of pepsin-like enzymes). Fish kept under low salinity (5 psu) condition showed a lower acid proteolytic activity compared to those maintained at the highest salinity tested (31 psu). These could indicate that fish kept at low salinity are feeding but intake rates are lower because the decreased proteolytic activity induced by low environmental salinity. In addition, the energy obtained from food is being used in other physiological processes such as osmoregulation more than in growth (Laiz-Carrión et al. 2005; Psochiou et al. 2007; Arjona et al. 2009). However, the lack of significant differences in other enzyme activities show that fish are feeding correctly, and that absorption of nutrients and nutritional status is similar in fish maintained at all salinities (Applebaum et al. 2001; Zambonino-Infante and Cahu 2001). In addition, the trypsin chymotrypsin−1 ratio (T/C ratio) exhibited significant differences between the highest salinity (31 psu) with respect to the other two salinities (5 and 15 psu). The T/C ratio has been related to increases in growth, food utilization and nutritional status of organisms (Rungruangsak-Torrisen et al. 2006; Rungruangsak-Torrisen 2007). We observed an inverse relationship in the T/C ratio of E. maclovinus with respect to environmental salinity. This result may suggest that food utilization and efficiency is better in fish acclimated to low salinities, leading not to better growth, but to increased energy available for physiological processes such osmoregulation.
Conclusion
Juvenile of E. maclovinus present a better growth rate in a range that goes from their iso-osmotic point (15 psu) to hyperosmotic environments (31 psu). Energy expenditure in osmoregulation is minimal at 15 psu, while increased at high salinity due to the activity of gills. At low salinity environments, osmoregulation processes (mainly at gills and intestine) consume energy reserves, compromising growth rate in this species. As the main metabolic stores were mobilized in extreme salinity environments, it would be necessary to investigate the nutritional requirements of this species at different salinities, in order to optimize its culture. Finally, it would be appropriate to further investigate the metabolic behaviour of E. maclovinus depending on its life stage under different environmental salinities.
References
Alarcón FJ, Díaz M, Moyano FJ, Abellán E (1998) Characterization and functional properties of digestive proteases in two sparids; gilthead seabream (Sparus aurata) and common dentex (Dentex dentex). Fish Physiol Biochem 19:257–267
Appel W (1974) Leucine aminopeptidase determination with L-leucinamide as substrate. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis. Academis Press, New York, pp 954–958
Applebaum SL, Peréz R, Lazo JP, Holt GJ (2001) Characterization of chymotrypsin activity during early ontogeny of larval red drum (Sciaenops ocellatus). Fish Physiol Biochem 25:291–300
Arjona FJ, Vargas-Chacoff L, Ruiz-Jarabo I, Martín del Río MP, Mancera JM (2007) Osmoregulatory response of Senegalase sole (Solea senegalensis, Kaup 1858) to changes in environmental salinity. Comp Biochem Physiol A 148:413–421
Arjona FJ, Vargas-Chacoff L, Ruiz-Jarabo I, Gonçalves O, Páscoa I, Martín del Río MP, Mancera JM (2009) Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup, 1858) to osmotic acclimation: implications for osmoregulation, energy metabolism and growth. Aquaculture 287:419–426
Asha-Devi R, Aravindan AM (1997) Influence of salinity on the digestive enzyme activity of Oreochromis mossambicus (Peters). J Inland Fish Soc India 29:1–6
Barman UK, Jana SN, Garg SK, Bhatnagar A, Arasu ART (2005) Effect of inland water salinity on growth, feed conversion efficiency and intestinal enzyme activity in growing grey mullet Mugil cephalus (Linn.): field and laboratory studies. Aquacult Int 13:241–256
Bath RN, Eddy FB (1979) Ionic and respiratory regulation in rainbow trout during rapid transfer to seawater. J Comp Physiol 134:351–357
Boeuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol C 130:411–423
Bolasina S, Perez A, Yamashita Y (2006) Digestive enzyme activity during ontogenetic development and e¡ect of starvation in Japanese founder, Paralichthys olivaceus. Aquaculture 252:503–515
Erlanger B, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Gjellesvik DR, Lombardo D, Walther BT (1992) Pancreatic bile salt dependent lipase from cod (Gadus morhua): purification and properties. Biochim Biophys Acta 1124:123–134
Herrera M, Vargas-Chacoff L, Hachero I, Ruíz-Jarabo I, Rodiles A, Navas J, Mancera JM (2009) Osmoregulatory changes in wedge sole (Dicologoglossa cuneata, Moreau 1881) after acclimation to different environmental salinities. Aquac Res 40:762–771
Hummel BCW (1959) A modified spectrophotometric determination of chymotrypsin, trypsin and thrombin. Can J Biochem Physiol 37:1393–1399
Imsland AK, Foss A, Gunnarsson S, Berntssen MHG, FitzGerald R, Wendelaar Bonga S, Ham E, Naevdal G, Stefansson SO (2001) The interaction of temperature and salinity on growth and food conversion in juvenile turbot (Scophthalmus maximus). Aquaculture 198:353–367
Jensen MK, Madsen SS, Kristiansen K (1998) Osmoregulation and salinity effects on the expression and activity of Na+-K+ ATPase in the gills of European sea bass, Dicentrarchus labrax (L.). J Exp Zool 282:290–300
Keppler D, Decker K (1974) Glycogen. Determination with amyloglucosidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic, New York, pp 1127–1131
Kirschner LB (1995) Energetics of osmoregulation in fresh water vertebrates. J Exp Zool 271:243–252
Laiz-Carrión R, Sangiao-Alvarellos S, Guzmán JM, Martín del Río MP, Soengas JL, Mancera JM (2005) Growth performance on gilthead sea bream Sparus aurata in different osmotic conditions: implications on osmoregulation and energy metabolism. Aquaculture 250:849–861
Mancera JM, McCormick SD (2007) Role of prolactin, growth hormone, insuline-like growth factor and cortisol in teleost osmoregulation. In: Baldisserotto B, Mancera JM, Kapoor BG (eds) Fish Osmoregulation. Science Publishers, New York, pp 497–515
Mancera JM, Laiz-Carrión R, Martín del Río MP (2002) Osmoregulatoryaction of PRL, GH, and cortisol in the gilthead seabream (Sparus aurata L.). Gen Comp Endocrinol 129:95–103
Marshall WS (2002) Na+, Cl−, Ca++ and Zn++ transport by fish gills: retrospective review and prospective synthesis. J Exp Zool 292:264–283
McCormick SD (1993) Methods for nonlethal gill biopsy and measurement of Na+-K+ ATPase activity. Can J Fish Aquat Sci 50:656–658
McCormick SD (1995) Hormonal control of gill Na+, K+-ATPase and chloride cell function. In: Wood CM, Shuttlewoth TJ (eds) Fish Physiology, vol XIV., Ionoregulation: cellular and molecular approachesAcademic Press, New York, pp 285–315
Moore S (1968) Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Chem Biol 1242:6281–6283
Moutou KA, Panagiotaki P, Mamuris Z (2004) Effects of salinity on digestive protease activity in the euryhaline sparid Sparus aurata L.: a preliminary study. Aquacult Int 35:912–914
Nikolopoulou D, Moutou KA, Fountoulaki E, Venou B, Adamidou S, Alexis MN (2011) Patterns of gastric evacuation, digesta characteristics and pH changes along the gastrointestinal tract of gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.). Comp Biochem Physiol A 158:406–414
Pequeño G, Pavés H, Bertrán C, Vargas-Chacoff L (2010) Seasonal limnetic feeding regime of the “robalo” Eleginops maclovinus (Valenciennes 1830), in the Valdivia river, Chile. Gayana 74:47–56
Psochiou E, Mamuris Z, Panagiotaki P, Kouretas D, Mountou KA (2007) The response of digestive proteases to abrupt salinity decrease in the euryhaline sparid Sparus aurata L. Comp Biochem Physiol B 147:156–163
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, United Kingdom
Rungruangsak-Torrisen K (2007) Digestive efficiency, growth and qualities of muscle and oocyte in Atlantic salmon (Salmo salar L.) fed on diets with krill meal as an alternative protein source. J Food Biochem 31:509–540
Rungruangsak-Torrisen K, Moss R, Andresen LH, Berg A, Waagbø R (2006) Different expressions of trypsin and chimotrypsin in relation to growth in salmon (Salmo salar). Fish Physiol Biochem 32:7–23
Sá R, Gavilán M, Rioseco MJ, Llancabure A, Vargas-Chacoff L, Augsburger A, Bas F (2014) Dietary protein requirement of Patagonian blennie (Eleginops maclovinus, Cuvier 1830) juveniles. Aquaculture 428–429:125–134
Salas-Leitón EA, Rodriguez-Rúa A, Asensio E, Infante C, Manchado M, Fernández-Díaz C, Cañavate JP (2012) Effect of salinity on egg hatching, yolk sac absorption and larval rearing of Senegalese sole (Solea senegalensis Kaup 1858). Rev Aquacult 4:49–58
Sangiao-Alvarellos S, Laiz-Carrión R, Guzmán JM, Martín del Río MP, Mancera JM, Soengas JL (2003) Acclimation of Sparus aurata to various salinities alters energy metabolism of osmoregulatory and and nonosmoregulatory organs. Am J Physiol 285:897–907
Sangiao-Alvarellos S, Arjona FJ, Martín del Río MP, Míguez MP, Mancera JM, Soengas JL (2005) Time course of osmoregulatory and metabolic changes during osmotic acclimation in Sparus auratus. J Exp Biol 208:4291–4304
Sarath G, De la Monte RS, Warner FW (1989) Protease assay methods. In: Beyon RJ, Bond JS (eds) Proteolytic enzymes: a practical approach. Oxford University Press, New York, pp 25–56
Soengas JL, Sangiao-Alvarellos S, Laiz-Carrión R, Mancera JM (2007) Energy metabolism and osmotic acclimation in teleost fish. In: Baldisserotto B, Mancera JM, Kapoor BG (eds) Fish osmoregulation. Science Publishers, Enfield, pp 277–308
Taylor JR, Grosell M (2006) Feeding and osmoregulation: dual function of the marine teleost intestine. J Exp Biol 209:2939–2951
Tipsmark CK, Madsen SS, Seidelin M, Christensen AS, Cutler CP, Cramb G (2002) Dynamics of Na+, K+, 2Cl− cotransporter and Na+, K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). J Exp Zool 293:106–118
Tsuzuki MY, Sugai JK, Maciel JC, Francisco CJ, Cerqueira VR (2007) Survival, growth and digestive enzyme activity of juveniles of the fat snook (Centropomus parallelus) reared at different salinities. Aquaculture 271:319–325
Vargas-Chacoff L, Arjona FJ, Polakof S, Martín del Río MP, Soengas JL, Mancera JM (2009a) Interactive effects of environmental salinity and temperature on metabolic responses of gilthead sea bream Sparus aurata. Comp Biochem Physiol A 154:417–424
Vargas-Chacoff L, Arjona FJ, Ruiz-Jarabo I, Páscoa I, Gonçalves O, Martín del Río MP, Mancera JM (2009b) Seasonal variation in osmoregulatory and metabolic parameters in earthen pond cultured gilthead sea bream Sparus aurata. Aquac Res 40:1279–1290
Vargas-Chacoff L, Calvo A, Ruiz-Jarabo I, Villarroel F, Muñoz JL, Tinoco AB, Cárdenas S, Mancera JM (2011) Growth performance, osmoregulatory and metabolic modifications in red porgy fry, Pagrus pagrus, under different environmental salinities and stocking densities. Aquac Res 42:1269–1278
Vargas-Chacoff L, Moneva F, Oyarzún R, Martínez D, Muñóz JLP, Bertrán C, Mancera JM (2014a) Environmental salinity-modified osmoregulatory response in the Sub-Antarctic notothenioid fish Eleginops maclovinus. Polar Biol 37:1235–1245
Vargas-Chacoff L, Ortíz E, Oyarzún R, Martínez D, Saavedra E, Sá R, Olavarría V, Yáñez A, Bertrán C, Mancera JM (2014b) Stocking density and Piscirickettsia salmonis infection affect the skeletal muscle intermediate metabolism in Eleginops maclovinus. Fish Physiol Biochem 40:1683–1691
Vargas-Chacoff L, Martínez D, Oyarzún R, Nualart D, Olavarría V, Yáñez A, Bertrán C, Ruiz-Jarabo I, Mancera JM (2014c) Combined effects of high stocking density and Piscirickettsia salmonis treatment on the immune system, metabolism and osmoregulatory responses of the Sub-Antarctic Notothenioid fish Eleginops maclovinus. Fish Shellfish Immun 40:424–434
Vijayan MM, Foster GD, Moon TW (1993) Effects of cortisol on hepatic carbohydrate metabolism and responsiveness to hormones in the sea raven Hemitripterus americanus. Fish Physiol Biochem 12:327–335
Vijayan MM, Reddy PK, Leatherland JF, Moon TW (1994) The effects of cortisol on hepatocyte metabolism in rainbow trout: a study using the steroid analogue RU486. Gen Comp Endocrinol 96:75–84
Walter HE (1984) Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: Bergmeyer HJ (ed) Methods of enzymatic analysis, vol V. Verlag Chemie, Weinham, pp 270–277
Wendelaar-Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Worthington K, Worthington V (2011) Worthington Biochemical Corporation date of access. Worthington Enzime Manual. (http://www.worthington-biochem.com/pap/default.html)
Zambonino-Infante JL, Cahu CL (2001) Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol C 130:477–487
Acknowledgments
This study was carried out in the framework of FONDECYT Project 1110235 and FONDAP-INCAR, No. 15110027. We thank Dr. Lafayette Eaton and the Dirección de Investigación of the Universidad Austral de Chile (DID) for their help checking this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Vargas-Chacoff, L., Saavedra, E., Oyarzún, R. et al. Effects on the metabolism, growth, digestive capacity and osmoregulation of juvenile of Sub-Antarctic Notothenioid fish Eleginops maclovinus acclimated at different salinities. Fish Physiol Biochem 41, 1369–1381 (2015). https://doi.org/10.1007/s10695-015-0092-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0092-3