Abstract
Juvenile sablefish, Anoplopoma fimbria (mean length 15.5 ± 1.9 cm, mean weight 68.5 ± 4.8 g), were used to evaluate the effects on growth, oxidative stress, and non-specific immune responses by changes of water temperature (8, 10, 12, 14, 16, 18, and 20 °C) and salinity (100 (35.0), 90 (31.5), 80 (28.0), 70 (24.5), 60 (21.0), 50 (17.5), and 40% (14.0) (‰)) for 4 months. The growth performance was significantly increased at the temperature of 12 and 14 °C, and the feed efficiency was notably decreased at the temperature of 18 °C. The growth performance and feed efficiency were also significantly decreased at low salinity. The antioxidant responses such as superoxide dismutase and catalase were significantly increased by the high temperature and decreased by the low salinity. The immune responses such as lysozyme and phagocytosis were elevated by the temperature of 18 °C and decreased by the salinity of 50%. The results of this study indicate that the growth performance of juvenile sablefish, A. fimbria, is influenced by the temperature and salinity, and the excessive temperature and salinity levels can affect the antioxidant and immune responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Of various environmental factors, water temperature is one of the most important factors in fish. Most fish are vulnerable to sharp thermal alterations, because the thermal changes affect ectothermal animals such as ventilation volume, cardiac output, and ventilation-perfusion ration, which can lead to a decrease in the effectiveness of oxygen removal, arterial and venous oxygen content, and hypoxia (Lushchak and Bagnyukova 2006). The higher temperature than a proper temperature can induce a progressive loss of equilibrium such as the rolling or swimming upwards or downwards in an uncoordinated manner (Logue and Cossins 1995). In addition, temperature is a critical physical regulatory factor in fish such as most reproductive procedure of gamete development, maturation, ovulation, spermiation, spawning, embryogenesis, and hatching, as well as survival (Pankhurst and Munday 2011). Salinity is also one of the most critical factors in fish, because most fish are euryhaline. The physiological responses by salinity are often much more influential than water temperature (Wuenschel et al. 2004a). Salinity as well as water temperature substantially influences the metabolism during phase II of the mass metabolism relationship in fish (Wuenschel et al. 2004b). Most teleost fish control their plasma ions for their body fluid homeostasis which is equivalent to approximately 10 to 15‰, and marine fish use their energy to fulfill the metabolic costs of ionic and osmotic regulation (Brett 1979). Therefore, the growth and development in fish can be significantly affected by the salinity (Imsland et al. 2001). In fish, the ability to handle fluctuating salinity is considered as a critical factor for maximized growth and reproduction (Sampaio and Bianchini 2002).
Thermal stress in fish also causes oxidative stress (Vinagre et al. 2012), because the high temperature induces the hyperthermia, which results in reactive oxygen species (ROS) generation by tissue re-oxygenation during recovery (Madeira et al. 2013). The metabolic rate in fish according to water temperature affects oxygen consumption, which is highly associated with ROS production such as superoxide anion, hydrogen peroxide, and hydroxyl radical (Madeira et al. 2013). The alterations of salinity induce physiological stress, which is closely related to the generation of ROS (Yin et al. 2011). In response to ROS generation, superoxide dismutase (SOD) decomposes superoxide anion to hydrogen peroxide, and catalase (CAT) decomposes hydrogen peroxide to oxygen and water (Kim and Kang 2015). Considering the important functions of the antioxidant enzymes, the antioxidant responses such as SOD and CAT suggest a reliable parameter to assess the oxidative stress by the water temperature and salinity alterations.
Environmental temperature affects the immunological processes of aquatic animals in addition to the metabolism and behavior (Jun et al. 2009). Temperature is a critical factor in cold-blooded animals, and thermal alterations cause the changes of fish immune responses (Martins et al. 2011). Changes in salinity also affect the immune responses in aquatic animals (Wang and Chen 2005). Matozzo et al. (2007) also suggested that salinity affects the immune responses in the aquatic animals of marine and freshwater. Among various immune responses, lysozyme is a crucial non-specific immune parameter in fish, and the lysozyme activity can be stimulated by temperature as well as health condition, stress, sex, and toxicants (Kim and Kang 2016a). Phagocytosis is also a major non-specific immune response, which engulfs large particles into intracellular vacuoles to remove pathogenic microorganisms, and it has been considered as a major immunological indicator to evaluate the health status and immune ability in fish (Risjani and Yunianta 2014). Therefore, the immune response parameters suggest a reliable indicator to assess the effects of water temperature and salinity.
Sablefish, Anoplopoma fimbria, is widely known as Alaskan black cod, which is a long-lived demersal fish species mainly between 200 and 1500 m inhabited in North Pacific Ocean (Rondeau 2013), and it is also one of the most important commercial fish species due to its high demand, appreciate flesh, and rapid growth. However, the insufficient study about the breeding water temperature and salinity for aquaculture has been conducted in the fish. In Korea, the coastal temperature in the summer goes up to the temperature of 28 °C. But, the coastal underground water in Korea maintains the temperature below 18 °C. Therefore, the purposes of this study were to evaluate the effects of growth performance, oxidative stress, and non-specific immune responses by changes of water temperature and salinity and verify the possibility to culture this species in Korea.
Materials and methods
Experimental fish and conditions
Juvenile sablefish (A. fimbria) were obtained from Troutlodge Sablefish LLC., USA, which were transported by plane maintaining the temperature of 5 °C. During the acclimation period, the fish were fed diet twice daily and maintained on a 24-h dark cycle and constant condition at all times. After acclimatization, 350 fish (body length, 15.5 ± 1.9 cm; body weight, 68.5 ± 4.8 g) were randomly selected for the study. The water temperature was adjusted from ambient at a rate of ±1 °C/day until a final temperature of 20 °C was reached. The acclimation period commenced once the final temperature had been sustained for 24 h and animals were feeding, while showing no sign of stress. The salinity concentrations were 100 (35.0‰), 90 (31.5‰), 80 (28.0‰), 70 (24.5‰), 60 (21.0‰), 50 (17.5‰), and 40% (14.0‰). The tank water temperature was maintained with a water cooling device. At the end of each period (at 2 and 4 months), animals were anesthetized in buffered 3-aminobenzoic acid ethyl ester methanesulfonate (Sigma Chemical, St. Louis, MO).
Growth performance
The weight and length of sablefish were measured just before exposure, at 2 and 4 months. Daily length gain, daily weight gain, condition factor, and feed efficiency were calculated by the following method.
Antioxidant enzyme analysis
Liver and gill tissues were excised and homogenized with 10 volumes of ice-cold homogenization buffer using a Teflon-glass homogenizer (099CK4424, Glass-Col, Germany). The homogenate was centrifuged at 10,000g for 30 min under refrigeration, and the obtained supernatants were stored at −80 °C for analysis.
Superoxide dismutase (SOD) activity was measured with 50% inhibitor rate about the reduction reaction of WST-1 using SOD Assay Kit (Dojindo Molecular Technologies, Inc.). One unit of SOD is defined as the amount of the enzyme in 20 μl of sample solution that inhibits the reduction reaction of WST-1 with superoxide anion by 50%. SOD activity was expressed as unit milligram per protein.
Catalase (CAT) activity was measured using the OxiSelect™ Catalase Assay Kit (Cell Biolabs, Inc.). The quinoneimine dye coupling product is measured at 520 nm, which correlated to the amount of hydrogen peroxide remaining in the reaction mixture. The CAT activity was expressed as unit/mg protein, and one unit of CAT is the amount of enzyme that will decompose 1 μM of H2O2 per minute at 25 °C.
Non-specific immune responses
The plasma for analysis was separated from the blood sample. Kidney tissues were excised and homogenized with 10 volumes of ice-cold homogenization buffer (0.004 M phosphate buffer, pH 6.6) using a Teflon-glass homogenizer (099CK4424, Glass-Col, Germany). The homogenate was centrifuged at 10,000g for 10 min under refrigeration, and the obtained supernatant was stored at −70 °C (MDF-U53V, SANYO Electric Co. Ltd., Japan) for analysis. Protein content was determined by the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories GmbH, Munich, Germany) based on the Bradford dye-binding procedure, using bovine serum albumin as standard. Lysozyme concentration was calculated through the measure of its enzyme activity.
Lysozyme activity was determined by a turbidimetric method (Ellis 1990) using Micrococcus lysodeikticus (Sigma) as a substrate (0.2 mg/ml 0.05 M phosphate buffer, pH 6.6 for kidney sample and pH 7.4 for plasma). A standard curve was made with lyophilized hen egg white lysozyme (Sigma), and the rate of change in turbidity was measured at 0.5- and 4.5-min intervals at 530 nm. The results were expressed as μg/ml and μg/g equivalent of hen egg white lysozyme activity (Anderson and Siwicki 1994).
Phagocytosis was measured using the phagocytosis assay kit (Cell Biolabs, Inc.). Add 200 μl of cold 1× PBS to each well, and promptly remove the PBS solution. Add 100 μl of fixation solution to each well, and incubate for 5 min. Promptly remove the fixation solution, and wash twice with 1× PBS. Add 100 μl of pre-diluted 1 blocking solution to each well, and incubate the plate for 30 min. Promptly remove the blocking solution, and wash three times with 1× PBS. Add 100 μl of pre-diluted 1× permeabilization solution to each well, incubate for 5 min, and promptly remove the PBS. Initiate the reaction by adding 100 μl of substrate, and incubate for 10–30 min. Stop the reaction by adding 100 μl of the stop solution, and read the absorbance at 450 nm.
Statistical analysis
The experiment was conducted in exposure period for 4 months and performed triplicate. Statistical analyses were performed using the SPSS/PC+ statistical package (SPSS Inc., Chicago, IL, USA). Significant differences between groups were identified using one-way ANOVA and Tukey’s test for multiple comparisons or Student’s t test for two groups (n = 10). The significance level was set at P < 0.05.
Results
Growth performance
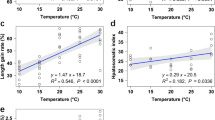
The growth rate and feed efficiency of A. fimbria by changes of water temperature are shown in Fig. 1. The daily length gain and daily weight gain of A. fimbria were significantly increased at the temperature of 14 °C after 2 months and at the temperature of 12 and 14 °C after 4 months. The condition factor was also notably increased at 14 °C after 2 months and at 12 and 14 °C after 4 months. In the feed efficiency, a significant reduction was observed at 18 °C after 2 and 4 months.
Daily length gain, daily weight gain, condition factor, and feed efficiency of sablefish, Anoplopoma fimbria, exposed to the different levels of water temperature for 4 months. Vertical bars denote a standard error. Values with different superscripts are significantly different at 2 and 4 months (P < 0.05) as determined by Tukey’s multiple range test (n = 10)
The growth rate and feed efficiency of A. fimbria by changes of water temperature are shown in Fig. 2. The daily length gain and daily weight gain of A. fimbria were significantly decreased at the salinity of 50% after 2 months and below salinity 60% after 4 months. The condition factor was substantially decreased at salinity of 50% after 2 and 4 months. In the feed efficiency, a significant reduction was observed at salinity of 50% after 2 months and below salinity 60% after 4 months.
Daily length gain, daily weight gain, condition factor, and feed efficiency of sablefish, Anoplopoma fimbria exposed to the different levels of salinity for 4 months. Vertical bars denote a standard error. Values with different superscripts are significantly different at 2 and 4 months (P < 0.05) as determined by Tukey’s multiple range test (n = 10)
Antioxidant enzyme analysis
The alterations of antioxidant responses in the liver and gill tissues of A. fimbria by changes of water temperature are demonstrated in Fig. 3. The liver SOD activity of A. fimbria was significantly increased at 18 °C after 2 months and over 16 °C after 4 months, and the gill SOD activity was also considerably increased at 18 °C after 2 and 4 months. In the CAT activity, a notable increase was observed in the liver at 18 °C after 2 months and over 16 °C after 4 months and in the gill at 18 °C after 2 and 4 months.
Changes of SOD and CAT activity in liver and gill of sablefish, Anoplopoma fimbria, exposed to the different levels of water temperature for 4 months. Vertical bars denote a standard error. Values with different superscripts are significantly different at 2 and 4 months (P < 0.05) as determined by Tukey’s multiple range test (n = 10)
The alterations of antioxidant responses in the liver and gill tissues of A. fimbria by changes of salinity are demonstrated in Fig. 4. The liver SOD was notably decreased at salinity of 50% after 2 months and below salinity 60% after 4 months, and the gill SOD was also significantly decreased at salinity of 50% after 2 and 4 months. The liver CAT was significantly decreased at salinity of 50% after 2 months and below salinity 60% after 4 months, and the gill CAT was substantially decreased at salinity of 50% after 2 and 4 months.
Changes of SOD and CAT activity in liver and gill of sablefish, Anoplopoma fimbria, exposed to the different levels of salinity for 4 months. Vertical bars denote a standard error. Values with different superscripts are significantly different at 2 and 4 months (P < 0.05) as determined by Tukey’s multiple range test (n = 10)
Non-specific immune responses
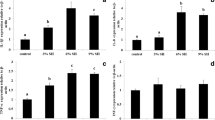
The non-specific immune responses in the plasma and kidney tissues of A. fimbria by changes of water temperature are shown in Fig. 5. The plasma and kidney lysozyme were notably increased at the temperature of 18 °C after 2 and 4 months. The plasma and kidney phagocytosis were significantly increased at 18 °C after 2 and 4 months.
Changes of non-specific immune responses (lysozyme and phagocytosis in plasma and kidney) of sablefish, Anoplopoma fimbria, exposed to the different levels of water temperature for 4 months. Vertical bars denote a standard error. Values with different superscripts are significantly different at 2 and 4 months (P < 0.05) as determined by Tukey’s multiple range test (n = 10)
The non-specific immune responses in the plasma and kidney tissues of A. fimbria by changes of salinity are shown in Fig. 6. The plasma lysozyme was significantly decreased at salinity of 50% after 2 months and below salinity 60% after 4 months, and the kidney lysozyme was also notably decreased at salinity of 50% after 2 and 4 months. The plasma and kidney phagocytosis were significantly decreased at salinity of 50% after 2 and 4 months.
Changes of non-specific immune responses (lysozyme and phagocytosis in plasma and kidney) of sablefish, Anoplopoma fimbria, exposed to the different levels of salinity for 4 months. Vertical bars denote a standard error. Values with different superscripts are significantly different at 2 and 4 months (P < 0.05) as determined by Tukey’s multiple range test (n = 10)
Discussion
In aquaculture, environmental factors such as temperature, pH, dissolved oxygen, and chemical oxygen demand have been considered as a critical indicator to cultivate various fish species for a proper growth and production. Of the environmental parameters, temperature is one of the most important fundamental factors, because water temperature affects the physiology in aquatic animals in addition to their growth, propagation, metabolism, and osmoregulation (An and Choi 2010). Therefore, the experiments regarding the proper temperature of the respective fish species should be conducted for aquaculture. In this study, the higher or lower temperature than a proper temperature (12 and 14 °C) induced a significant reduction in the growth performance of sablefish, A. fimbria. Susan and Bori (2001) also reported a significant increase in the growth rate of bluefish, A. fimbria (size: mean length 50–60 mm, mean weight 0.7–1.5 g), at high temperature, and the growth increased rapidly as temperature increased from 14 to 22 °C. But, a significant reduction in the growth performance was observed at 24 °C. Although the species used in the Susan and Bori (2001)’s article was the same as the species in this study, the difference in the proper temperature may be due to the size of the fish. Handeland et al. (2000) reported a linear relationship between growth performance and temperature in the Atlantic salmon, Salmo salar, but a rapid reduction in growth rate was observed at the higher temperature (18.9 °C) than a proper temperature (4.6–14.4 °C). An optimal temperature for a respective species results in maximum growth, which is due to its covariation of food intake and food conversion with growth performance as thermal alterations (Handeland et al. 2008).
Of various ecological factors, salinity is also one of the most critical indicators to aquatic environment, and many authors reported the effects of external salinity on growth performance in fish (Boeuf and Payan 2001). The low salinity below 50% induced a significant reduction in the growth performance of sablefish, A. fimbria. The salinity is widely known to affect the growth of aquatic animals. Brown and Hartwick (1988) reported a significant reduction in the growth of Pacific oyster, Crassostrea gigas, by the low salinity. Deane and Woo also suggested that salinity alterations can cause a significant reduction in the growth of fish, because changes in environmental salinity cause osmotic stress in fish to maintain water and ionic homeostasis. Chen and Chen (1999) suggested that salinity in the range of 25 to 35 psu is optimal levels to many species. However, several authors reported a significant increase in the growth performance at lower salinity (Bray et al. 1994; Imsland et al. 2001).
Antioxidant responses have been widely considered as a reliable biomarker to assess the heat shock stress in aquatic animals, because the high water temperature affects the synthesis of relevant antioxidant systems in marine fish (Abelea and Puntarulo 2004). In addition, the increase in environmental water temperature induces an increase of oxygen consumption, which promotes the ROS production (Lushchak and Bagnyukova 2006). In this study, the high temperature over 16 °C affects the antioxidant responses such as the superoxide dismutase (SOD) and catalase (CAT) of A. fimbria. Verlecar et al. (2007) reported also the increased activities of antioxidants such as SOD and CAT in Green-lipped mussels, Perna viridis, by the higher temperature than their thermal optimum. Parihar et al. (1997) reported significant increases in the SOD activity of freshwater catfish, Heteropneustes fossilis, by the high thermal exposure. In this study, the thermal stress caused by the heat shock of the high water temperature affected significant alterations in the redox homeostasis of A. fimbria.
In aquatic animals, salinity changes also can induce oxidative stress by generating ROS induced by the salinity change stress (Lushchak 2011). Li et al. (2007) suggested that stress caused by salinity alterations is closely related to increased ROS production, which induces oxidative injury. The low salinity induced a significant decrease in the SOD activity of A. fimbria. Wang and Chen (2005) reported a significant decrease in the SOD activity of white shrimp, Litopenaeus vannamei, exposed to low salinity. Wang and Chen (2006) also reported a notable decrease in the SOD activity of tiger shrimp, Penaeus monodon, exposed to low salinity, which is responsible for scavenging superoxide anion. Ma et al. (2016) reported a substantial decrease in the CAT activity of golden pompano, Trachinotus ovatus, by the low salinity. Roche and Boge (1996) suggested that the alterations in antioxidant enzyme activities in fish are closely related to hypo-osmotic shock. Considering the results of the antioxidant responses, the higher temperature and lower salinity than their moderate scope affect the experimental animal as oxidative stress, and the redox-related biomarkers can be a reliable indicator to assess the thermal stress in aquatic animals.
Thermal stress by severe thermal alterations can influence the non-specific immunity in aquatic animals, whereas optimal temperature helps enhance the immunity (Morvan et al. 1998). The thermal stress in fish can stimulate the immune system by increasing host susceptibility to infectious disease and increasing white blood cell circulating (Prophete et al. 2006). In this study, the high temperature induced a significant increase in the lysozyme activity of sablefish, A. fimbria. Many authors reported a notable increase in the lysozyme activity of fish species by the higher temperature exposure than their temperature scope (Langston et al. 2002; Dominguez et al. 2005; Ndong et al. 2007). The high temperature (18 °C) caused a significant increase in the lysozyme activity of A. fimbria. Harrahy (2001) suggested that lysozyme relies on temperature in most fish species, and low water temperature induced a decrease in lysozyme activity, whereas the increased water temperature caused an increase in lysozyme. The phagocytosis activity of sablefish, A. fimbria, was also significantly increased by the high temperature exposure. Carballal et al. (1997) reported a higher phagocytosis activity of Mytilus galloprovincialis at the higher temperature. Considering that the non-specific immune responses can be a sensitive indicator for the thermal stress as well as chemical toxicants (Prophete et al. 2006), the results of this study for the non-specific immune responses should suggest a good indicator to evaluate the thermal stress in sablefish, A. fimbria, by the high thermal exposure.
The low salinity stress as well as thermal stress can cause significant decreases in the immune responses of aquatic animals (Li et al. 2010). Wang et al. (2008) suggested that alterations in salinity influence the immune parameters in aquatic animals. The lysozyme and phagocytosis activities of sablefish, A. fimbria, were significantly decreased by the low salinity. Lysozyme activity can be significantly changed by salinity, and Matozzo et al. (2007) reported a significant alteration in the lysozyme activity of the clam, Chamelea gallina, by the salinity change. Lin et al. (2012) reported a notable reduction in the lysozyme activity of white shrimp, L. vannamei, exposed to long-term low salinity. Wang and Chen (2005) reported that the low salinity induced a significant reduction in the immune responses such as phagocytic activity and phenoloxidase activity and respiratory burst of white shrimp, L. vannamei. Cheng et al. (2004) also reported a significant decrease in the phagocytic activity of Taiwan abalone, Haliotis diversicolor supertexta, exposed to low salinity. In the results of this study, the low salinity substantially affected the immune responses of sablefish, A. fimbria.
In conclusion, the proper temperature for the growth of juvenile sablefish, A. fimbria, was between 12 and 14 °C, and the higher temperature at 16 °C affects the antioxidant responses as an oxidative stress factor. In addition, the temperature of 18 °C alters the non-specific immune responses. The salinity from 100 to 60% did not induce significant alterations in the growth, antioxidant, and immune responses of A. fimbria. But, the salinity below 50% caused notable reductions in the growth, antioxidant, and immune responses. Considering the results of this study, the proper temperature for the best growth performance of sablefish, A. fimbria, not to influence the antioxidant and immune responses was at 12 and 14 °C. In salinity, even though the salinity at certain levels did not affect the experimental fish, the exceeded optimum levels of low salinity (below 50%) influenced the growth, antioxidant, and immune responses of A. fimbria.
References
Abelea D, Puntarulo S (2004) Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A 138:405–415
An MI, Choi CY (2010) Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comp Biochem Physiol B: Biochem Mol Biol 155(1):34–42
Anderson DP, Siwicki AK (1994) Simplified assays for measuring nonspecific defense mechanisms in fish. Fish Health Section. Am. Fisheries Soc, Meeting, Seattle, WA 26pp
Boeuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol C: Toxicol Pharmacol 130:411–423
Bray WA, Lawrence AL, Leung-Trujillo JR (1994) The effect of salinity on growth and survival of Penaeus vannamei, with observations on the interaction of IHHN virus and salinity. Aquaculture 122:133–146
Brett JR (1979) Environmental factors and growth. Fish Physiol Bioenerg Growth 8:599–675
Brown JR, Hartwick EB (1988) Influences of temperature, salinity, and available food upon suspended culture of the Pacific oyster, Crassostrea gigas: I. Absolute and allometric growth. Aquaculture 70:231-251
Carballal MJ, López C, Azevedo C, Villalba A (1997) In vitrostudy of phagocytic ability of Mytilus galloprovincialis Lmk. Haemocytes. Fish Shellfish Immunol 7(6):403–416
Chen JC, Chen WC (1999) Temperature tolerance of Haliotis diversicolor supertexta at different salinity and temperature levels. Comp Biochem Physiol A 124:73–80
Cheng W, Juang FM, Chen JC (2004) The immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus at different salinity levels. Fish Shellfish Immunol 16:295–306
Dominguez M, Takemura A, Tsuchiya M (2005) Effects of changes in environmental factors on the non-specific immune response of Nile tilapia, Oreochromis niloticus L. Aquac Res 36:391–397
Ellis AE (1990) Lysozyme assay. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology, vol 1990. ISOS Publications, NJ, pp 101–103
Handeland SO, Berge A, Bjornsson BT, Lie O, Stefansson SO (2000) Seawater adaptation by out-of-season Atlantic salmon (Salmo salar L.) smolts at different temperatures. Aquaculture 181:377–396
Handeland OS, Albert KI, Stefansson OS (2008) The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283:36–42
Harrahy LNM (2001) The effects of elevated temperature and stress on immune function in juvenile chinook salmon (Oncorhynchus tshawytscha). Master’s thesis. Oregon State University, Corvallis
Imsland AK, Foss A, Gunnarsson S, Berntssen MHG, FitzGerald R, Bonga SW, vHam E, Naevdal G, Stefansson SO (2001) The interaction of temperature and salinity on growth and food conversion in juvenile turbot (Scophthalmus maximus). Aquaculture 198:353–367
Jun LJ, Jeong JB, Kim JH, Nam JH, Shin KW, Kim JK, Kang JC, Jeong HD (2009) Influence of temperature shifts on the onset and development of red sea bream iridoviral disease in rock bream Oplegnathus fasciatus. Dis Aquat Org 84:201–208
Kim JH, Kang JC (2015) Oxidative stress, neurotoxicity, and non-specific immune responses in juvenile red sea bream, Pagrus major, exposed to different waterborne selenium concentrations. Chemosphere 135:46–52
Kim JH, Kang JC (2016a) The toxic effects on the stress and immune responses in juvenile rockfish, Sebastes schlegelii exposed to hexavalent chromium. Environ Toxicol Pharmacol 43:128–133
Langston AL, Hoare R, Stefansson M, Fitzgerald R, Wergeland H, Mulcahy M (2002) The effect of temperature on non-specific defence parameters of three strains of juvenile Atlantic halibut (Hippoglossus hippoglossus L.) Fish Shellfish Immunol 12(1):61–76
Li CC, Yeh ST, Chen JC (2010) Innate immunity of the white shrimp Litopenaeus vannamei weakened by the combination of a Vibrio alginolyticus injection and low-salinity stress. Fish Shellfish Immunol 28:121–127
Li L, Xie P, Li S, Qiu T, Guo L (2007) Sequential ultrastructural and biochemical changes induced in vivo by the hepatotoxic microcystins in liver of the phytoplanktivorous silver carp Hypophthalmichthys molitrix. Comp Biochem Physiol C: Toxicol Pharmacol 146:357–367
Lin YC, Chen JC, Li CC, Morni WZW, Suhaili ASNA, Kuo YH, Chang YH, Chen LL, Tsui WC, Chen YY, Huang CL (2012) Modulation of the innate immune system in white shrimp Litopenaeus vannamei following long-term low salinity exposure. Fish Shellfish Immunol 33:324–331
Logue JPT, Cossins AR (1995) Heat injury and resistance adaptation in fish. J Ther Biol 20:191–197
Lushchak VI, Bagnyukova TV (2006) Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comp Biochem Physiol C: Toxicol Pharmacol 143:30–35
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Ma Z, Zheng P, Guo H, Jiang S, Qin JG, Zhang D, Liu X (2016) Salinity regulates antioxidant enzyme and Na+ K+-ATPase activities of juvenile golden pompano Trachinotus ovatus (Linnaeus 1758). Aquac Res 47:1481–1487
Madeira A, Camps M, Zorzano A, Moura TF, Soveral G (2013) Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS One 8:e83442. doi:10.1371/journal.pone.0083442
Martins ML, Xu DH, Shoemaker CA, Klesius PH (2011) Temperature effects on immune response and hematological parameters of channel catfish Ictalurus punctatus vaccinated with live theronts of Ichthyophthirius multifiliis. Fish Shellfish Immunol 31:774–780
Matozzo V, Monari M, Foschi J, Serrazanetti GP, Cattani O, Marin MG (2007) Effects of salinity on the clam Chamelea gallina. Part I: alterations in immune responses. Mar Biol 151:1051–1058
Morvan CL, Troutaud D, Deschaux P (1998) Differential effects of temperature on specific and nonspecific immune defences in fish. J Exp Biol 201:165–168
Ndong D, Chen YY, Lin YH, Vaseeharan B, Chen JC (2007) The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol 22(6):686–694
Pankhurst NW, Munday PL (2011) Effects of climate change on fish reproduction and early life history stages. Mar Freshw Res 62(9):1015–1026
Parihar MS, Javeri T, Hemnani T, Dubey AK, Prakash P (1997) Responses of superoxide dismutase, glutathione peroxidase and reduced glutathione antioxidant defenses in gills of the freshwater catfish (Heteropneustes fossilis) to short-term elevated temperature. J Therm Biol 22(2:151–156
Prophete C, Carlson EA, Li Y, Duffy J, Steinetz B, Lasano S, Zelikoff JT (2006) Effects of elevated temperature and nickel pollution on the immune status of Japanese medaka. Fish Shellfish Immunol 21 (3:325–334
Risjani Y, Yunianta, Couteau J, Minier C (2014) Cellular immune responses and phagocytic activity of fishes exposed to pollution of volcano mud. Mar Environ Res 96:73–80
Roche H, Boge G (1996) Fish blood parameters as a potential tool for identification of stress caused by environmental factors and chemical intoxication. Mar Environ Res 41:27–43
Rondeau EB (2013) Genomics of sablefish (Anoplopoma fimbria): expressed genes, mitochondrial phylogeny, linkage map and identification of a putative sex gene. BMC Genomics 14:452
Sampaio LA, Bianchini A (2002) Salinity effects on osmoregulation and growth of the euryhaline flounder Paralichthys orbignyanus. J Exp Mar Biol Ecol 269:187–196
Susan MS, Bori LO (2001) Growth and behavioral responses to elevated temperatures by juvenile sablefish Anoplopoma fimbria and the interactive role of food availability. Mar Ecol Prog Ser 217:121–134
Vinagre C, Madeira D, Narciso L, Cabral HN, Diniz M (2012) Effect of temperature on oxidative stress in fish: lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol Indic 23:274–279
Verlecar XN, Jena KB, Chainy GBN (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem Biol Interact 167(3):219–226
Wang LU, Chen JC (2005) The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus at different salinity levels. Fish Shellfish Immunol 18:269–278
Wang FI, Chen JC (2006) Effect of salinity on the immune response of tiger shrimp Penaeus monodon and its susceptibility to Photobacterium damselae subsp. Damselae. Fish Shellfish Immunol 20:671–681
Wang F, Yang H, Gao F, Liu G (2008) Effects of acute temperature or salinity stress on the immune response in sea cucumber, Apostichopus japonicas. Comp Biochem Physiol A Mol Integr Physiol 151:491–498
Wuenschel MJ, Jugovich AR, Hare JA (2004a) Effect of temperature and salinity on the energetics of juvenile gray snapper (Lutjanus griseus): implications for nursery habitat value. J Exp Mar Biol Ecol 312:333–347
Wuenschel MJ, Werner RG, Hoss DE (2004b) Effect of body size, temperature, and salinity on the routine metabolism of larval and juvenile spotted seatrout. J Fish Biol 64:1088–1102
Yin F, Peng S, Sun P, Shi Z (2011) Effects of low salinity on antioxidant enzymes activities in kidney and muscle of juvenile silver pomfret Pampus argenteus. Acta Ecol Sin 31:55–60
Acknowledgments
This research was a part of the project titled “Development of practical techniques for the artificial seeding production of sablefish,” funded by the Ministry of Oceans and Fisheries, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, JH., Park, HJ., Kim, KW. et al. Growth performance, oxidative stress, and non-specific immune responses in juvenile sablefish, Anoplopoma fimbria, by changes of water temperature and salinity. Fish Physiol Biochem 43, 1421–1431 (2017). https://doi.org/10.1007/s10695-017-0382-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0382-z