Abstract
Astronotus ocellatus, commonly called the oscar, is one of the popular cichlids among aquarium hobby. The present study deals with the development and characterization of a new cell line from caudal fin of A. ocellatus. The cell line was cultured in Leibovitz’s L−15 medium supplemented with 10% fetal bovine serum at 28 °C. The optimum temperature and FBS concentration for cell growth were tested with temperature ranges from 20 to 37 °C and FBS concentrations of 5–20% at 28 °C. The Astronotus ocellatus fin cell line has been subcultured 45 times since its development and the modal chromosome number (2n) is 48. The cell line is composed mainly of epithelial cells as confirmed by immunocytological technique using anti-cytokeratin antibodies. The cell line was cryopreserved at different passage levels and the revival efficiency showed 80% survival rate. Partial sequence amplification and sequencing of two genes, mitochondrial 16S ribosomal RNA and cytochrome oxidase I, confirmed the origin of cell line. The cell line did not show Mycoplasma contamination. The cells showed good transfection efficiency when transfected with 2 μg of pAcGFP1-N1 expression vector. The extracellular products of fish bacterial pathogens viz., Aeromonas hydrophila and A. caviae, were cytotoxic to AOF cells but were not susceptible to Cyprinid herpes virus 2. The development of AOF cell line will have significant applications in fish virology and will prove useful to isolate pathogens in the event of sudden viral disease outbreak and for the development of vaccines and diagnostic kits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ornamental fish farming is a developing industry all over the world, with an average annual growth rate of international trade in export of ornamental fish of 14% (FAO 2013). Astronotus ocellatus is among the most important members of the cichlid family in the ornamental fish trade, with a wide range of colors and forms. It is considered among the most intelligent ornamental fish because of its interactive behavior in the aquarium (Pronek 1982). With the growth of the ornamental fish sector, more attention has been given to the emerging diseases that have caused losses in ornamental fisheries. There are four reports of viral diseases of aquarium cichlids, but only the megalocytiviruses (Yanong and Waltzek 2013) and iridoviruses (Yanong and Terrell 2003) have been reported previously in Oscar. The diagnosis of fish viral pathogens is primarily based on clinical signs, histopathology, and electron microscopic studies of diseased tissues. Their basic biology and pathogenicity are often difficult to study due to lack of cell lines for culturing viruses.
Cell lines offer a huge advantage over in vivo experiments where a number of animals have to be sacrificed for each study. Maintaining such large numbers of animals is also troublesome. Numerous cell lines have been developed and characterized from different fish species (Lakra et al. 2011). India has contributed 14 fish cell lines from 9 different freshwater and brackishwater species such as Labeo rohita, Catla catla, Cirrhinus mrigala, Clarias gariepinus, Tor putitora, Etroplus suratensis, Epinephelus coioides, Lates calcarifer, and Chanos chanos (Lakra et al. 2011). These cell lines have contributed greatly to the detection of viral pathogens of fish in India, viz. viral nervous necrosis (VNN) (Banerjee et al. 2014), carp edema virus (CEV) (Swaminathan et al. 2016), Cyprinid herpes virus 2 (CyHV-2) (Sahoo et al. 2016). Considering that several emerging viral pathogens have been reported in recent years from India, cell lines from important ornamental fish are essential for isolating viruses and studying virus-host interactions. There is no report on the development of a cell line from oscar till date. In this study, a novel cell line from fin of oscar (AOF, A. ocellatus fin) was established and characterized. The AOF cell line will be useful for studying viral pathogens and other gene functions in vitro.
Materials and methods
Generation of primary culture and maintenance
Apparently, healthy A. ocellatus were purchased from local ornamental fish farms and kept in plastic tanks for acclimatization for a week. The fish were provided with proper aeration and feed and one third of water was changed every alternate day. A primary culture from caudal fin was prepared following the explant method, which is more advantageous than the enzymatic method (Avella et al. 1994). Briefly, the caudal fin was wiped with 70% alcohol and cut aseptically into a petriplate with 1 mL of phosphate buffer saline (PBS) (Life Technologies, Grand Island, NY) with antibiotics-antimycotics (100×) (Life Technologies, Grand Island, NY) and Gentamicin/Amphotericin B (500×) (Life Technologies, Grand Island, NY). The fin sample was washed thrice with PBS by gentle pipetting and finally transferred to a fresh Petri plate with PBS. The tissue sample was then minced into small pieces of 1 mm3 using a sterile scalpel blade. The tissue fragments were then explanted with 200 μL of fetal bovine serum (FBS) (Life Technologies, Paisley, UK) into a 25-cm2 cell culture flask (Thermo Scientific, Roskilde, Denmark) and allowed to attach to the surface in a vertical position for 2 h. Finally, 7 mL of complete medium Leibovitz’s L−15 (L-15) (HiMedia, Nashik, India) with 20% FBS and 100 μL of antibiotic and antimycotic solution were added to the flask and incubated at 28 °C.
Once the primary culture achieved 90% confluency, the cells were passaged into another flask. Briefly, the confluent flask was first washed with PBS twice and finally treated with 0.25% trypsin-EDTA (Life Technologies, Grand Island, NY) in PBS. The cells were harvested by vigorous pipetting with 2 mL of fresh L-15 medium with 5% FBS, 1 mL of which was seeded into a fresh flask. The volume was made up to 7 mL in both the flasks with fresh L-15 medium containing 5% FBS. No antibiotics were used hereafter. The passage number was recorded every time.
Cell growth studies
The stability of cell growth pattern was studied at different FBS concentrations and temperatures at 27th passage level. The cells were seeded in six-well tissue culture plates at a density of 5 × 104 cells per well. Plates were incubated at 20, 25, 28, 35, and 37 °C. Another set of plates had the cells grown in 5, 7.5, 10, 15, and 20% FBS concentration at 28 °C in duplicate. The growth pattern was observed up to day 14. Finally, cells were harvested and counted with a hemocytometer.
Cryopreservation and revival
The cells were cryopreserved at 5, 15, 20, and 35th passage levels for further use. When the cells achieved 70–80% confluency in a flask, they were trypsinized (0·25% trypsin-EDTA in PBS) and collected in a 15-mL centrifuge tube by centrifuging at 367×g at 4 °C. The cells were then washed thrice with 2 mL PBS with antibiotics and finally resuspended in Recovery™ cell-culture freezing medium (Life Technologies, Grand Island, NY) at a density of 5 × 106 cells/mL. The cells were then aliquoted to 1.5 mL pre-sterilized cryovials and stored at − 20 °C for 2 h. The cells were then kept at − 70 °C overnight and then transferred to liquid nitrogen (LN2) for cryopreservation.
After 6 months, the cells were revived and checked for cell viability. Briefly, the frozen cells were thawed quickly at 37 °C in a water bath and mixed drop-wise with complete medium in a 15-mL centrifuge tube. Then, the cells were centrifuged at 825×g at 4 °C and the pellet resuspended in 10 mL of complete medium. Cell viability was checked with a hemocytometer following trypan blue staining. The revived cells were seeded into 25-cm2 flask and incubated at 28 °C for further culture.
Immunophenotyping of the cells
AOF cells at 32nd passage were grown on cover slips placed on 6-well plates (Thermo Scientific, Jiansu, P. R. China) for immunotyping. After a 24-h culture at 28 °C, the cells were washed with PBS and fixed in methanol for 30 min at − 20 °C. The methanol was removed, the cells were washed with PBS again, and finally incubated in 1% BSA dissolved in PBS for 1 h at 37 °C. The cells were incubated with primary antibodies (1:200), mouse anticytokeratin (pan), clone AE1/AE3 antibodies (Life Technologies, Grand Island, NY), or mouse anti-fibronectin antibodies (Life Technologies, Grand island, NY) overnight at 4 °C. For control group, 1% BSA was used in PBS in place of primary antibodies. Then, the cells were washed with PBS and incubated with rabbit anti-mouse immunoglobulin (IgG) fluorescein isothiocyanate conjugate (Life Technologies, Grand Island, NY) diluted 1:50 times in PBS containing 1% BSA for 1 h. After a final wash with PBS, the cover slips were mounted in VECTASHIELD mounting medium (Vector Laboratories, USA) and monitored under a fluorescence microscope (Nikon, Japan).
Chromosome analysis
Chomosome analysis was done at 12th and 34th passage level. Briefly, 24-h grown culture with 80% confluency was washed with PBS and treated with colchicine at a final concentration of 1 μg/mL in complete medium for 4–6 h at 28 °C. Then, the cells were trypsinized and pelleted down in a tube by centrifuging at 367×g for 10 min at 25 °C. The supernatant was removed and the cells were treated with 5 mL of 0.56% KCl for 25 min. Then, the cells were pre-fixed for 5 min in 10-mL fixative solution (3:1 methanol and acetic acid). The cells were pelleted again at 367×g at 4 °C for 10 min and washed four times with methanol and acetic acid solution. Finally, the cells were resuspended in 2-mL fresh fixative solution and dropped onto a clean pre-cooled glass slide to make them spread. The cells were stained with 10% Giemsa solution for 20 min and dried for 20 min at room temperature. A total of 100 chromosome spreads were counted using a light microscope.
Mitochondrial gene sequencing
To authenticate the origin of the cell line, COI and 16S rRNA gene were amplified from DNA isolated from cells at 30th passage level. Mitochondrial DNA (mtDNA) isolated from muscle tissue of A. ocellatus according to the salting out method (Miller et al. 1988) served as positive control for amplified DNA sequences. Universal primers for COI (F 5′CGC CTG TTT ATC AAA AAC AT 3′ and R 5′CCG GTC TGA ACT CAG ATC ACG T 3′) (Ward et al. 2005) and 16s rRNA (F 5′TCA ACC AAC CAC AAA GAC ATT GGC AC 3′ and R 5′TAG ACT TCT GGG TGG CCA AAG AAT CA3′) (Palumbi et al. 1991) produced bands of 642 and 562 bp, respectively. The PCR reaction and temperature conditions were as per Swaminathan et al. (2013). The PCR products were analyzed in 1.5% agarose gel and visualized under UV transilluminator (Bio-Rad, USA). The PCR products were then purified and sequenced using ABI 3730 DNA analyzer (Applied Biosystems, USA). The resulting DNA sequences for both the fragments were aligned with known sequences of A. ocellatus obtained from National Centre for Biotechnology Information (NCBI, USA) database.
Cytotoxicity test and bacterial extracellular products
For cytotoxicity studies, Aeromonas hydrophila and Aeromonas caviae isolated from diseased fish in our laboratory were used. The extracellular products (ECPs) from these bacteria were prepared as per Liu (1957) with some modifications. Briefly, the bacterial cells were incubated for 48 h at 28 °C after spreading onto a sterile cellophane sheet overlaying an agar plate. The cells were harvested with PBS and sonicated for 10 min on ice. The cell suspensions were centrifuged at 13,000×g for 20 min at 4 °C, and the supernatant was filtered through 0.22-μm filters (Satorious, Göttingen, Germany) and stored at − 80 °C until further use.
Cytotoxicity was tested as per Fornelli et al. (2004). Briefly, 2 × 105 cells from AOF at 32nd passage level were seeded into a 6-well plate and incubated for 18 h. Then, the medium was replaced with 100 μL of ECP samples and 900 μL fresh medium and incubated for 48 h. Later, the cells were harvested and cell viability was measured by MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Invitrogen, USA) assay (Borenfreund et al. 1988). After 48 h, the medium was replaced with 20 μL of 5 mg/mL MTT in PBS and incubated for 4 h at 20 °C. The MTT solution was aspirated and the cells were washed twice with PBS. Then, dimethyl sulfoxide was added at 150 μL/well to solubilize the produced intracellular purple formazan crystals and absorbance was measured at 570 nm. The percentage viability was then calculated.
Viral susceptibility study
Cyprinid herpesvirus 2 (CyHV-2), the viral pathogen isolated in India, from the goldfish, Carassius auratus (Sahoo et al. 2016), was used for viral susceptibility studies. Viral inoculum was prepared by homogenizing the infected tissue samples in L-15 medium with antibiotics and without FBS. The tissue homogenate was then frozen and thawed four times and centrifuged at 12,000×g for 45 min at 4 °C. The supernatant was filtered using 0.22-μm membrane filter and stored at − 20 °C for further use. Oscar cell line at its 30th passage was incubated with tissue filtrate for 1 h at 37 °C. After 1 h, the tissue filtrates were aspirated and fresh L-15 medium with 5% FBS was added and the cells were incubated at 28 °C. The cells were observed for cytopathic effects (CPE) under an inverted microscope (Nikon, Japan) for 15 successive days.
Transfection
For transfection studies, cells were cultured in a 6-well plate at a density of 1 × 105 cells well−1 at 40th passage level. After 24 h, the sub-confluent monolayers were transfected with 2 μg of pAcGFP1-N1 expression vector (Clontech, USA) using Lipofectamine 2000 Transfection Reagent (Invitrogen, USA) following the manufacturer’s instructions. After 52 h, the transfected cells were checked for the green fluorescence signals under a fluorescent microscope.
Mycoplasma detection
To check Mycoplasma contamination, cells at 36th passage level were grown without antibiotics in the medium for 5 days. Then, the cells were harvested and centrifuged at 200×g for 10 min. Supernatant was transferred into new micro-centrifuge tubes and centrifuged further at 250×g to remove debris. Finally, the supernatant was centrifuged at 15,000×g for 10 min and the pellet was resuspended in 50 μL of buffer solution. Then, the solution was heated at 95 °C for 3 min and stored at − 20 °C until use. Tests for Mycoplasma contamination were run using EZdetectTM PCR Kit (HiMedia, USA) based on amplification of spacer region between 16S and 23S ribosomal RNA (rRNA) genomic DNA sequence. The amplification products were analyzed in 1% agarose gel.
Results
Primary culture
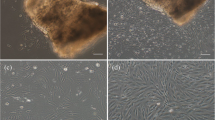
Explant method was followed to develop the cell culture from caudal fin of Oscar. Cell growth and migration to form a complete monolayer took place after day 15 of the culture (Fig. 1a). The monolayer subcultured at initial passages (10th passage) comprised both fibroblastic-like and epithelial-like cells (Fig. 1b). Later, after about 22nd passage, epithelial-like cells dominated over fibroblastic cells. The developed cell line was named A. ocellatus fin (AOF) and passaged up to 45th passage level. The immunostaining results showed a strong positive signal in the cells incubated with cytokeratin, but not in fibronectin which confirmed that AOF cells are epithelial cells (Fig. 1d).
Phase contrast and fluorescent photomicrographs of freshwater Oscar, Astronotus ocellatus caudal fin cell line (AOF). a Explant culture of caudal fin from A. ocellatus showing radiation of cells, b AOF cell line at 10th passage, c AOF cell line at 22nd passage, and d AOF cells showing presence of cytokeratin marker
Effects of temperature and FBS on growth
The AOF cells grown at varying temperatures ranging from 20 to 37 °C showed maximum growth at 28 °C. The AOF cells grown at different FBS concentration showed increase in growth rate with increased FBS concentration. The cells showed comparatively less growth at 5% FBS concentration, relatively moderate growth was seen at 10 and 15%, and maximum growth was observed at 20% FBS concentration (Fig. 2a, b).
Cryopreservation
The AOF cells that were cryopreserved at 5th, 15th, 20th, and 35th passages were successfully revived after 6 months with 80% viability (Fig. 3). The revived cells formed a monolayer at 28 °C by about day 10 without showing any morphological change.
Species authentication
Most of the AOF cells at 12th and 34th passage level were arrested in metaphase after treatment with colchicine. The chromosome count of hundred cells revealed 32 to 55 and 30 to 54 chromosomes in 12th and 34th passage level, respectively, showing a peak at 48 diploid chromosome number. The majority of cells (53%) showed a haploid chromosome number (2n = 48), that is in accordance with the published chromosome number of the species (Fig. 4a, b).
The COI and 16S rRNA amplification produced products of 642 and 562 bp, respectively. The sequence information obtained for these two fragments showed a 98–99% match with the known A. ocellatus mtDNA sequences in NCBI database in the basic local alignment search tool (BLAST; Altschul et al. 1990). This confirmed that the cells as originating from A. ocellatus and the sequences were submitted to GenBank.
Cytotoxicity to bacterial ECPs and virus susceptibility tests
The cytotoxicity of ECPs from A. hydrophila and A. caviae was noticed in AOF cells after 12 h post-inoculation (hpi). Change in cell morphology, such as cell shrinking and rounding and floating of dead cells (Fig. 3), was observed at 48 hpi whereas no significant change was observed in the PBS-treated control group. The percentage viability of AOF cells observed in MTT assay showed an inverse relationship with duration of ECP incubation time. The AOF cells did not show any CPE to CyHV-2, even after 2 weeks and 10 blind passages.
Transfection
The AOF cells transfected at 40th passage with 2 μg of pAcGFP1-N1 expression vector showed fluorescent signals at 52 h post-transfection. The estimated transfection efficiency of AOF cells was c. 12%, indicating its potential to be used in transfection studies for gene expression (Fig. 5).
Detection of Mycoplasma contamination
At 36th passage, the AOF cells did not give any amplification, thus confirming they are free from any Mycoplasma contamination.
Discussion
In this work, a cell line was established and characterized from caudal fin of Oscar for its use in virus isolation studies. The AOF cell line was passaged for more than 45 times over a period of 1 year. Emerging viral diseases are causing huge economic losses to ornamental fish and commercial aquaculture, and they may become a threat to wild aquatic animals too (Walker and Winton 2010). Astronotus ocellatus, commonly called the oscar, is a popular ornamental fish not only for its varying body color and form, also for its interactive behavior with the hobbyist (Pronek 1982). This species is susceptible to iridoviral infections that can cause huge losses in production as well as wild stocks (Yanong and Tarrell 2003). A cell line from the caudal fin of A. ocellatus was developed and characterized for use in virus isolation studies.
The cell line was maintained in L-15 medium with 10% FBS. L-15 was used since it supports better growth of fish cell lines compared to other media (Fernandez-Puentes et al. 1993) and is widely used as a preferred media in many fish cell lines (Lakra et al. 2011). The AOF cells have not shown any morphological changes up to the 45th passage level. The passage number was restricted to 45th since more passages and high volume of trypsin exposure may affect the phenotypic and genotypic characteristics of the cells. Though both epithelial-like and fibroblastic-like cells were observed during the initial passages, a homogeneous population of epithelial cells was noticed after the 22nd passage. This was further confirmed with a strong positive reaction to epithelial marker, cytokeratin, and a negative reaction to fibroblastic marker, fibronectin. The AOF cells showed temperature-dependent growth between 20 and 37 °C with optimum growth at 28 °C. Previously, Ossum et al. (2004) reported that the cells from warm water fishes can grow at 15–37 °C incubation temperature. The growth pattern of AOF cells checked at different concentration of FBS (5–20%) showed an optimal growth at 10% FBS concentration, which again reduced the cost of FBS in the culture. Cryopreservation of AOF cell lines for 6 months at − 196 °C showed 80% viability after revival. Previous reports also showed similar results (70–90% viability) after storage at − 196 °C (Chaudhary et al. 2013; Swaminathan et al. 2013). The chromosome number of AOF was found to be 2n = 48 at 12th and 34th passage numbers, which was in accordance with the modal chromosome number reported earlier by Nascimento et al. (2006). Mitochondrial DNA genes, e.g., COI and 16S rRNA amplified and sequenced from the AOF cell line, further confirmed the origin to be A. ocellatus. These genes are mostly used for species identification and deriving phylogenetic relationships, among aquatic and mammalian species. The AOF cells were free from Mycoplasma contamination as checked by screening by PCR at 36th passage as described by Frerichs (1996). Screening for Mycoplasma contamination is important since 1–35% of cells are prone to this kind of contamination in primary early passage and in continuous cell cultures (Uphoff and Drexler 2002).
ECPs are important virulent factors of many fish pathogenic bacteria that serve to resist the host defense mechanism (Ellis 1991). The cytotoxicity showed by ECPs of A. hydrophila and A. caviae in AOF cells was almost similar to CPE produced by other pathogenic bacteria studied earlier (Swaminathan et al. 2010; Sood et al. 2015). The cytotoxic effects of ECPs of A. hydrophila and A. caviae on AOF cells in vitro can be used as an alternative to in vivo bioassays to determine cytotoxic effects. However, AOF cell lines cannot be used as an isolating cell line for CyHV-2, since it did not show any susceptibility to this virus. Other viral species should also be tested, which may show CPE in AOF cells. The transfection efficiency of AOF cells was found to be really good which allows it to be an efficient cell line for gene expression studies and assessing promoter efficiency in various plasmid constructs. Additionally, having a cell line of A. ocellatus will also give a platform for further studies on the pathogenesis of many fish pathogens. Some pathogenic viruses are species specific which makes the establishment of the host cell line necessary for proper monitoring of viral diseases (Luc Rougee et al. 2007). Therefore, the development of AOF cell line will help in isolating viruses in case of any report on disease outbreaks of A. ocellatus. The AOF cell line was deposited at National Repository of Fish Cell line (NRFC), National Bureau of Fish Genetic Resources (NBFGR), Lucknow, India for further availability and dissemination.
In conclusion, a cell line, AOF has been successfully developed from the caudal fin of A. ocellatus. The ECPs from pathogenic fish bacteria, A. hydrophila and A. caviae, are cytotoxic to AOF cells and AOF cells are suitable for gene expression studies. The novel AOF cells could also be used in viral isolation studies and for the development of antiviral health management strategies.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Bio 215:403–410
Avella M, Berhaut J, Payan P (1994) Primary culture of gill epithelial cells from the sea bass Dicentrarchus labrax. In Vitro Cell Dev Biol Anim 30:41–49
Banerjee D, Hamod MA, Suresh T, Karunasagar I (2014) Isolation and characterization of a nodavirus associated with mass mortality in Asian seabass (Lates calcarifer) from the west coast of India. Virus Dis 25:425–429
Borenfreund E, Babich H, Martin-Alguacil N (1988) Comparison of two in vitro cytotoxicity assays: the neutral red and tetrazolium (MTT) tests. Toxicol in Vitro 2:1–6
Chaudhary DK, Sood N, Swaminathan TR, Rathore G, Pradhan PK, Agarwal NK, Jena JK (2013) Establishment and characterization of an epithelial cell line from thymus of Catla catla (Hamilton, 1822). Gene 512:546–553
Ellis AE (1991) An appraisal of the extracellular toxins of Aeromonas salmonicida sp. salmonicida. J Fish Dis 14:265–277
FAO (2013) Fisheries Department publications. FAO Fisheries and Aquaculture Department. (http://www.fao.org/fishery/topic/13611/en. 12 Mar, 2013), Rome
Fernandez-Puentes C, Novoa B, Figueras A (1993) Initiation of a cell line from turbot (Scophthalmus maximus L). In Vitro Cell Dev Biol Anim 29:899–900
Fornelli F, Minervini F, Logrieco A (2004) Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF-9). J Invertebr Pathol 85:74–79
Frerichs GN (1996) Identification and elimination of mycoplasmas in fish cell line cultures. J Fish Dis 19:435–439
Lakra WS, Swaminathan TR, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37:1–20
Liu PV (1957) Survey of haemolysin production among species of pseudomonads. J Bacteriol 74:718–727
Luc Rougee GK, Ostrander RH, Richmond YL (2007) Establishment, characterization and viral susceptibility of two cell lines derived from goldfish, Carassius auratus muscle and swim bladder. Dis Aquat Org 77:127–135
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Nascimento AL, Souza ACP, Feldberg E, Carvalho JR, Barros RMS, Pieczarka JC, Nagamachi CY (2006) Cytogenetic analysis on Pterophyllum scalare (Perciformes, Cichlidae) from Jari River, Parástate. Caryologia 59:138–143
Ossum GC, Hoffmann EK, Vijayan MM, Holt SE, Bols NC (2004) Characterization of a novel fibroblast-like cell line from rainbow trout and responses to sublethal anoxia. J Fish Biol 64:1103–1116
Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G (1991) The simple Fool’s guide to PCR. University of Hawaii, Honolulu, HI
Pronek N (1982) Oscars. TFH Publications, Neptune City
Sahoo PK, Swaminathan TR, Abraham TJ, Kumar R, Pattanayak S, Mohapatra A, Ratha SS, Patra A, Adikesavalu H, Sood N, Pradhan PK, Das BK, Jayasankar P, Jena JK (2016) Detection of goldfish haematopoietic necrosis herpes virus (cyprinid herpesvirus-2) with multi-drug resistant Aeromonas hydrophila infection in goldfish: first evidence of any viral disease outbreak in ornamental freshwater aquaculture farms in India. Acta Trop 161:8–17
Sood N, Chaudhary DK, Pradhan PK, Verma DK, Swaminathan TR, Kushwaha B, Punia P, Jena JK (2015) Establishment and characterization of a continuous cell line from thymus of striped snakehead, Channa striatus (Bloch 1793). In Vitro Cell Dev Biol Anim 51:787–796
Swaminathan TR, Basheer VS, Gopalakrishnan A, Rathore G, Chaudhary DK, Kumar R, Jena JK (2013) Establishment of caudal fin cell lines from tropical ornamental fishes Puntius fasciatus and Pristolepis fasciata endemic to the western Ghats of India. Acta Trop 128:536–541
Swaminathan TR, Kumar R, Jency PME, Charan R, Syamkrishnan MU, Basheer VS, Sood N, Jena JK (2016) A new fish cell line derived from the caudal fin of freshwater angelfish Pterophyllum scalare: development and characterization. J Fish Biol 89:1769–1781
Swaminathan TR, Lakra WS, Gopalakrishnan A, Basheer VS, Kushwaha B, Sajeela K (2010) Development and characterization of a new epithelial cell line PSF from caudal fin of green chromide, Etroplus suratensis (Bloch, 1790). In Vitro Cell Dev Biol Anim 46:647–656
Uphoff CC, Drexler HG (2002) Comparative antibiotic eradication of mycoplasma infections from continuous cell lines. In Vitro Cell Dev Biol Anim 38:86–89
Walker PJ, Winton JR (2010) Emerging viral diseases of fish and shrimp. Vet Res 41:51
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia’s fish species. Philos Trans R Soc Lond B 360:1847–1857
Yanong RPE, Waltzek TB (2013) Megalocytivirus infections in fish, with emphasis on ornamental species. UF/IFAS extension FA182. University of Florida, Gainesville, FL
Yanong, RPE and Terrell SP (2003) Iridoviral-associated disease in oscars (Astronotus ocellatus). Proceedings of the 34th Annual Conference of the International Association for Aquatic Animal Medicine, Waikoloa, Hawaii
Acknowledgements
The authors express their thanks to Director, ICAR-NBFGR and Deputy Director General (Fy. Sc.), ICAR for the support, guidance, and encouragement. The authors are also thankful to National Fisheries Development Board, DADF, Ministry of Agriculture and Farmers Welfare for the financial support under NSPAAD (Grant Number: NFDB/Coord/NBFGR/2012-13/16720 dated 11.02.2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Ravi, C., Das, S. et al. Establishment and characterization of a caudal fin-derived cell line, AOF, from the Oscar, Astronotus ocellatus. Fish Physiol Biochem 45, 123–131 (2019). https://doi.org/10.1007/s10695-018-0542-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0542-9