Abstract
The establishment and characterization of a continuous cell line from the thymus of air-breathing fish Channa striatus are described. The cell line, designated C. striatus thymus (CST), has been subcultured over 71 times and shows optimal growth at 28°C in Leibovitz’s-15 (L-15) medium supplemented with 20% fetal bovine serum. The CST cells exhibited low plating efficiency which improved with increase in seeding density. The karyotype analysis revealed that CST cells have a normal diploid karyotype with 2n = 40. Partial amplification and sequencing of two mitochondrial genes, viz. 16S ribosomal RNA (rRNA) and cytochrome oxidase I, confirmed that the cell line originated from C. striatus. CST cells were successfully transfected indicating their potential application for expression of recombinant proteins. In immunocytochemical staining, CST cells showed characteristics of epithelial cells. These cells were sensitive to extracellular products of Vibrio cholerae MTCC 3904 as well as to heavy metal mercuric chloride. The CST cell line would be a useful tool in functional genomic studies such as RNA interference and gene knockout as well as for cytotoxicity studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Striped snakehead, Channa striatus (Bloch 1793), belonging to the family Channidae, is a popular and the most economically important species of the genus Channa (Talwar and Jhingran 1992). This species has an extensive natural distribution particularly in Southeast Asian countries and has many advantageous characteristics, notably high market price, hardiness, and high tolerance to adverse environmental conditions. In addition, its flesh has high nutritive value and is said to have wound-healing effect and recuperative attributes (Courtenay and Williams 2004). C. striatus is highly suitable for aquaculture due to its air-breathing habit and is considered as a candidate species for aquaculture (NBFGR 2011). One of the major objectives of fish culture is to breed and cultivate fish to limit the impact on wild populations. However, various infectious diseases tend to occur during aquaculture. In this context, easily manageable tools such as in vitro test systems and optimal cell growth parameters are necessary (Grunow et al. 2011).
In vivo bioassays using fish are often applied, but assays using whole fish are inconvenient, time consuming, difficult to reproduce and require sacrificing the organisms (Wang et al. 2004). The inherent economic and ethical constraints associated with tests on live animals have encouraged the use of fish cell lines (Ní Shúilleabháin et al. 2004) which seem to be potential surrogates for the whole fish (Wang et al. 2004). The fish cell lines provide a readily available, stable, cost-effective, and reproducible system for analyzing identical cells that genetically resemble animal tissues and species (Bols et al. 1994; Smith 2006) and, therefore, are valuable for studying tissue- and species-specific responses at the cellular level to different stimuli (Dewitte-Orr et al. 2006; Higaki et al. 2013). Moreover, the cell lines allow cellular phenomena to be studied in a controlled and completely defined environment, independent of the complexities and variability of systemic or larger physiological controls (Bols et al. 2005). The cultured cells also have the potential to be used as nucleus carriers for fish bearing valuable phenotype or genotype or when their gametes or embryos are not available (Han et al. 2011).
Fish cell lines have been widely used as in vitro models in physiology, nutrition, virology, immunology, and functional genomics ( Fent 2001; Wise et al. 2002; Ní Shúilleabháin et al. 2004; Ariel et al. 2009; Cheng et al. 2010; Buonocore et al. 2011). The susceptibility of fish viruses can be species-specific and this makes a cell line derived from a particular fish species more appropriate for isolation of viruses (Dewitte-Orr et al. 2006; Swaminathan et al. 2010). In addition, virus isolation using cell lines can detect emerging or previously unknown viruses for which molecular assays are yet to be developed. The cell lines also allow to produce a sufficient stock of virus for infectivity trials and vaccine production (Frerichs et al. 1996; Ariel et al. 2009). Furthermore, cell lines are useful tools to study the cellular and molecular basis of physiological processes such as analysis of function of important immune genes (Thompson et al. 1999 Dewitte-Orr et al. 2006; Wang et al. 2010). Permanent cell lines have been employed for screening and relative toxicity ranking of chemicals and environmental samples (Davoren et al. 2005; Schirmer et al. 2008; Tan et al. 2008).
The present paper describes the establishment of a cell line from the thymus of C. striatus, using the explant method. The developed cell line was characterized in terms of growth at different temperatures and varying concentrations of fetal bovine serum. Confirmation of species of origin, histological origin, karyotyping, susceptibility to bacterial extracellular products and heavy metal as well as expression of foreign gene was also assessed.
Materials and Methods
Preparation of tissue for primary cell culture.
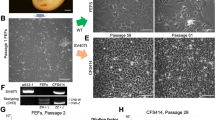
Apparently healthy C. striatus, weighing 90 g, was procured from a local fish market. The fish was euthanized with an overdose of MS222 (Sigma-Aldrich, St. Louis, MO) and wiped with 70% alcohol. The thymus which is located on the dorsal body wall of the gill cavity (Fig. 1) was aseptically removed. The thymus tissue was washed thrice with phosphate buffer saline (PBS) containing 2× concentration of antibiotic-antimycotic solution (Gibco, Grand Island, NY) and transferred to a petri dish containing PBS with 1× concentration of antibiotic-antimycotic solution. The tissue was minced into small pieces (approximately 1 mm3) with sterile scissors and transferred to a 25-cm2 flask (Nunc, Roskilde, Denmark). The excess PBS was removed and 100 μl of heat-inactivated fetal bovine serum (FBS, Gibco) was added to facilitate the attachment of tissue explants to the surface of the flask which was incubated at 28°C. After 2 h, excess FBS was drained from the flask and 5 ml of growth medium, Leibovitz’s-15 (L-15) medium supplemented with 10% FBS and 1× concentration of antibiotic-antimycotic solution, was added. About one third of the medium was replaced every 4th day till the radiating cells attained 90% confluency. After formation of a monolayer, the cells were trypsinized with 0.05% trypsin-EDTA solution (Gibco) and subcultured at a split ratio of 1:2 in growth medium.
Cryopreservation.
The subcultures were stored in liquid nitrogen after every 10th passage in the freezing medium, which consisted of FBS containing 10% dimethyl sulfoxide. After trypsinization, the cells were centrifuged at 1500g for 5 min. The pellet was resuspended in 1 ml of the freezing medium and the cell suspension was transferred to a cryovial (Nunc). The cryovial was kept at −80°C overnight in a Mr. Frosty Freezing container and finally stored in liquid nitrogen (−196°C). For revival, the cryovial was thawed quickly in a water bath at 28°C and transferred to a 25-cm2 tissue culture flask containing about 10 ml of growth medium. The viability of the revived cells was estimated by trypan blue staining using a Neubauer hemocytometer. After overnight incubation, the medium in the flasks was replaced with fresh medium.
Effect of temperature and FBS concentration on cell growth.
Growth studies were carried out to determine the optimum temperature and FBS concentration for the C. striatus thymus (CST) cell line. A total of 1 × 105 cells ml−1 at passage 45 were inoculated into 25-cm2 cell culture flasks and incubated at 28°C for 2 h to allow attachment of cells. Afterwards, the batches of culture flasks were incubated at selected temperatures of 24, 28, 32, and 37°C for growth studies. This study was performed using growth medium. Every day, three flasks at each temperature were trypsinized to count the number of cells and the study was carried out for 4 d. A similar study was carried out to determine the effect of different concentrations of FBS (5, 10, 15, and 20%) on cell growth with passage 46 cells at 28°C.
Plating efficiency (PE).
The plating efficiency of the CST cell line was determined at passage 67 in 8.8-cm2 tissue culture dishes (Nunc). The wells were seeded with CST cells at densities of 100 and 250 cells dish−1 and cultured in L-15 medium at 28°C. Half of the medium was replaced every 4th day. After 10 d, the medium was discarded and cells were washed with PBS. Thereafter, the cells were fixed with methanol and stained with crystal violet. The individual colonies were counted under a microscope, and plating efficiency was calculated using the formula: PE (%) = number of cell colonies / number of cells seeded × 100 (Freshney 2005).
Chromosome analysis.
The karyotype was prepared using the standard procedure with passage 64 cells (Freshney 2005). Briefly, the cells were grown in a 25-cm2 tissue culture flask till 70–80% confluency was attained. Colchicine solution (Life Technologies, Carlsbad, CA) was added to the cells at a final concentration of 0.2 μg ml−1. The flask was incubated for 2 h at 28°C. After trypsinization, the cells were collected by centrifugation at 200g for 5 min and treated with a hypotonic solution of 0.56% potassium chloride for 20 min. Thereafter, the cells were fixed in acetic acid/methanol solution (1:3) for 5 min at room temperature. Slides were prepared using a conventional drop technique and stained with 5% Giemsa solution. Chromosomes were observed and counted under a light microscope.
Confirmation of the origin of the cell line.
The origin of the developed CST cell line was authenticated by partial amplification and sequencing of two mitochondrial genes, viz. 16S ribosomal RNA (rRNA) and cytochrome oxidase I (COI), from CST cells following Swaminathan et al. (2010). Briefly, DNA was isolated from CST cells (5 × 106) at passage 42 as well as from the muscle of C. striatus, and fragments of the two genes were amplified by PCR using the published primers (Table 1). The thermal cycling conditions included an initial denaturation at 95°C for 5 min, followed by 30 cycles of 95°C for 45 s, 50°C for 30 s, 72°C for 45 s, and a final extension of 5 min at 72°C. The amplified PCR products were sequenced in Applied Biosystems ABI 3730xl capillary sequencer through a commercial sequencing facility. The DNA sequences from the CST cell line were aligned with sequences from C. striatus muscle. The sequences for both the mitochondrial genes amplified from the CST cell line were also checked for similarity with existing sequences submitted in NCBI GenBank.
Transfection.
The CST cells at the 60th passage were seeded in a six-well plate at a density of 1 × 105 cells well−1. After 24 h, the subconfluent monolayers were transfected with 2 μg of phrGFP II-N mammalian expression vector (Stratagene, La Jolla, CA) which contains a CMV promoter, bovine growth hormone polyadenylation signal, and a gene for neomycin/kanamycin resistance. The CST cells were transfected using SatisFection reagent (Stratagene), and the green fluorescence signals were observed under a fluorescent microscope 48 h after transfection (Qin et al. 2006).
Morphological confirmation with immunocytochemistry.
Immunophenotyping of the CST cell line was carried out following Mauger et al. (2009) at passage 47. Briefly, CST cells were grown on sterile coverslips for 24 h. The cells were subsequently fixed and permeabilized with methanol at −20°C for 30 min. For immunostaining, the coverslips were preincubated with PBS containing 1% bovine serum albumin (BSA) for 1 h at 37°C and then incubated overnight at 4°C with mouse anticytokeratin (pan), clone AE1/AE3 antibodies (Invitrogen), or mouse antivimentin antibodies (Invitrogen). In control coverslips, only PBS with 1% BSA was used in place of the primary antibodies. After PBS washing, cells were incubated for 1 h with rabbit antimouse IgG FITC conjugate (diluted 1:50 in PBS containing 1% BSA). The coverslips were washed again in PBS, mounted in VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA), and observed under a fluorescent microscope.
Cytotoxicity test.
Bacterial extracellular products.
The cytotoxicity of bacterial extracellular products (ECPs) from Vibrio cholerae MTCC 3904 was tested with CST cells. The ECPs from V. cholerae were prepared following Balebona et al. (1998). Briefly, 0.5 ml of a 24-h-old broth culture of V. cholerae was spread on sterile cellophane sheet, overlaying brain heart infusion agar plate, and incubated at 37°C for 48 h. Bacterial cells were harvested from the cellophane sheet with PBS. The cell suspension was centrifuged at l3,000g for 20 min. The supernatant was filtered through a 0.22-μm membrane filter and used as crude preparation of ECPs. The monolayers of CST cells grown in a 24-well plate using growth medium were inoculated with 0.1 ml of a twofold serial dilution of crude ECPs in triplicates. For negative controls, sterile saline was used in place of ECPs. Plates were incubated at 28°C and the effects of ECPs on the cells were observed up to 48 h.
Heavy metal.
Neutral red uptake assay was carried out to measure plasma membrane integrity in CST cells following exposure to different concentrations of mercuric chloride, as per Repetto et al. (2008). Briefly, wells of a 96-well plate were seeded with 100 μl of 1 × 105 CST cells ml−1 and incubated overnight at 28°C. Thereafter, the culture supernatant was removed and 100 μl of L-15 containing mercuric chloride (SRL, Mumbai, India) in increasing concentration (1.95, 3.91, 7.81, 15.63, 31.25, 62.5, 125, 250, and 500 μg ml−1) was added to the wells in triplicates and incubated at 28°C for 24 h. In control wells, 100 μl of growth medium was added. Following washing with PBS, 100 μl of neutral red working solution (40 μg ml−1 of growth medium) was added to the wells. The plate was again incubated for 2 h at 28°C and then the neutral red solution was decanted. The wells were washed with 150 μl of PBS followed by fixation of cells by adding 5% glutaraldehyde for 2 min. Then, 150 μl of neutral red destaining solution was added to each well and the plate was kept on a plate shaker for 10 min. The optical density (OD) of the neutral red extract was taken at 540 nm in a microplate reader.
Results
Primary cell culture and subculture.
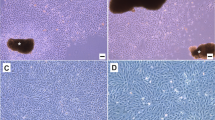
The cells migrated out from the edges of thymus explants of C. striatus and formed a complete monolayer by 3 wk. Subsequently, the cells were split at a ratio of 1:2 and monolayer formation took about 10–11 d during earlier passages. After about 50 passages, the monolayer was formed in 6–7 d. The cells have been subcultured for 71 passages and the cell line has been designated CST cell line. Observation of radiating cells from the explants revealed cells with epithelial as well as fibroblastic morphology (Fig. 2). During the initial passages, there was a mixed population of cells: cells with long cytoplasmic processes which were quite prominent, and in between these cells, there were cells with epithelial morphology which appeared very light. After 40 passages, the epithelial cells became predominant with a few cells having long cytoplasmic processes.
Growth studies.
The CST cells grew well at 28 and 32°C; however, the maximum growth was observed at 28°C. The cells did not grow at 37°C (Fig. 3a ). Growth of the cell line in media supplemented with varying concentrations of FBS is shown in Fig. 3b . Faster growth of cells was observed with increasing concentration of FBS, with maximum growth in L-15 medium supplemented with 20% FBS. After 4 d of culture, the growth of CST cells was very slow in the medium containing 5% FBS than in the media containing a higher concentration of FBS.
Cryopreservation and plating efficiency.
Revival of CST cells after 8 mo of storage in liquid nitrogen showed over 90% viability after thawing. The cells grew to form a monolayer in 5 d and did not show any change in morphology. Plating efficiency of CST cells was determined to be 2.5 and 6.4% at seeding concentrations of 100 and 250 cells, respectively, in 8.8-cm2 tissue culture dishes. The efficiency was low but improved with increase in seeding density.
Chromosome analysis.
The results of chromosome counts of 100 metaphase spreads from CST cells at passage 64 revealed that the number of chromosomes varied from 31 to 49 (Fig. 4a ). However, the majority of CST cells (62%) had a modal chromosome number of 40. The metaphase spreads with normal diploid number of 40 displayed normal karyotype morphology (Fig. 4b ).
Karyotype analysis of CST cells at passage 64. (a) Chromosome number distribution, metaphase spread; and (b) diploid karyotype of CST cell. The main chromosome number was 40 which consisted of 4 pairs of metacentrics, 1 pair of submetacentrics, 8 pairs of subtelocentrics, and 7 pairs of telocentrics (2n = 8 m + 2 sm + 16 st + 14 t).
Identification of the cell line.
Amplification of cytochrome oxidase I and 16S rRNA genes from CST cells and C. striatus muscle yielded PCR products of approximately 654 and 569 bp, respectively (Fig. 5). Comparative sequence analysis revealed 100% identity among the COI genes from CST cells and C. striatus muscle, as well as with the known C. striatus mitochondrial DNA sequences available in NCBI GenBank. Similarly, the sequence of the 16S rRNA gene of CST cells showed 100% similarity with 16S sequence from C. striatus muscle and known mitochondrial DNA sequence available in GenBank. The sequences of COI and 16S rRNA gene of CST cells have been submitted to GenBank (accession numbers KP226701 and KP226702). The data confirm that the CST cell line is derived from C. striatus.
PCR amplification of ~600 and 700 bp sequences of the Channa striatus genome using universal oligonucleotide primers of the 16S rRNA and cytochrome oxidase I (COI) genes, respectively. Mitochondrial DNA amplification with 16S rRNA primers: lane 1—negative control, lane 2—C. striatus muscle, lane 3—CST cells, lane 4—100 bp DNA ladder (Thermo Scientific, Waltham, MA USA); mitochondrial DNA amplification with COI primers: lane 5—CST cells, lane 6—C. striatus muscle, lane 7—negative control.
Cell transfection.
CST cells were successfully transfected with phrGFP II-N vector. The expression of GFP in the form of clear and strong green fluorescence signals was observed 48 h after transfection (Fig. 6).
Morphological confirmation by immunocytochemistry.
The CST cells exhibited a strong reactivity for cytokeratin, an epithelial cell marker (Fig. 7). No reactivity was observed in CST cells incubated with antivimentin antibodies or in control coverslips in which PBS with 1% BSA was used in place of primary antibodies.
Cytotoxicity studies.
The ECPs from V. cholerae MTCC 3904 proved to be cytotoxic to the CST cell line. The cytotoxic effects could be observed even with a dilution of 1:16, within 12 h of inoculation of ECPs. The morphological alterations in CST cells included rounding, detachment, and destruction of the monolayer by 48 h (Fig. 8). In addition, neutral red uptake assay was used to measure the integrity of the plasma membrane of CST cells in response to a toxicant, viz. mercuric chloride. The cells were incubated with growth medium containing increasing concentration of mercuric chloride and subsequently examined for its effect on neutral red uptake. A decrease in the uptake of neutral red was evident with increasing concentration of mercuric chloride (Fig. 9).
Discussion
In the present study, a cell line has been established from the thymus of an air-breathing fish, C. striatus, using the explant method. The cell line, designated CST cell line, has been subcultured over 71 passages and the cells exhibit epithelial morphology. Till date, there are only a few reports on the establishment of cell lines from the thymus of fishes (Katakura et al. 2009; Chaudhary et al. 2013, 2014; Rebello et al. 2014). In teleosts, the thymus is a paired lymphoid organ and primary site for the development and maturation of T lymphocytes. Thymic parenchyma consists of leukocytic cells called thymocytes, and stromal cells (Le Douari and Jotereau 1975). The stromal cells are composed of thymic epithelial cells, macrophages, interdigitating/dendritic cells, myoid cells, and also other cell types in fewer numbers (Zapata et al. 1996) and provide signals to support the diverse processes of thymocyte development that are essential for the supply of circulating T cells (Williams et al. 1986). The thymic cell lines of epithelial origin can be critical to evaluate the role of these cells in the induction and/or regulation of thymocyte differentiation and maturation (Chaudhary et al. 2013).
Previously, a number of cell lines have been established from C. striatus, viz. SSN-1 from whole fry tissue (Frerichs et al. 1991): SHMS, SHHT, and SHSB from muscle, heart, and swim bladder, respectively (Zhao et al. 2004); C. striatus kidney (CSK) from kidney (Abdul Majeed et al. 2013); and C. striatus gill (CSG) from gill tissue (Abdul Majeed et al. 2014). The cells from all these cell lines have fibroblast morphology except CSG which has epithelioid morphology.
The optimum temperature for growth of CST cells was determined by incubating the flasks at different temperatures. The cells show maximum growth at 28°C. Most of the cell lines derived from freshwater fishes from tropical countries have been reported to grow optimally at 25–30°C (Cheng et al. 2010; Ku et al. 2010; Chaudhary et al. 2014, 2013; Ma et al. 2013; Swaminathan et al. 2014). Similarly, maximum growth of CSK cells was observed in L-15 medium supplemented with 20% FBS. However, the cells also showed good growth in the medium supplemented with 10% FBS which can be used for routine growth and maintenance of this cell line. These results are in conformity with earlier reports on different fish cell lines (Ku et al. 2009; Cheng et al. 2010; Ma et al. 2013 Swaminathan et al. 2014). In the present study, the CST cells showed very good viability (>90%) even after 8 mo of storage in liquid nitrogen and formed a monolayer within 5 d of revival. Furthermore, the cells did not show any alterations in morphology following freezing and thawing. These results are in accordance with most of earlier studies (Ku et al. 2010; Abdul Majeed et al. 2013, 2014; Swaminathan et al. 2014).
Plating efficiency or clonogenic assay is a measure of colonies originating from single cells and is a phenotypic marker of transformed cells. This assay essentially tests every cell in the population for its ability to undergo unlimited division. It is a very sensitive test, often used for determining the nutritional requirements of cells, testing serum lots, measuring the effects of growth factors, and testing toxicity. This is also the method of choice to determine cell reproductive death after treatment with ionizing radiation (Franken et al. 2006). Plating efficiency was low but improved with increase in seeding density. In conformity with our results, low plating efficiency has been reported previously for the cell line from red sea bream (Ku et al. 2010) and rockfish grouper (Ku et al. 2009). On the contrary, very high plating efficiency has been observed for cell lines derived from the kidney and gills of C. striatus (Abdul Majeed et al. 2013, 2014).
The main purposes of transfection are to study the function of genes or gene products, by enhancing or inhibiting specific gene expression in cells, and to produce recombinant proteins in the cells (Wurm 2004). The high transfection efficiency of CST cells indicated that conventional transfection procedures and heterologous CMV promoter adapted to mammalian cells can be applied to CMT cells. Therefore, it can be inferred that this cell line can be used as an in vitro model for exogenous gene manipulation. Similar results have been reported earlier for many fish cell lines (Qin et al. 2006; Ku et al. 2009, 2010; Cheng et al. 2010; Wang et al. 2010).
The modal chromosome number in the CST cell line was reported to be 40 which is identical to the chromosome number reported for C. striatus (Kumar et al. 2013) as well as cell lines derived from C. striatus (Abdul Majeed et al. 2013, 2014). On the contrary, Zhao et al. (2004) reported that all the three cell lines derived from different tissues of C. striatus had a chromosome count of 44. Furthermore, Courtenay and Williams (2004) observed that chromosome counts of C. striatus had been reported to be 40 as well as 44, indicating that C. striatus represents a complex species. Mitochondrial DNA typing has been recommended for identifying the species of origin of established cell lines at molecular level (Cooper et al. 2007; O’Donoghue et al. 2011). A number of mitochondrial genes, viz. 12S rRNA, 16S rRNA, 18S rRNA, and cytochrome oxidase I gene, have been used for authenticating the origin of fish cell lines (Ding et al. 2006; Rougée et al. 2007; Ishaq Ahmed et al. 2009; Cheng et al. 2010; Chaudhary et al. 2014). In this study, we analyzed and compared partial mitochondrial 16S and COI gene sequences from CST cells with genes of C. striatus. A BLAST search indicated 100% sequence identity among the 16S and COI genes from CST cells and C. striatus indicating that this cell line was indeed derived from C. striatus.
The dominant cell types in fish cell lines have been reported to be fibroblast-like cells or epithelial-like cells (Lakra et al. 2011). The presumed origin of cells is mostly based on cell morphology (Bols et al. 2005); however, the relevance of cell morphology criterion to characterize the cultured cell population is questionable as cultured cell morphology changes quickly (Mauger et al. 2009). Therefore, immunochemical markers of a cytoskeleton have been developed in mammals to distinguish the two cell types and these antibodies of fibroblastic or epithelial markers have also been applied to confirm the lineage of fish cell lines (Himizu et al. 2003; Butler and Nowak 2004; Ishaq Ahmed et al. 2009; Chaudhary et al. 2014). In the present study, CST cells showed strong reactivity to anticytokeratin antibodies indicating that the cell line is epithelial in origin.
Many cell lines have proven suitable for demonstration of cytotoxic effects exerted by extracellular products of fish pathogenic bacteria (Ku et al. 2009, 2010). The ECPs from V. cholerae were cytotoxic to CST cells and the morphological changes in subconfluent monolayers were similar to those described previously (Bejar et al. 1997; Ishaq Ahmed et al. 2009; Swaminathan et al. 2010; Chaudhary et al. 2014).
Neutral red (NR) uptake assay is a viability assay, based on the ability of viable cells to incorporate and bind supravital dye NR which concentrates in the lysosomes. The dye is then extracted from viable cells using an acidified ethanol solution and absorbance of solubilized dye is quantified using a spectrophotometer (Repetto et al. 2008). Any alterations of the cell surface or lysosomal membrane produced by toxic substances cause decreased uptake and binding of NR, making it possible to distinguish between viable, damaged, or dead cells via spectrophotometric measurements. It is one of the most used cytotoxicity tests (Brandão et al. 1992; Repetto and Sanz 1993) and believed to be a highly sensitive test in detecting cytotoxic events (Fotakis and Timbrell 2006). In the present experiment, lysosomal integrity of CST cells appeared to have been affected following incubation with L-15 medium containing varying concentrations of mercuric chloride, and it was evidenced by the concentration-dependent decrease in uptake of NR by CST cells. Hence, the CST cell line can be used for in vitro screening of chemicals for cytotoxicity and successfully employed as a biological alternative to the use of whole fish in toxicity screening, in accordance with earlier studies (Ní Shúilleabháin et al. 2004; Davoren et al. 2005; Fotakis and Timbrell 2006). There are a number of reports on evaluating cytotoxic effects of mercury salts using cell lines (Devlin and Clary 1998; Issa et al. 2003).
The results suggest that the CST cell line appears as a suitable system for expression of recombinant vertebrate proteins as well as an in vitro monitoring system for short-term cytotoxicity assays. The cell line can also be a useful tool to study and identify new biomarkers and can provide experimental insights into their basis.
References
Abdul Majeed S, Nambi KSN, Taju G, Sahul Hameed AS (2013) Development, characterization and application of a new fibroblastic-like cell line from kidney of a freshwater air breathing fish Channa striatus (Bloch, 1793). Acta Trop 127(1):25–32
Abdul Majeed S, Nambi KSN, Taju G, Sarath Babu V, Farook MA, Sahul Hameed AS (2014) Development and characterization of a new gill cell line from air breathing fish Channa striatus (Bloch 1793) and its application in toxicology: direct comparison to the acute fish toxicity. Chemosphere 96:89–98
Ariel E, Skall HF, Olesen NJ (2009) Susceptibility testing of fish cell lines for virus isolation. Aquaculture 298:125–130
Balebona MC, Andreu MJ, Bordas MA, Zorrilla I, Moriñigo MA, Borrego JJ (1998) Pathogenicity of Vibrio alginolyticus from cultured gilt-head sea bream (Sparus aurata L.). Appl Environ Microbiol 64:4269–4275
Bejar J, Borrego JJ, Alvarez MC (1997) A continuous cell line from the cultured marine fish gilt-head sea bream (Sparus aurata). Aquaculture 150:143–153
Bols NC, Barlian AM, Chirino-Trejo M, Caldwell SJ, Goegan P, Lee LEJ (1994) Development of a cell line from primary cultures of rainbow trout, Oncorhynchus mykiss (Walbaum), gills. J Fish Dis 17:601–611
Bols NC, Dayeh VR, Lee LEJ, Schirmer K (2005) Use of fish cell lines in the toxicology and ecotoxicology of fish. In: Moon TW, Mommsen TP (eds) Biochemistry and molecular biology of fishes—environmental toxicology, vol 6. Elsevier, Amsterdam, pp 43–84
Brandão JC, Bohets HHL, Van De Vyver IE, Dierickx PJ (1992) Correlation between the in vitro cytotoxicity to cultured fathead minnow fish cells and fish lethality data for 50 chemicals. Chemosphere 25:553–562
Buonocore F, Randelli E, Lorenzen N, Einer-Jensen K, Scapigliati G (2011) Analysis of the expression and modulation of selected immune-related gene transcripts in the DLEC cell line from European sea bass (Dicentrarchus labrax L.). J Aquac Res Development 2:1
Butler R, Nowak BF (2004) A dual enzyme method for the establishment of long and medium-term primary cultures of epithelial and fibroblastic cells from Atlantic salmon gills. J Fish Biol 65:1108–1125
Chaudhary DK, Sood N, Rathore G, Pradhan PK, Punia P, Agarwal NK, Jena JK (2014) Establishment and characterization of macrophage cell line from thymus of Catla catla (Hamilton, 1822). Aquacult Res 45:299–311
Chaudhary DK, Sood N, Swaminathan TR, Rathore G, Pradhan PK, Agarwal NK, Jena JK (2013) Establishment and characterization of an epithelial cell line from thymus of Catla catla (Hamilton, 1822). Gene 512:546–553
Cheng TC, Lai YS, Lin IY, Wu CP, Chang SL, Chen TI, Su MS (2010) Establishment, characterization, virus susceptibility and transfection of cell lines from cobia, Rachycentron canadum (L.), brain and fin. J Fish Dis 33:161–169
Cooper JK, Sykes G, King S, Cottrill K, Ivanova NV, Hanner R, Ikonomi P (2007) Species identification in cell culture: a two-pronged molecular approach. In Vitro Cell Dev Biol Anim 43(10):344–351
Courtenay WR Jr, Williams JD (2004) Snakeheads (Pisces, Channidae)—a biological synopsis and risk assessment. U.S. Geological Survey Circular; 1251 vi+143 pp
Davoren M, Ní Shúilleabháin S, Hartl MGJ, Sheehan D, O’Brien NM, O’Halloran J, Van Pelt FNAM, Mothersill C (2005) Assessing the potential of fish cell lines as tools for the cytotoxicity testing of estuarine sediment aqueous elutriates. Toxicol in Vitro 19:421–431
Devlin EW, Clary B (1998) ln vitro toxicity of methyl mercury to Fathead Minnow cells. Bull Environ Contam Toxicol 61:527–533
DeWitte-Orr SJ, Lepic K, Bryson SP, Walsh SK, Lee LEJ, Bols NC (2006) Development of a continuous cell line, PBLE, from an American eel peripheral blood leukocyte preparation. In Vitro Cell Dev Biol Anim 42:263–272
Ding S, Zhuang X, Guo F, Wang J, Su Y, Zhang Q, Li Q (2006) Molecular phylogenetic relationships of China Seas groupers based on cytochrome b gene fragment sequences. Sci China C Life Sci 49:235–242
Fent K (2001) Fish cell lines as versatile tools in ecotoxicology: assessment of cytotoxicity, cytochrome P4501A induction potential and estrogenic activity of chemicals and environmental samples. Toxicol in Vitro 15(4–5):477–488
Fotakis G, Timbrell JA (2006) In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 160(2):171–177
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1:2315–2319
Frerichs GN, Morgean D, Hart D, Sherrow C, Roberts RJ, Onions DE (1991) Spontaneously productive C-type retrovirus infection of fish cell line. J Gen Virol 72:2537–2539
Frerichs GN, Roger HD, Peric Z (1996) Cell culture isolation of piscine neuropathy nodavirus from juvenile sea bass, Dicentrarchus labrax. J Gen Virol 77:2067–2071
Freshney RI (2005) Culture of animal cells: a manual of basic technique. Wiley, New Jersey
Grunow B, Noglick S, Kruse C, Gebert M (2011) Isolation of cells from Atlantic sturgeon Acipenser oxyrinchus oxyrinchus and optimization of culture conditions. Aquat Biol 14:67–75
Han JE, Choresca CH, Koo OJ, Oh HJ, Hong SG, Kim JH, Shin SP, Jun JW, Lee BC, Park SC (2011) Establishment of glass catfish (Kryptopterus bicirrhis) fin-derived cells. Cell Biol Int Rep 18(1):e00008
Higaki S, Koyama Y, Shirai E, Yokota T, Fujioka Y, Sakai N, Takada T (2013) Establishment of testicular and ovarian cell lines from Honmoroko (Gnathopogon caerulescens). Fish Physiol Biochem 39(3):701–711
Himizu C, Hike H, Denise MM, Breisch E, Westerman M, Buchanan J, Ligman HR, Phillips RB, Carlberg JM, Olst JV, Burns JC (2003) Characterization of a white bass (Morone chrysops) embryonic cell line with epithelial features. In Vitro Cell Dev Biol Anim 39:29–35
Ishaq Ahmed VP, Chandra V, Sudhakaran R, Rajesh Kumar S, Sarathi M, Sarath Babu V, Ramesh B, Sahul Hameed AS (2009) Development and characterization of cell lines derived from rohu, Labeo rohita (Hamilton), and catla, Catla catla (Hamilton). J Fish Dis 32:211–218
Issa Y, Watts DC, Duxbury AJ, Brunton PA, Watson MB, Waters CM (2003) Mercuric chloride: toxicity and apoptosis in a human oligodendroglial cell line MO3.13. Biomaterials 24(6):981–987
Katakura F, Takizawa F, Yoshida M, Yamaguchi T, Araki K, Tomana M, Nakao M, Moritomo T, Nakanishi T (2009) Co-culture of carp (Cyprinus carpio) kidney haematopoietic cells with feeder cells resulting in long-term proliferation of T-cell lineages. Vet Immunol Immunopathol 131:127–136
Ku CC, Lu CH, Wang CS (2010) Establishment and characterization of a fibroblast cell line derived from the dorsal fin of red sea bream, Pagrus major (Temminck & Schlegel). J Fish Dis 33(3):187–196
Ku CC, Teng YC, Wang CS, Lu CH (2009) Establishment and characterization of three cell lines derived from the rockfish grouper Epinephelus quoyanus: use for transgenic studies and cytotoxicity testing. Aquaculture 294:147–151
Kumar R, Kushwaha B, Nagpure NS, Behera BK, Lakra WS (2013) Karyological and molecular diversity in three freshwater species of the genus Channa (Teleostei, Perciformes) from India. Caryologia 66(2):109–119
Lakra WS, Swaminathan TR, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37:1–20
Le Douari NM, Jotereau FV (1975) Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J Exp Med 142:17–40
Ma J, Sun S, Zeng L, Lu Y (2013) Establishment, characterization and viral susceptibility of two cell lines derived from leopard wrasse Macropharyngodon geoffroy. J Fish Biol 83(3):560–573
Mauger PE, Labbé C, Bobe J, Cauty C, Leguen I, Baffet G, Le Bail PY (2009) Characterization of goldfish fin cells in culture: some evidence of an epithelial cell profile. Comp Biochem Physiol B Biochem Mol Biol 152(3):205–215
NBFGR (2011) Proceedings of national consultation on species prioritization for ex situ conservation and freshwater aquaculture, 17–18 September 2011. NBFGR, Lucknow
Ní Shúilleabháin S, Mothersill C, Sheehan D, O’Brien NM, O’Halloran J, Van Pelt FN, Davoren M (2004) In vitro cytotoxicity testing of three zinc metal salts using established fish cell lines. Toxicol In Vitro 18(3):365–376
O’Donoghue LE, Rivest JP, Duval DL (2011) Polymerase chain reaction-based species verification and microsatellite analysis for canine cell line validation. J Vet Diagn Invest 23:780–785
Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G (1991) The simple fool’s guide to PCR. University of Hawaii, Honolulu
Qin QW, Wu TH, Jia TL, Hegde A, Zhang RQ (2006) Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus. J Virol Methods 131:58–64
Rebello SC, Rathore G, Punia P, Sood N, Elangovan V (2014) Development and characterization of a continuous macrophage cell line, LRTM, derived from thymus of Labeo rohita (Hamilton 1822). In Vitro Cell Dev Biol Anim 50(1):22–38
Repetto G, Peso AD, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3:1125–1131
Repetto G, Sanz P (1993) Neutral red uptake, cellular growth and lysosomal function: in vitro effects of 24 metals. Altern Lab Anim 21:501–507
Rougée L, Ostrander GK, Richmond RH, Lu Y (2007) Establishment, characterization, and viral susceptibility of two cell lines derived from goldfish Carassius auratus muscle and swim bladder. Dis Aquat Org 77:127–135
Schirmer K, Tanneberger K, Kramer NI, Völker D, Scholz S, Hafner C, Lee LEJ, Bols NCJ, Hermens LM (2008) Developing a list of reference chemicals for testing alternatives to whole fish toxicity tests. Aquat Toxicol 90:128–137
Smith CL (2006) Mammalian cell culture. Curr Protoc Mol Biol 28.0.1–28.0.2. doi:10.1002/0471142727.mb2800s73
Swaminathan TR, Basheer VS, Gopalakrishnan A, Sood N, Pradhan PK (2014) A new epithelial cell line, HBF from caudal fin of endangered yellow catfish, Horabagrus brachysoma (Gunther, 1864). Cytotechnology. doi:10.1007/s10616-014-9804-2
Swaminathan TR, Lakra WS, Gopalakrishnan A, Basheer VS, Kushwaha B, Sajeela K (2010) Development and characterization of a new epithelial cell line PSF from caudal fin of Green chromide, Etroplus suratensis (Bloch, 1790). In Vitro Cell Dev Biol Anim 46:647–656
Talwar PK, Jhingran AG (1992) Inland fishes of India and adjacent countries. A. A. Balkema, Rotterdam, Two volumes
Tan F, Wang M, Wang W, Lu Y (2008) Comparative evaluation of the cytotoxicity sensitivity of six fish cell lines for four heavy metals in vitro. Toxicol in Vitro 22:164–170
Thompson KD, Lilley JH, Chen SC, Adams A, Richards RH (1999) The immune response of rainbow trout (Oncorhynchus mykiss) against Aphanomyces invadans. Fish Shellfish Immunol 9:195–210
Wang N, Wang XL, Sha ZX, Tian YS, Chen SL (2010) Establishment, characterization and virus susceptibility of a kidney-derived cell line from southern flounder, Paralichthys lethostigma Jordan & Gilbert. J Fish Dis 34:81–85
Wang YC, Chaung RH, Tung LC (2004) Comparison of the cytotoxicity induced by different exposure to sodium arsenite in two fish cell lines. Aquat Toxicol 69:67–79
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia’s fish species. Philos Trans R Soc Lond B Biol Sci 360:1847–1857
Williams GT, Kingston R, Owen MJ, Jenkinson EJ, Owen JJ (1986) A single micromanipulated stem cell gives rise to multiple T-cell receptor gene rearrangements in the thymus in vitro. Nature 324:63–64
Wise JPSR, Winn RN, Renfro JL (2002) Generating new marine cell lines and transgenic species. J Exp Zool 15:292–295
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22:1393–1398
Zapata AG, Chiba A, Varas A (1996) Cells and tissues of the immune system of fish. In: Iwama G, Nakanishi T (eds) The fish immune system. Organism, pathogen and environment. Academic, San Diego, pp 1–62
Zhao Z, Montgomery-Brock D, Lee CS, Lu Y (2004) Establishment, characterization and viral susceptibility of 3 new cell lines from snakehead, Channa striatus (Blooch). Methods Cell Sci 25:155–166
Acknowledgments
The authors are thankful to Dr. S. Ayyappan, Secretary, DARE and Director-General, ICAR and Dr. B. Meenakumari, DDG (Fy) ICAR, New Delhi, India, for their support, encouragement, and guidance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Sood, N., Chaudhary, D.K., Pradhan, P.K. et al. Establishment and characterization of a continuous cell line from thymus of striped snakehead, Channa striatus (Bloch 1793). In Vitro Cell.Dev.Biol.-Animal 51, 787–796 (2015). https://doi.org/10.1007/s11626-015-9891-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9891-1