Abstract
High-fat diets may have favorable effects on growth and cost, but high-fat diets often induce excessive fat deposition, resulting in liver damage. This study aimed to identify the hepatoprotective of a Chinese herb (berberine) for blunt snout bream (Megalobrama amblycephala). Fish were fed with a normal diet (LFD, 5 % fat), high-fat diet (HFD, 15 % fat) or berberine-supplemented diets (BSD, 15 % fat with berberine 50 or 100 mg/kg level) for 8 weeks. After the feeding, histology, oxidative status and mitochondrial function of liver were assessed. The results showed that HFD caused fat accumulation, oxidative stress and apoptosis in hepatocytes of fish. Hepatocytes in HFD group appeared to be hypertrophied, with larger liver cells diameter than these of LFD group. Berberine-supplemented diets could attenuate oxidative stress and hepatocytes apoptosis. HFD induced the decreasing mitochondrial complexes activities and bulk density and surface area density. Berberine improved function of mitochondrial respiratory chain via increasing the complex activities. Moreover, the histological results showed that berberine has the potential to repair mitochondrial ultrastructural damage and elevate the density in cells. In conclusion, our study demonstrated that berberine has attenuated liver damage induced by the high fat mainly via the protection for mitochondria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary lipids play a prominent role in fish nutrition to provide energy, essential fatty acids (EFA) and phospholipids (Sargent et al. 1999; Watanabe 1982). Increasing dietary lipid levels could improve feed efficiency and growth (Li et al. 2012). Moreover, more lipid used for energy can also reduce nitrogenous waste production (Vergara et al. 1999). Therefore, fat-rich diets have been widely used with the development of intensive fish farming. However, dietary lipid toward an upper limit often leads unwanted fat deposition in the liver (or other tissues), referred as fatty liver (Du et al. 2006; Lu et al. 2013a). The health of fish is often affected by fatty liver, which often closely positively correlates with mortality and immune suppression (Bolla et al. 2011; Lu et al. 2014a; Roberts 2012). According to our previous study, fatty liver of fish has an important characteristic of lipid metabolism disorder (Lu et al. 2013b, 2014b; Zhang et al. 2014). In order to prevent fatty liver, thousands of ways have been tried by the fish nutritionist. Some Chinese herbs have attracted great interest as reverting hepatic lipid dysfunction and few side effects (Chen et al. 2006; Mei and Huang 2006; Zhou et al. 2015). One of projects in our laboratory is to select Chinese herb and supplement into diets to improve lipid metabolism of fish. Berberine, an alkaloid isolated from huanglian (Coptis chinensis), has been extensively used in traditional Chinese medicine (Zhang et al. 2008). Many in vitro and in vivo studies showed that berberine has potentially beneficial effects in the treatment of fatty liver and obesity in human (Kong et al. 2004; Lee et al. 2006). However, the effect of berberine on fish lipid metabolism is still unknown and maybe it also can revert the metabolic syndrome in fish.
Blunt snout bream (Megalobrama amblycephala) is an herbivorous freshwater fish native to China. Due to its fast growth, tender flesh, and high disease resistance, this species has been widely favored for aquaculture in China. However, compared to a number of other commercially produced fishes, its artificial rearing is often associated with fatty liver that correlates closely with a high rate of mortality or poor growth. Based on our previous studies, fatty liver impaired mitochondrial functions, and subsequently mitochondrial dysfunction mediated oxidative stress and hepatocyte apoptosis (Lu et al. 2014a). Some previous studies showed that berberine can revert hepatic mitochondrial dysfunction in high-fat-fed rats (Teodoro et al. 2013). The principle goal of this study was therefore to investigate the effect of berberine on lipid metabolism, mitochondrial function and hepatocyte apoptosis. The result may have implications for a theoretical basis for research and development of hepatoprotective herbal treatments in fish farming.
Materials and methods
Experimental fish and feeding trial
Juvenile blunt snout bream were obtained from the fish hatchery of Wuhan (Hubei, China). The experiment was performed in a recirculating aquaculture system in laboratory. Prior to the experiment, fish were reared in several 250-l tanks (60 juveniles per tank) for 2 weeks to acclimate to the experiment conditions. After the acclimation period, 240 fish of similar size (average weight of 8.15 ± 0.10 g) were randomly distributed into twelve 100-l tanks at the rate of 20 juveniles per tank. Water temperature, dissolved oxygen (DO), and pH were monitored daily. During the feeding period, fish were reared under the following conditions: water temperature, 25–27 °C; DO, 5.0–6.0 mg/l; pH 7.2–7.6; photoperiod, 12:12 h (dark: light). Fish were hand-fed to apparent satiation three times daily (08:00, 12:00, and 16:00 h) using four experimental diets. The experimental diets are 5 % fat diet (LFD), 15 % fat diet (HFD), 15 % fat with 50 or 100 mg/kg berberine diets [BSD (50/100)]. Each treatment was tested in triplicate, and the trial lasted 8 weeks. Formulation and proximate composition of the experimental diets are presented in Table 1. All experimental diets were prepared in the laboratory. Ingredients were carefully weighed, thoroughly mixed and blended with oil (soybean oil and fish oil) and then added an appropriate amount of water to form stiff dough. The dough was then pelleted using a pillet mill with a 2-mm-diameter die and then air-dried at room temperature. After drying, the diets were stored in sealed plastic bags at −20 °C until used.
Sample collection
At the end of the feeding trial, fish were starved overnight prior to sampling. Then, ten fish per tank were sampled and immediately euthanized by 100 mg/l MS-222 (tricaine methanesulfonate; Sigma, USA). Blood was rapidly taken from caudal vessel into heparinized Eppendorf tubes, centrifuged (850×g, 10 min, 4 °C), and the plasma was stored at −70 °C until analysis. Liver was removed (placed on ice) and then stored at −70 °C until analysis. Additionally, the liver samples for the histology observations were fixed in the relevant buffer.
Measures of plasma biochemical parameters
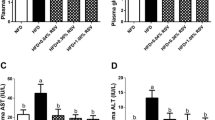
Concentrations of triglycerides and cholesterol in plasma were determined by colorimetric enzymatic methods using commercial kits (Beijing BHKT Clinical Reagent Co., Ltd, China). Activities of plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by enzymatic colorimetric methods according to the method of Reitman and Frankel (1957).
Ultrastructure study
Samples for electron microscopy observation were fixed in 2.5 % glutaraldehyde for 24 h, post-fixed in 1 % osmium tetroxide (OsO4) for 1 h and stored at 4 °C. Sections were embedded in epoxy resin Epon812, cut into70-nm-thick sections with a RMC PowerTome XL microtome, stained with uranyl acetate and lead citrate, and examined under a Hitachi H-7650 (Hitachi, Tokyo, Japan) transmission electron microscope. Morphometry of hepatocyte was examined by Image-Pro Plus 6.0. The morphometric analysis of the mitochondrion was done according to the methods described by the method of Weibel et al. (1969).
Apoptosis detection
For hepatocyte apoptosis determination, the 5-µm-thick sections were further treated using the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay following the protocol of Apoptosis Detection Kit (Nanjing JianCheng Bioengineering Institute, China). Briefly, the sections were deparaffinized, hydrated and incubated with freshly prepared 3 % hydrogen peroxide (H2O2) in PBS for 10 min to block the endogenous peroxidase activity. Each slide was permeated with 10 µg/mL proteinase K solution at 37 °C for 10 min. The tissues were immersed in 20 µl terminal deoxynucleotidyl transferase (TdT) which was marked with biotin on dUTP and incubated at 37 °C for 2 h. The slide was covered with horseradish peroxidase-labeled streptavidin (HRP-streptavidin) at 37 °C for 30 min and then stained with 3,3′-Diaminobenziine (DAB) for 15 min. Finally, the nucleus was counterstained with hematoxylin for 30 s. Brown–yellow granules were found in the nucleus of positive cell. The DNase1-treated tissue was used as the positive control. The reaction without TdT enzyme was used as the negative control. For the quantitative measurement of the number of hepatocytes using 200× microscopic magnifications, 100 cells were counted in different areas (five fields in each histological slide) and the percentage of cells with DNA damage was calculated.
Assays for antioxidant status in liver
For the determination of antioxidant status, liver samples were prepared as described by Lu et al. (2014a). Total superoxide dismutase (SOD) activity was measured with the commercial kit (Nanjing JianCheng Bioengineering Institute, China), according to Nanton et al. (2001). The method of Maral et al. (1977) for catalase (CAT) assay was adapted as described by Rueda-Jasso et al. (2004). Glutathione peroxidase (GPX) activity was measured using the method described by Dabas et al. (2012). Thiobarbituric acid reactive substances (TBARS) were performed as described by Rueda-Jasso et al. (2004), using a malondialdehyde (MDA) kit (Nanjing JianCheng Bioengineering Institute, China). The reduced glutathione (GSH) were determined enzimatically with a commercial kit (Nanjing JianCheng Bioengineering Institute, China) based on the recycling reaction of reduced glutathione (GSH) with DNTB (5,5′-dithios-2-205 nitrobenzoic acid) in the presence of an excess of glutathione reductase. Measurements were taken in a microplate reader. Protein concentration in liver homogenates was determined using Lowry et al.’s method (1951).
Measurement of mitochondrial respiratory chain complexes activities
The activities of respiratory chain complexes were determined according to the methods of Zhang et al. (2004), using the commercial kits (Nanjing JianCheng Bioengineering Institute, China).
Total RNA extraction, reverse transcription and real-time PCR
Total RNA was extracted from the liver tissue using RNAiso Plus (Takara Co. Ltd, Japan). RNA samples were treated by RQ1 RNase-Free DNase prior to RT-PCR (Takara Co. Ltd, Japan) to avoid genomic DNA amplification. cDNA was generated from 500 ng DNase-treated RNA using ExScriptTM RT-PCR kit (Takara Co. Ltd, Japan), and the mixture consisted of 500 ng RNA, 2 µl buffer (5×), 0.5 µl dNTP mixture (10 mM each), 0.25 µl RNase inhibitor (40 U/µl), 0.5 µl dT-AP primer (50 mM), 0.25 µl ExScriptTM RTase (200 U/µl) and DEPC H2O, with total volume up to 10 µl. The reaction conditions were as follows: 42 °C for 40 min, 90 °C for 2 min and 4 °C thereafter.
Real-time PCR was employed to determine mRNA levels based on the SYBR Green I fluorescence kit. Specific primers were designed using Primer 5.0 version. Primer characteristics used for real-time PCR are listed at the Supplementary Material. Real-time PCR was performed in a Mini Option real-time detector (BIO-RAD, USA). The fluorescent quantitative PCR reaction solution consisted of 12.5 µl SYBR® premix Ex TaqTM (2×), 0.5 µl PCR forward primer (10 µM), 0.5 µl PCR reverse primer (10 µM), 2.0 µl RT reaction (cDNA solution) and 9.5 µl dH2O. The reaction conditions were as follows: 95 °C for 3 min followed by 45 cycles consisting of 95 °C for 10 s and 60 °C for 20 s. The fluorescent flux was then recorded, and the reaction continued at 72 °C for 3 min. The dissolution rate was measured between 65 and 90 °C. Each increase of 0.2 °C was maintained for 1 s, and the fluorescent flux was recorded. All amplicons were initially separated by agarose gel electrophoresis to ensure that they were of correct size. A dissociation curve was determined during the PCR program to make sure that specific products were obtained in each run. The gene expression levels were normalized toward the mean of the reference gene (β-actin). Normalized gene expressions of the low-fat group were set to 1, and the expression of each target gene for the others groups were expressed relative to low-fat group (as 2−△△Ct method).
Statistical analysis
Data were analyzed by one-way ANOVA using the SPSS 16.0 for Windows. Duncan’s test was used for the multiple comparisons. The level of significance was set at P < 0.05. All data were presented as mean ± S.E. (standard error of the mean).
Results
Ultrastructure of hepatocytes
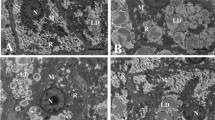
Liver of fish fed LFD showed normal ultrastructure (Fig. 1a). In these fish, the nucleus was round and the nucleolus was visible. Hepatocyte displayed dark and slender mitochondria. All individuals fed LFD presented few and small vacuoles giving a uniform degree of hepatic steatosis score (+). However, fish fed HFD showed a severe degree of vacuolization (+++). Moreover, hepatocytes in HFD group appeared to be hypertrophied, with larger liver cells diameter than these of LFD group (Table 2). In addition, the abnormal ultrastructure was found in livers of fish fed high-fat diet, such as mitochondria swell and nucleus atrophy. HFD-induced lipid vacuoles can be significantly reduced in BSD-fed fish, as shown by the decrease in the steatosis score.
Transmission electron microscope images of blunt snout bream hepatocyte and mitochondrion ultrastructure: N (Nucleus), L (lipid droplet), M (mitochondrion). Photomicrographs and main findings: a hepatocytes of fish fed low-fat diet with normal structure; b hepatocytes presenting extensive intracellular lipid droplets of fish fed high-fat diet; c hepatocytes of fish fed high-fat with berberine die; d hepatocytes of fish fed low-fat diet displaying dark and slender mitochondria; e mitochondria showing highly hydropic changes (→) of fish fed high-fat diet. f Mitochondria of fish fed high-fat with berberine diet
Blood biochemistry
Blood biochemistry of blunt snout bream is shown in Table 3. AST and ALT activities and triglycerides concentrations were significantly (P < 0.05) affected by diet treatments. Higher triglycerides concentration was observed in fish given HFD than those of fish fed other diets. AST and ALT activities of fish fed high-fat diet were significantly (P < 0.05) higher than those of other groups. From the results, we found that the berberine-supplemented diet can lower the triglycerides concentrations and AST and ALT activities than those of the HFD group.
Hepatic oxidative status
Hepatic oxidative status parameters are shown in Table 4. As can be seen, MDA content and SOD and GPX activities were significantly (P < 0.05) higher in fish fed with HFD than those of fish fed by LFD. However, the alteration of GSH level is opposite. The berberine-supplemented diets could decrease MDA level and SOD and GPX activities in liver and elevate the content of hepatic GSH, compared to HFD group.
Hepatocyte apoptosis
TUNEL-stained hepatocytes showed the characteristic features of DNA damage. Normal hepatocytes were stained amethyst by hematoxylin; however, apoptotic cells were labeled brown (Fig. 2). In the LFD group, the apoptotic cells of fish were just 2 % of total hepatocytes. However, fish fed HFD has about 9 % apoptotic cells of total hepatocytes. Thus, TUNEL positive cells of fish fed HFD were significantly more (P < 0.05) than those of control fish. Moreover, the distribution of lots of apoptotic cell of fish fed the high-fat diet was observed around the central vein. The steatotic hepatocyte seems more likely to be apoptotic in the high-fat group. The TUNEL positive cells of fish fed the berberine-supplemented diets were significantly less than (P < 0.05) those of fish with high-fat diet.
Hepatocyte apoptosis of blunt snout bream. a low-fat diet group (×200), normal cells, amethyst; b high-fat diet group (×200), normal cells, amethyst (blue arrow), apoptotic cells, brown (yellow arrow); c high-fat+berberine (50) diet group (×200), normal cells, amethyst (blue arrow), apoptotic cells, brown (yellow arrow); d high-fat+berberine (100) diet group (×200), normal cells, amethyst (blue arrow), apoptotic cells, brown (yellow arrow)
Mitochondria status
Activities of mitochondrial complexes in the liver of blunt snout bream are presented in Table 5. Activities of complex I, II and III were significantly (P < 0.05) affected by diet treatments. LFD-fed fish showed significantly higher activities of complex I and II than the HFD-fed fish. BSD significantly elevated the HFD-induced decreasing of mitochondrial complexes activities. In addition, complex III activities in fish fed with berberine-supplemented diet were significantly higher than those of LFD and HFD groups. However, there was no significant difference of activities of complex IV and V among all the treatments.
Morphometric analysis of mitochondria is presented in Fig. 3. The result showed that fish fed with HFD significantly (P < 0.05) lowered the bulk density and surface area density of mitochondria. Moreover, BSD could improve the bulk density, compared to that of HFD group.
Morphometric analysis of mitochondria of blunt snout bream. a Bulk density of mitochondria of fish fed different diets; b surface area density of mitochondria of fish fed different diets. Mean values and standard error (±SE) are presented for each parameter. Significant differences within the diets are indicated by different letters
Gene expression
The expression of genes involved in apoptosis and mitochondrial complexes is shown in Fig. 4. HFD-fed fish were characterized by drastic increases in the hepatic mRNA expression of Bax and Caspase 3. By contrast, berberine supplementation significantly decreased the mRNA levels for both Bax and Caspase 3. Moreover, the expressions of Sirt 3, ND1 and Cyt B in fish fed high-fat diet were significantly lower than did in LFD-fed fish. Berberine significantly reversed the HFD-induced down-regulation of these expressions.
Relative mRNA expressions of genes. Mean values and standard error (±SE) are present for each parameter. The values of the expression of the target genes are presented as relative to value of low-fat group (set to 1). Data were normalized by β-actin. Significant differences within the diets are indicated by different letters
Discussion
The histological examination is considered as the golden standard of liver damage. In the present study, profound alterations in the ultrastructure of liver were noted after the intake of a high-fat diet. The excessive lipid droplets even caused nuclear polarization. In present study, the berberine-supplemented diets could attenuate these alterations, especially in decreasing the steatosis scores. The blood biochemistry also supports this conclusion as lower TG level and AST and ALT activities appeared after feeding the berberine-supplemented diets. In previous in vivo and in vitro studies, lipid-lowering effect of berberine in human subjects, rats and other mammals have been proved (Hu and Davies 2009, 2010; Hu et al. 2012b). In hamster, treatment of hyperlipidemic hamsters with berberine reduced serum cholesterol by 40 %, and the mechanism is that it elevated LDLR expression through a post-transcriptional way stabilizing the mRNA (Kong et al. 2004). In high-fat-fed mice, berberine exerts anti-hyperlipidemic, and anti-obesity effects likely mediated by inhibiting gut microbes via decreasing degradation of dietary polysaccharide (Xie et al. 2011). Now, we did not know how berberine lowered the lipid contents in liver of high-fat-fed fish. In the future study, we will focus on the mechanism of berberine lipid-lowering effect in fish.
Numerous studies have indicated that accumulation of fat in fish tissues can increase the rate of lipid oxidation and affect the health of fish (Chaiyapechara et al. 2003; Rueda-Jasso et al. 2004). The fish body has several physiological ways to eliminate oxidative stress, including counterbalance such as enzymes (SOD, CAT and GPX) and functionalized molecules (GSH). Oxidative stress occurs when the generation of ROS exceeds the antioxidant defense system’s capacity to neutralize and eliminate them. After intake of high-fat diet, ROS is often over produced due to the elevation of oxidative phosphorylation in mitochondria. In the high-fat group, the elevated SOD activity due to overproduction of ROS resulted in increased H2O2 levels and thus a rise in GPX activity happened. MDA is an indicator commonly used to evaluate lipid peroxidation (Parvez and Raisuddin 2005). In the present study, the elevated MDA level of fish fed high-fat diet indicated an imbalance between the generation and removal of ROS. GSH also plays an important role in detoxifying ROS. A lower GSH level in liver of HFD-fed fish is also indicative of ROS-induced tissue damage. Our result showed that berberine-supplemented diets decreased oxidative damage in liver as the attenuating the production of MDA. Berberine has a high free radical scavenging effect to quenches superoxide anions and nitric oxide (Shan et al. 2011; Siow et al. 2011). Moreover, berberine inhibits ROS production in cell-based systems (Hur et al. 2009). Numerous reports showed that berberine also could induce antioxidant defenses by increasing the levels of non-enzymatic antioxidants (Du et al. 2003). We think that the above effects of berberine played the important roles in attenuating the oxidative stress in blunt snout bream.
Due to mitochondrial oxidative phosphorylation, mitochondrion is the prime site of ROS generation and endogenous oxidative stress (Qian et al. 2005). Thus, an imbalance of the cell’s redox environment will most likely originate in and affect the cell’s mitochondria. In fact, blunt snout bream fed with high-fat diets has a higher number of damaged mitochondria with lost cristae, matrix and metrical density with highly hydropic changes. Concomitantly, this leads to a vicious cycle due to the fact that excessive ROS generation leads to mitochondrial dysfunction, decreasing the electronic transport capacity and thus increasing ROS generation. Most ROS was produced by complex I, occurring primarily on the matrix side of the inner mitochondrial membrane. ROS can deactivate the iron-sulfur centers of complexes I, II and III, causing rapid loss of enzyme activity. Therefore, in the present study, the decreased activities of complexes I and II in fish fed with high-fat diet might indicate the over production of ROS. These decreased activities would induce the impaired energy metabolism of mitochondria. In addition, the expressions of ND 1 and CYT B were down-regulated in the high-fat group. According to the literature, the reduced expression of the ND 1 subunit of complex I suggests that the assembly of the whole complex I is impaired (Eya et al. 2011). Sirtuin 3 (SirT3) is localized within the matrix of mitochondrion, where it controls acetylation levels of enzymes involved in energy metabolism processes, including the respiratory chain (Teodoro et al. 2013). The loss of SirT3 could cause hyperacetylation of mitochondrial proteins, such as complex I and II with concomitant decrease in their activity (Ahn et al. 2008). This study showed that high-fat diet reduced SirT3 expression, which may reduce efficiency of mitochondrial oxidative phosphorylation and then induce the over production of ROS. Therefore, in the present study, berberine appears to improve the activities of complex I and II via the increase in SirT3 expression. Moreover, morphometric analysis showed that berberine could also improve the mitochondria density in hepatocytes.
Moreover, hepatocytes apoptosis is often associated with fatty liver, and the assessment of apoptosis is considered as a novel biomarker of disease severity of fatty liver in human (Wieckowska et al. 2006). In the present study, TUNEL positive cells of fish fed the high-fat diet were significantly more than those of control fish. Moreover, the steatotic hepatocyte seems more likely to be apoptotic. The apoptotic pathway is composed of the extrinsic pathway stimulated by death receptors and the intrinsic pathway initiated by cellular stress (Wieckowska et al. 2006). Mitochondrial dysfunction could release pro-apoptotic proteins into cytosol and then form an activation complex with apoptotic-protein activation factor and caspase 9. This can activate the downstream effector caspase 3 which execute the final apoptotic changes. So, the caspase 3 expression in liver of high-fat group is highly increased. According to the result, TUNEL positive cells of fish fed the berberine-supplemented diets were significantly less than those of fish with high-fat diet. It seems that berberine could inhibit hepatocytes apoptosis induced by over fat intake. Some previous studies have confirmed that berberine has the anti-apoptotic effect on thymocyte and primary neurons (Hu et al. 2012a; Miura et al. 1997). The anti-apoptotic effects of berberine were mediated by increasing phosphor-activation of Akt, leading to the decreased cleavage of the caspase 3 (Hu et al. 2012a). Bax, a member of the Bcl-2 protein family, can accelerate apoptosis by caspase activation and cytochrome C release from mitochondria (Finucane et al. 1999; Wolter et al. 1997). In this study, the decreased Bax expression in fish fed the berberine-supplemented diets might also imply the anti-apoptotic effect of berberine. In addition, we think that the anti-apoptotic effect of berberine also based on the protection to mitochondrial function and integrality as the above mentioned.
In summary, our results showed that berberine-supplemented diets could attenuate oxidative stress and hepatocytes apoptosis and improve mitochondrial function. Moreover, berberine has the potential to elevate the density in cells. In conclusion, berberine has the potential to attenuate the liver damage induced by the high-fat intake mainly via the protection for mitochondrial function.
References
Ahn BH et al (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci 105:14447–14452
Bolla S, Nicolaisen O, Amin A (2011) Liver alterations induced by long term feeding on commercial diets in Atlantic halibut (Hippoglossus hippoglossus L.) females. Histological and biochemical aspects. Aquaculture 312:117–125
Chaiyapechara S, Casten MT, Hardy RW, Dong FM (2003) Fish performance, fillet characteristics, and health assessment index of rainbow trout (Oncorhynchus mykiss) fed diets containing adequate and high concentrations of lipid and vitamin E. Aquaculture 219:715–738
Chen P, Chen J, Guo Z (2006) Preliminary research on the effect of Si–Ni-San on carp’s liver protection and recovery. Feed Ind 27:28–31
Dabas A, Nagpure N, Kumar R, Kushwaha B, Kumar P, Lakra W (2012) Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel, Channa punctatus. Fish Physiol Biochem 38:469–482
Du L, Kang Y, Park MK et al (2003) Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II. TNF-alpha. iNOS and IL-12 production in LPS-stimulated macrophages. Life Sci 73:1401–1412
Du ZY, Clouet P, Zheng WH, Degrace P, Tian LX, Liu YJ (2006) Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp (Ctenopharyngodon idella) fed high-fat diets. Brit J Nutr 95:905–915
Eya JC, Ashame MF, Pomeroy CF (2011) Association of mitochondrial function with feed efficiency in rainbow trout: diets and family effects. Aquaculture 321:71–84
Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR (1999) Bax-induced caspase activation and apoptosis via cytochromec release from mitochondria is inhibitable by Bcl-xL. J Biol Chem 274:2225–2233
Hu Y, Davies G (2009) Berberine increases expression of GATA-2 and GATA-3 during inhibition of adipocyte differentiation. Phytomedicine 16:864–873
Hu Y, Davies GE (2010) Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia 81:358–366
Hu J et al (2012a) PI3 K p55γ promoter activity enhancement is involved in the anti-apoptotic effect of berberine against cerebral ischemia-reperfusion. Eur J Pharmacol 674:132–142. doi:10.1016/j.ejphar.2011.11.014
Hu Y et al (2012b) Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine 19:861–867
Hur JM, Hyun MS, Lim SY, Lee WY, Kim D (2009) The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J Cell Biochem 107:955–964
Kong W et al (2004) Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 10:1344–1351
Lee YS et al (2006) Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55:2256–2264
Li X, Jiang Y, Liu W, Ge X (2012) Protein-sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingerlings: effects on digestive and metabolic responses. Fish Physiol Biochem 38:529–541
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu KL, Xu WN, Li JY, Li XF, Huang GQ, Liu WB (2013a) Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Sci 79:661–671
Lu KL, Xu WN, Li XF, Liu WB, Wang LN, Zhang CN (2013b) Hepatic triacylglycerol secretion, lipid transport and tissue lipid uptake in blunt snout bream (Megalobrama amblycephala) fed high-fat diet. Aquaculture 408:160–168
Lu KL, Xu WN, Liu WB, Wang LN, Zhang CN, Li XF (2014a) Association of mitochondrial dysfunction with oxidative stress and immune suppression in Blunt Snout Bream Megalobrama amblycephala fed a high-fat diet. J Aquat Anim Health 26:100–112
Lu KL, Xu WN, Wang LN, Zhang DD, Zhang CN, Liu WB (2014b) Hepatic β-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in Blunt Snout Bream Megalobrama amblycephala fed a high fat diet. PLoS ONE 9:e93135
Maral J, Puget K, Michelson A (1977) Comparative study of superoxide dismutase, catalase and glutathione peroxidase levels in erythrocytes of different animals. Biochem Biophys Res Commun 77:1525–1535
Mei J, Huang Y (2006) Studies on enzymatic mechanism of the liver-protective effect of Baogan Jiedu decoction on experimental hepatosis in Anguilla anguilla. Acta Vet Zootech Sin 37:1353
Miura N, Yamamoto M, Ueki T, Kitani T, Fukcuda K, Komatsu Y (1997) Inhibition of thymocyte apoptosis by berberine. Biochem Pharmacol 53:1315–1322. doi:10.1016/S0006-2952(97)87955-5
Nanton D, Lall S, McNiven MA (2001) Effects of dietary lipid level on liver and muscle lipid deposition in juvenile haddock, Melanogrammus aeglefinus L. Aquac Res 32:225–234
Parvez S, Raisuddin S (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environ Toxicol Phar 20:112–117
Qian W, Nishikawa M, Haque AM, Hirose M, Mashimo M, Sato E, Inoue M (2005) Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am J Physiol Cell Physiol 289:C1466–C1475
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56
Roberts RJ (2012) Fish pathology. Wiley, Oxford
Rueda-Jasso R et al (2004) Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 231:417–433
Sargent J, Bell G, McEvoy L, Tocher D, Estevez A (1999) Recent developments in the essential fatty acid nutrition of fish. Aquaculture 177:191–199
Shan WJ, Huang L, Zhou Q, Meng FC, Li XS (2011) Synthesis, biological evaluation of 9-N-substituted berberine derivatives as multi-functional agents of antioxidant, inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-β aggregation. Eur J Med Chem 46:5885–5893
Siow YL, Sarna L, Karmin O (2011) Redox regulation in health and disease-therapeutic potential of berberine. Food Res Int 44:2409–2417
Teodoro JS, Duarte FV, Gomes AP, Varela AT, Peixoto FM, Rolo AP, Palmeira CM (2013) Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: a possible role for SirT3 activation. Mitochondrion 13:637–646
Vergara J, Lopez-Calero G, Robaina L, Caballero M, Montero D, Izquierdo M, Aksnes A (1999) Growth, feed utilization and body lipid content of gilthead seabream (Sparus aurata) fed increasing lipid levels and fish meals of different quality. Aquaculture 179:35–44
Watanabe T (1982) Lipid nutrition in fish. Comp Biochem Physiol Part B: Comp Biochem 73:3–15
Weibel ER, Stäubli W, Gnägi HR, Hess FA (1969) Correlated morphometric and biochemical studies on the liver cell I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol 42:68–91
Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE (2006) In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 44:27–33
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292
Xie W, Gu D, Li J, Cui K, Zhang Y (2011) Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6 J mice. PLoS ONE 6:e24520
Zhang S, Fu J, Zhou Z (2004) In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol In Vitro 18:71–77
Zhang Y et al (2008) Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocr Metab 93:2559–2565
Zhang D, Lu K, Dong Z, Jiang G, Xu W, Liu W (2014) The effect of exposure to a high-fat diet on MicroRNA expression in the liver of Blunt Snout Bream (Megalobrama amblycephala). PLoS ONE 9:e96132
Zhou J, Li C, Wang L, Ji H, Zhu T (2015) Hepatoprotective effects of a Chinese herbal formulation, Yingchen decoction, on olaquindox-induced hepatopancreas injury in Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem 41:153–163
Acknowledgments
This work was funded by the National Nature Science Foundation of China (31172418, 31202005).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, KL., Wang, LN., Zhang, DD. et al. Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Physiol Biochem 43, 65–76 (2017). https://doi.org/10.1007/s10695-016-0268-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0268-5