Abstract
Here, we aimed to investigate whether resveratrol (RSV) can ameliorate high-fat diet (HFD)-induced metabolic disorder in fish. Blunt snout bream (Megalobrama amblycephala) with average weight 27.99 ± 0.56 g were fed a normal fat diet (NFD, 5% fat, w/w), a HFD (11% fat), or a HFD supplemented with 0.04, 0.36, or 1.08% RSV for 10 weeks. As expected, fish fed a HFD developed hepatic steatosis, as shown by elevated hepatic and plasma triglycerides, raised whole body fat, intraperitoneal fat ratio and hepatosomatic index, and increased plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST). RSV supplementation lessened increases in body mass, whole body fat, and intraperitoneal fat, and alleviated development of hepatic steatosis, elevations of plasma triglyceride and glucose, and abnormalities of ALT and AST in HFD-fed fish. RSV supplementation increased SIRT1 messenger RNA (mRNA) expression and consequently hepatic mRNA expression of adipose triglyceride lipase (ATGL), carnitine palmitoyltransferase (CPT1a), and microsomal triglyceride transfer protein (MTTP), implying upregulation of lipolysis, β-oxidation, and lipid transport, respectively, in the liver. Conversely, hepatic lipoprotein lipase (LPL), sterol regulatory element-binding protein 1 (SREBP-1c), peroxisome proliferator-activated receptor γ (PPARγ), and ATP citrate lyase (ACLY) mRNA expression were decreased, implying suppression of fatty acid uptake, lipogenesis, and fatty acid synthesis. Additionally, RSV downregulated glucokinase (GCK) and sodium-dependent glucose cotransporter 1 (SGLT1) and upregulated glucose transporter 2 (GLUT2) mRNA expression, thus restoring normal glucose fluxes. Thus, RSV improves lipid and glucose metabolisms in blunt snout bream, which are potentially mediated by activation of SIRT1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The blunt snout bream (Megalobrama amblycephala) is a commercially important fish species farmed in China. Since aqua-feed became more available and affordable in the past few decades, the total output of blunt snout bream in China has increased each year and reached 705,821,000 kg in 2012, according to the latest Food and Agriculture Organization of the United Nations (FAO) Fishery and Aquaculture Statistics yearbook (FAO 2014). However, it has become apparent that this species is highly susceptible to hepatic steatosis in intensive culture, probably as a result of the feeding of nutritional imbalance diet, especially high fat protein-sparing diet (Lu et al. 2013). This kind of metabolic syndrome affects growth performance, the stress response, anti-oxidant capacity, and innate immunity of blunt snout bream and causes great economic loss every year (Lu et al. 2014). Thus, novel approaches are required to prevent and treat hepatic steatosis in this species.
Resveratrol (3,4′,5-trihydroxystilbene, RSV) is a natural polyphenol and phytoalexin that is found in grapes, red wine, peanuts, other groundnuts, and some berries. Previous studies demonstrated that RSV has potential anti-oxidant, anti-inflammatory, anti-tumorigenic, and cardioprotective properties (de la Lastral and Villegas 2007; Delmas et al. 2006; Hung et al. 2000; Schwager et al. 2017) and can exert important effects on lipid and glucose metabolism (Baur et al. 2006; Ganjam et al. 2009; Zhang et al. 2012). Due to its beneficial effects on energy metabolism, RSV has been suggested to be a promising therapeutic agent for metabolic diseases. Adenosine monophosphate-activated protein kinase (AMPK) and sirtuin-1 (SIRT1) are two key molecular targets of RSV (Kulkarni and Canto 2015). AMPK is a sensor of energy homeostasis, and it is activated by phosphorylation in response to RSV, thereby increasing mitochondrial biogenesis through its interaction with SIRT1 in vivo and in vitro (Gencoglu et al. 2015). SIRT1 deacetylase functions as a master switch to maintain lipid and glucose homeostasis and energy balance via important metabolic regulators, such as peroxisome proliferator-activated receptor γ coactivator α (PGC-1α), forkhead box protein O1 (FOXO-1), and liver X receptor (Li et al. 2007; Rodgers et al. 2008; Rodgers and Puigserver 2007). When challenged with a high-fat diet (HFD), transgenic mice overexpressing Sirt1 show lower levels of lipid-induced inflammation, better glucose tolerance, and near-total protection from hepatic steatosis versus wild-type littermates (Pfluger et al. 2008).
Hepatic steatosis, which is characterized by the accumulation of triglycerides (TG) in hepatocytes, is strongly associated with metabolic impairment in this species. Animal models of diet-induced hepatic steatosis are particularly useful to investigate the mechanisms underlying this metabolic disease. Previous studies report that RSV protects against diet-induced hepatic steatosis in murine models (Andrade et al. 2014). For example, RSV reduced body fat, total cholesterol, TG, plasma transaminases, and insulin in mice fed for 60 days with a HFD. In addition, messenger RNA (mRNA) expression of acetyl CoA carboxylase (ACC), peroxisome proliferator-activated receptor γ (PPARγ), and sterol regulatory element-binding protein 1 (SREBP-1) was significantly suppressed in these mice (Andrade et al. 2014), all of which encode proteins involved in adipogenesis. Ponugoti et al. showed that SREBP-1c acetylation was elevated in diet-induced obese mice and that hepatic overexpression of SIRT1 or treatment with RSV ameliorated this change, supporting the use of RSV for the treatment of metabolic disorders (Ponugoti et al. 2010). RSV also increased metabolic rate and reduced fat mass in wild-type but not in AMPKα1−/− mice fed a HFD and failed to increase insulin sensitivity, glucose tolerance, and mitochondrial biogenesis if either AMPKα1 or α2 was absent (Um et al. 2010). Furthermore, RSV maintained glucose homeostasis in mice fed a HFD by activating the phosphoinositol 3-kinase (PI3K) and SIRT1 signaling pathways (Wang et al. 2014).
Although the effects of RSV have been widely studied in terrestrial animals, it is not known whether RSV supplementation has beneficial effects in HFD-fed fish. Accordingly, the present study aimed to investigate the potential benefits and possible mechanisms whereby RSV might regulate lipid and glucose metabolism in blunt snout bream. A deeper understanding of the role of RSV in modulating biological markers of HFD-induced hepatic steatosis should indicate its potential as a therapeutic substance for fish.
Materials and methods
Experimental fish and feeding trial
Juvenile blunt snout bream obtained from the Fish Hatchery of Guangling (Yangzhou, China) were reared with commercial diet in floating net cages in a pond. The nutritional ingredients of commercial diet are very similar to those in the normal diet we used in our experiment (Table 1). After 2 weeks of acclimation, 320 fish (weight 27.99 ± 0.56 g; length 11.13 ± 0.78 cm) were randomly assigned into five groups and fed with a diet containing normal levels of fat (NFD, 5% fat, w/w), a HFD (11% fat, w/w), or a HFD supplemented with 0.04, 0.36, or 1.08% RSV (purity 99%; Hubei Jusheng Technology Co., Ltd., Hubei, China) for 10 weeks. Each group had four net cage replicates (L × W × H: 1.0 m × 1.0 m × 2.0 m), each housing 16 fish. The fish were hand-fed to apparent satiation three times a day (6:00–6:30, 12:00–12:30, and 18:00–18:30). During the trial period, fish were reared under the following conditions: water temperature, 25–29 °C; dissolved oxygen, 5.0–7.0 mg/L; pH, 7.2–7.6; photoperiod; and natural light. The formulations and approximate compositions of the experimental diets are described in Table 1. All dietary ingredients were finely ground, well mixed, and pelleted by a laboratory pellet machine (MUZL 180, Jiangsu Muyang Group Co., Ltd., Yangzhou, China). The extrusion temperature of the diets is 80–90 °C. After drying, all diets were kept at − 20 °C in a fridge until used.
Sample collection
Prior to sampling, fish were fasted for 24 h. Ten fish per tank were anesthetized using 100 mg/L MS-222 (tricaine methanesulfonate; Sigma Chemical Company, St. Louis, MO, USA) and sacrificed for blood and tissue collection according to the Chinese Regulation on Animal Experimentation (Experimental Animal Protect Guide of the Ministry of Science and Technology of China, No. 398 of September 30, 2006). Blood samples were rapidly collected from the caudal vessel into heparinized tubes and centrifuged at 590×g for 10 min at 4 °C. The supernatant was then stored at − 70 °C for subsequent analysis of plasma biochemical parameters. Liver samples were removed from the fish, immediately frozen in liquid nitrogen, and then stored at − 70 °C until use. To obtain the final weight data, fish were batch-weighed by cage. Proximate composition of diets was analyzed according to the standard methods (AOAC 1990). Moisture was determined by oven drying until constant weight (105 °C); crude protein (nitrogen × 6.25) was detected by the Kjeldahl method using an Auto Kjeldahl System (FOSS KT260, Hillerød, Denmark, Switzerland); crude lipid was measured by ether extraction using Soxtec System HT (Soxtec System HT6, Tecator, Sweden); ash was detected by combustion at 550 °C for 4 h; and crude fiber was measured by fritted glass crucible method using an automatic analyzer (ANKOMA2000i, Macedon, NY, USA). Whole body fats were extracted with a chloroform: methanol (2:1) mixture according to the previous study (Folch et al. 1957). Body weight gain (BWG), feed intake, intraperitoneal fat index (IFI), and hepatosomatic index (HSI) were measured using the following formulas:
Analysis of plasma and tissue biochemical parameters
Activities of plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST), concentrations of plasma and liver TG and concentrations of plasma free fatty acid were determined using commercial kits (C009, C010, F001 and A042-1 respectively; Jiancheng Bioengineering Institute, Nanjing, China). Plasma glucose levels were measured by GOD/POD method (Trinder 1969) using a commercial glucose kit (F006; Jiancheng Bioengineering Institute, Nanjing, China). Hepatic very low-density lipoproteins (VLDLs) were detected using an ELISA kit (H249; Jiancheng Bioengineering Institute, Nanjing, China). Liver fat was extracted with a chloroform/methanol (2:1) mixture and quantified as previously described (Folch et al. 1957).
RNA extraction and qPCR
Liver total RNA was extracted using TRIzol reagent (Life Technologies, CA, USA) according to the manufacturer’s instructions. RNA quantity and purity were determined by absorbance at 260 and 280 nm, and its integrity was assessed by electrophoresis in 1.0% formaldehyde denaturing agarose gel. Quantitative real-time PCR (qPCR) was performed using the PrimeScript RT Master Mix Kit and SYBR Premix Ex Taq II Kit (Takara Bio, Dalian, China). The following thermal cycling process was carried out on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA): 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 40 s. The primers used in this study are listed in Table S1. To confirm the specificity of the amplification reaction, a melting curve was performed after the last amplification cycle. The data were analyzed using the 2−ΔΔCt method, and the expression level of β-actin was used as the endogenous reference.
Statistical analysis
Data were analyzed using SPSS software, ver. 20.0 (SPSS Inc., Chicago, IL, USA). The data are expressed as means ± SEM (standard error of the mean). Shapiro-Wilk test was performed to test the normality of the variables. One-way ANOVA was performed to compare NFD group and HFD groups that did or did not receive RSV supplementation, and Duncan’s Multiple Test was performed when significant differences were identified in the groups. Significance was accepted at P < 0.05.
Results
Effect of RSV on growth performance
To evaluate the effect of RSV on growth performance of HFD-fed fish, blunt snout bream were fed a NFD, a HFD, or RSV-supplemented HFDs for 10 weeks. HFD feeding tended to increased body mass, although there was no significant difference in body mass gain between NFD and HFD groups. However, body mass gain was significantly lower in fish fed a HFD supplemented with 1.08% RSV than in fish fed a HFD (Table 2). There was no difference in food intake among all the groups, but food intake tended to reduce as the levels of RSV supplementation increased from 0 to 1.08%.
Effect of RSV on fat accumulation
HFD-feeding resulted in a significant increase in intraperitoneal fat percentage versus NFD-feeding, whereas HFD supplemented with 1.08% RSV significantly reduced intraperitoneal fat accumulation versus HFD (Table 2). RSV supplementation (0.36 and 1.08%) significantly ameliorated the HFD-induced elevations in whole body crude fat and HSI.
RSV improves plasma biochemistry parameters
Plasma biochemical parameters are presented in Fig. 1. Higher plasma TG was observed in HFD-fed fish than in NFD-fed fish, while 1.08% RSV supplementation significantly reduced this effect. Dietary RSV supplementation from 0.04 to 1.08% significantly ameliorated the HFD-induced elevations in plasma glucose. Furthermore, RSV significantly attenuated the HFD-induced increases in AST and ALT activities, indicating that HFD feeding resulted in liver damage, while RSV intake limited this.
Effect of diet and resveratrol on plasma biochemistry analysis. a Plasma triglyceride (TG), b plasma glucose, c plasma aspartate aminotransferase (AST), and d plasma alanine aminotransferase (ALT) in blunt snout bream fed a NFD or HFD ± resveratrol (RSV) supplementation (n = 16 replicates per group). Mean values with different lowercase letters are significantly different among all the groups (P < 0.05)
RSV ameliorates HFD-induced hepatic lipidosis
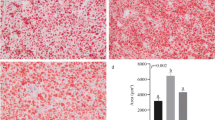
The hepatic TG content of HFD-fed fish was significantly higher than that of NFD-fed fish (Fig. 2a), but this was reduced as the level of RSV supplementation was increased from 0 to 1.08%; 0.36 and 1.08% RSV supplementation significantly ameliorated the HFD-induced elevations. HFD-fed fish tended to have higher hepatic lipid content versus NFD-fed fish, although this effect did not reach statistical significance (Fig. 2b). High levels of dietary RSV supplementation (0.36 and 1.08%) significantly reduced hepatic lipid content versus the HFD group. Additionally, hepatic VLDL levels in the HFD group were significantly lower versus the NFD group, while a high dose of RSV supplementation improved the secretion of VLDL (Fig. 2c).
Effect of RSV on the expression of lipid metabolism-related genes
Real-time PCR was used to measure hepatic mRNA expression of selected genes involved in lipid metabolism. As shown in Fig. 3, hepatic SIRT1 and adipose triglyceride lipase (ATGL) mRNA levels were significantly decreased in the HFD group compared with the NFD group, but this suppression was ameliorated by 1.08% RSV supplementation. Dietary RSV supplementation from 0.04 to 1.08% significantly increased the HFD-induced suppression of carnitine palmitoyltransferase 1a (CPT1a) and microsomal triglyceride transfer protein (MTTP) mRNA levels and significantly reduced the HFD-induced elevations in lipoprotein lipase (LPL), SREBP-1c, PPARγ, and ATP citrate lyase (ACLY) mRNA expression in the liver.
Effect of diet and resveratrol on hepatic lipid metabolism-related gene expression. a Sirtuin-1 (SIRT1), b adipose triglyceride lipase (ATGL), c carnitine palmitoyltransferase 1 (CPT1), d microsomal triglyceride transfer protein (MTTP), e lipoprotein lipase (LPL), f sterol regulatory element-binding protein-1c (SREBP-1c), g peroxisome proliferator-activated receptor γ (PPARγ), and h ATP citrate lyase (ACLY) mRNA expression in blunt snout bream. The expression level of each gene was normalized to that of the gene encoding β-actin. Data are represented as the mean ± SD of n = 16 replicates. Mean values with different lowercase letters are significantly different among all the groups (P < 0.05)
Effect of RSV on the expression of carbohydrate metabolism-related genes
Next, we aimed to determine how RSV attenuates the HFD-induced elevation in plasma glucose. To this end, we measured the hepatic mRNA expression of three carbohydrate metabolism-related genes, glucokinase (GCK), glucose transporter 2 (GLUT2), and sodium-dependent glucose cotransporter 1 (SGLT1) in blunt snout bream. GCK expression in the HFD group was higher than that in the NFD group (P < 0.05; Fig. 4), but RSV supplementation significantly reduced this. In addition, 1.08% RSV supplementation significantly reversed the HFD-induced suppression of GLUT2 mRNA expression and reduced SGLT1 mRNA expression versus the HFD group. The observed decreases in GCK and SGLT1 and the increase in GLUT2 mRNA expression likely explain the reduction in plasma glucose achieved by RSV supplementation in the HFD-fed fish.
Effect of diet and resveratrol on hepatic expression of genes involved in glucose flux. a Glucokinase (GCK), b glucose transporter 2 (GLUT2), and c sodium-dependent glucose cotransporter 1 (SGLT1) mRNA expression in blunt snout bream. The expression level of each gene was normalized to that of the gene encoding β-actin. Data are represented as the mean ± SD of n = 16 replicates. Mean values with different lowercase letters are significantly different among all the groups (P < 0.05)
Discussion
Consumption of a diet high in fat is a major contributing factor to the development of hepatic steatosis in animals (Nakamura and Terauchi 2013). Recently, the natural compound RSV was shown to suppress diet-induced liver steatosis in humans and terrestrial animals (Andrade et al. 2014; Faghihzadeh et al. 2014). However, whether RSV supplementation is a viable strategy for regulating lipid metabolism in fish has not been investigated. Blunt snout bream are susceptible to hepatic steatosis when consuming a HFD; thus, the aim of this study was to investigate whether HFD-induced hepatic steatosis in blunt snout bream could be effectively ameliorated by dietary RSV supplementation.
We first determined whether RSV exerted any effects on growth performance of HFD-fed fish. We found that there was no significant difference in body mass gain among fish fed with a HFD or a HFD supplemented with 0.04 or 0.36% RSV. However, body mass gain was significantly lower in fish fed a HFD supplemented with 1.08% RSV than in fish fed a HFD or a HFD supplemented with 0.04% RSV. Ha et al. demonstrated that the final body weights in the rats fed a HFD that includes high levels of RSV were significantly lower than those in the rats fed with a HFD (Ha et al. 2015). They thought that the unique flavor of RSV might affect the appetites of the rats fed a HFD supplemented with high levels of RSV, resulting in a decrease in food intake and weight reduction. It is known that resveratrol is able to cross the blood-brain barrier (Sasaki and Kitamura 2010) and reduce food intake in rodents (Kim et al. 2010). RSV can downregulate neuropeptide Y (NPY) gene expression to suppress the appetite and increase satiety in mice (Kim et al. 2010). Consistent with these results, we observed in this study that the food intake tended to decrease with the increasing of RSV supplementation, although there was no significant difference in food intake between HFD group and RSV supplemented groups (Table 2). Additionally, Lagouge et al. reported that RSV significantly increases the aerobic capacity of mice, as evidenced by increased running time and oxygen consumption by muscle fibers (Lagouge et al. 2006). The body weight reduction was because of reduced adipose tissue in the HFD-fed mice treated with RSV, even though food intake was similar to that of the control group. In our case, the losing of body fat and visceral fat may contribute to the weight reduction of fish with dietary RSV, after 10 weeks of HFD consumption (Table 2). These results suggest that RSV, a highly studied natural compound that activates sirtuins in terrestrial animals (Sinclair and Guarente 2014), might reduce energy storage efficiency and/or increase energy expenditure rate in HFD-fed blunt snout bream.
The hallmark of liver steatosis is TG accumulation in hepatocytes. After 10 weeks of HFD feeding, blunt snout bream showed higher levels of plasma and hepatic TG in comparison with NFD-fed fish, whereas supplementation with RSV significantly lowered plasma and hepatic TG. Furthermore, RSV supplementation (1.08%) significantly ameliorated the HFD-induced elevations in visceral fat of blunt snout bream. Consistent with this phenomenon, Pardal et al. observed that RSV can promote rapid consumption of the fat reserves in zebrafish (Danio rerio) larvae (Pardal et al. 2014). Moreover, the increase in plasma glucose levels resulting from HFD feeding was ameliorated by 10 weeks of RSV consumption. These improvements in glycemic control and blood lipid profile were also shown in previous studies (Yang and Lim 2014), and modulation of AMPK activity is suggested as one of the underlying mechanisms for this (Choi et al. 2014). Another characteristic of the hepatic steatosis induced by HFD feeding is the enhanced plasma activities of the markers of hepatic injury AST and ALT. Both of these enzyme activities were significantly reduced in the RSV-treated groups in this study. These findings collectively implicate RSV as a promising compound to relieve features of hepatic steatosis features in our fish model.
Hepatic steatosis is associated with an imbalance of lipid availability and disposal, which may be caused by enhanced free fatty acid release from adipose tissue or dietary lipids, increased de novo lipogenesis, impaired fatty acid oxidation, or decreased VLDL secretion. Therefore, RSV may ameliorate hepatic steatosis through modulation of one or more of these metabolic pathways. ATGL and LPL are key enzymes that regulate the disposal of lipid in the body. ATGL selectively performs the first and rate-limiting step, hydrolyzing TGs to generate diacylglycerols and fatty acids (Zimmermann et al. 2004), while LPL hydrolyzes TGs transported in the bloodstream as VLDLs and chylomicrons, providing free fatty acids for uptake by adipose tissue for storage or by other organs such as the muscle and heart for oxidation (Mead and Ramji 2002). In the present study, RSV supplementation increased hepatic mRNA expression of ATGL, potentially resulting in decreased accumulation of TG in the liver. RSV also significantly decreased hepatic mRNA expression of LPL in HFD-fed fish, consistent with the suppression previously observed in RSV-treated HFD-fed mice (Qiao et al. 2014).
CPT1 catalyzes a crucial step in mitochondrial fatty acid oxidation; therefore, the expression of CPT1 has implications for liver β-oxidation. Alberdi et al. demonstrated that RSV supplementation significantly elevated CPT1 activity in HFD-fed rats and prevented the increase in liver fat accumulation, suggesting that the decrease in TG accumulation induced by RSV was at least partly due to an increase in fatty acid oxidation. SIRT1 is a NAD+-dependent deacetylase and acts as a modulator of various metabolic pathways, including those involved in lipid and glucose homeostasis (Colak et al. 2011). RSV is a potent agonist of SIRT1, promoting the cellular expression of the SIRT1 protein and dramatically stimulating SIRT1 activity (Borra et al. 2005; Cohen et al. 2004). Recent studies demonstrated that RSV is able to alter the SIRT1 expression in zebrafish and RSV might attenuate fat deposition by reducing SREBP-1c expression via the SIRT1-FOXO-1 pathway in vitro and in vivo (Ponugoti et al. 2010; Wang et al. 2009). In this study, RSV significantly reversed the HFD-induced SIRT1 downregulation and SREBP-1c upregulation in blunt snout bream. PPARγ is a nuclear receptor central to glucose and lipid homeostasis, and it is generally overexpressed in steatotic liver (Moran-Salvador et al. 2011). SIRT1 activation increases lipolysis by suppressing PPARγ expression in adipose tissue, thereby altering expression of PPARγ-responsive genes, which mediate lipid anabolism (Picard et al. 2004). Here, RSV supplementation inhibited PPARγ expression and therefore impacted upon expression of its target gene, ACLY, which encodes a key enzyme of fatty acid oxidation. Hepatic ACLY expression in HFD-fed fish was significantly higher than in NFD-fed fish, and both 0.36 and 1.08% RSV supplementation significantly ameliorated this effect, which would also be predicted to reduce hepatic fat accumulation. Finally, MTTP plays a pivotal role in the assembly and secretion of apolipoprotein B-containing lipoproteins, including VLDL. Han et al. showed that RSV treatment promotes MTTP expression via SIRT1 activation in goose primary hepatocytes, while the SIRT1 inhibitor, nicotinamide, downregulates expression of MTTP (Han et al. 2014). Our data also imply that MTTP might be a SIRT1 target gene. The upregulation of MTTP as a result of RSV treatment was associated with increased VLDL secretion in the liver, suggesting that RSV supplementation can enhance hepatic lipid transport in blunt snout bream, which might also contribute to steatosis resistance.
High levels of dietary fat can impair glucose homeostasis in fish, mice, and humans (Figueiredo-Silva et al. 2012; Ikemoto et al. 1996), and a 10-week HFD was sufficient to elevate plasma glucose levels in this study. A hypoglycemic effect of RSV has been described in wild-type rats and mice, high-fat fed mice, and both mice and humans with type 2 diabetes (Yonamine et al. 2016). GCK, Glut2, and SGLT1 are key hepatic mediators of glucose flux and thus blood glucose homeostasis (Ganjam et al. 2009). In this study, RSV treatment of blunt snout bream suppressed expression of GCK, consistent with previous results in rats (Ganjam et al. 2009). Previous studies indicate that PPARγ and SREBP-1c regulate liver GCK expression (Kim et al. 2004a; Kim et al. 2004b); thus, the RSV-induced effect on GCK might be mediated through suppression of PPARγ and SREBP-1c via SIRT1 in this study.
Glucose enters eukaryotic cells via two different types of membrane-associated carrier proteins, the Na+-coupled glucose transporters and the facilitative glucose transporters. Glut2 is trans-membrane protein that can passively transport glucose through cell membranes, and it is mainly expressed by hepatocytes and pancreatic beta cells. Previous studies of the effect of RSV on Glut2 have been limited. However, RSV treatment improved pancreatic GLUT2 expression in diabetic rats (Gencoglu et al. 2015) and treatment of INS-1E cells for 24 h with 25 μM RSV resulted in marked potentiation of glucose-stimulated insulin secretion and GLUT2 expression through a SIRT1-dependent mechanism (Vetterli et al. 2011). Glucose derived either from hydrolysis of starch or from sucrose is taken up into cells by SGLT1, which is located in the brush border membrane at the apical side of enterocytes (Roder et al. 2014). Dietary polyphenols can downregulate the expression of SGLT1 in small intestine, thereby decreasing hepatic gluconeogenesis and glucose release indirectly (Kim et al. 2016). Here, we demonstrate for the first time that RSV supplementation can reduce hepatic SGLT1 expression.
Conclusions
RSV supplementation alleviated the increases in body mass, whole body and intraperitoneal fat, plasma TG, glucose, ALT, and AST associated with hepatic steatosis in HFD-fed blunt snout bream. We propose that this was achieved through an increase in SIRT1 mRNA expression, resulting in upregulation of hepatic ATGL, CPT1a, and MTTP mRNA expressions, thereby promoting liver lipolysis, β-oxidation, and lipid transport, respectively, and downregulation of hepatic LPL, SREBP-1c, PPARγ, and ACLY mRNA expressions, thereby suppressing fatty acid uptake and fatty acid synthesis. Additionally, RSV restored glucose homeostasis in the liver through downregulation of GCK and SGLT1 and upregulation of GLUT2 mRNA. Thus, 1.08% RSV supplementation ameliorates the defects in lipid and glucose metabolism resulting from feeding of a HFD to blunt snout bream.
References
Andrade JM et al (2014) Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 30:915–919
AOAC (1990) Official methods of analysis. Association of Official Analytical Chemists. Arlington, Virginia
Baur JA et al (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342
Borra MT, Smith BC, Denu JM (2005) Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280:17187–17195
Choi YJ, Suh HR, Yoon Y, Lee KJ, Kim DG, Kim S, Lee BH (2014) Protective effect of resveratrol derivatives on high-fat diet induced fatty liver by activating AMP-activated protein kinase. Arch Pharm Res 37:1169–1176
Cohen HY et al (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392
Colak Y et al (2011) SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit 17:Hy5–Hy9
de la Lastral CA, Villegas I (2007) Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans 35:1156–1160
Delmas D, Lancon A, Colin D, Jannin B, Latruffe N (2006) Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets 7:423–442
Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A (2014) Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res 34:837–843
FAO (2014) 2012 yearbook of fishery and aquaculture statistics. FAO, Rome
Figueiredo-Silva AC, Panserat S, Kaushik S, Geurden I, Polakof S (2012) High levels of dietary fat impair glucose homeostasis in rainbow trout. J Exp Biol 215:169–178
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Ganjam GK, Dimova EY, Unterman TG, Kietzmann T (2009) FoxO1 and HNF-4 are involved in regulation of hepatic glucokinase gene expression by resveratrol. J Biol Chem 284:30783–30797
Gencoglu H, Tuzcu M, Hayirli A, Sahin K (2015) Protective effects of resveratrol against streptozotocin-induced diabetes in rats by modulation of visfatin/sirtuin-1 pathway and glucose transporters. Int J Food Sci Nutr 66:314–320
Ha AW, Kang NE, Kim WK (2015) Ethanol extract of peanut sprout lowers blood triglyceride levels, possibly through a pathway involving SREBP-1c in rats fed a high-fat diet. J Med Food 18:850–855
Han CC et al (2014) Role of mammalian sirtuin 1 (SIRT1) in lipids metabolism and cell proliferation of goose primary hepatocytes. Mol Cell Endocrinol 382:282–291
Hung LM, Chen JK, Huang SS, Lee RS, Su MJ (2000) Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res 47:549–555
Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O (1996) High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metab-Clin Exp 45:1539–1546
Kim S, Kim H, Park SK, Im SS, Li TZ, Cheon HG, Ahn Y (2004a) Liver glucokinase can be activated by peroxisome proliferator-activated receptor-gamma. Diabetes 53:S66–S70
Kim SJ, Lee YH, Han MD, Mar W, Kim WK, Nam KW (2010) Resveratrol, purified from the stem of Vitis coignetiae Pulliat, inhibits food intake in C57BL/6J mice. Arch Pharm Res 33:775–780
Kim SY et al (2004b) SREBP-1c mediates the insulin-dependent hepatic glucokinase expression. J Biol Chem 279:30823–30829
Kim Y, Keogh JB, Clifton PM (2016) Polyphenols and glycemic control. Nutrients 8:17
Kulkarni SS, Canto C (2015) The molecular targets of resveratrol. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1852:1114–1123
Lagouge M et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122
Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L (2007) SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell 28:91–106
Lu KL, Xu WN, Li JY, Li XF, Huang GQ, Liu WB (2013) Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Sci 79:661–671
Lu KL, Xu WN, Liu WB, Wang LN, Zhang CN, Li XF (2014) Association of mitochondrial dysfunction with oxidative stress and immune suppression in blunt snout bream Megalobrama amblycephala fed a high-fat diet. J Aquat Anim Health 26:100–112
Mead JR, Ramji DP (2002) The pivotal role of lipoprotein lipase in atherosclerosis. Cardiovasc Res 55:261–269
Moran-Salvador E et al (2011) Role for PPAR gamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J 25:2538–2550
Nakamura A, Terauchi Y (2013) Lessons from mouse models of high-fat diet-induced NAFLD. Int J Mol Sci 14:21240–21257
Pardal D, Caro M, Tueros I, Barranco A, Navarro V (2014) Resveratrol and piceid metabolites and their fat-reduction effects in Zebrafish larvae. Zebrafish 11:32–40
Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 105:9793–9798
Picard F et al (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 430:921–921
Ponugoti B et al (2010) SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 285:33959–33970
Qiao Y, Sun J, Xia SF, Tang X, Shi YH, Le GW (2014) Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct 5:1241–1249
Roder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H (2014) The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 9:e89977
Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P (2008) Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett 582:46–53
Rodgers JT, Puigserver P (2007) Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A 104:12861–12866
Sasaki T, Kitamura T (2010) Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr J 57:939–946
Schwager J, Richard N, Widmer F, Raederstorff D (2017) Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement Alternat Med 17:309
Sinclair DA, Guarente L (2014) Small-molecule allosteric activators of sirtuins. Ann Rev Pharmacol Toxicol 54:363–380
Trinder P (1969) Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161
Um JH et al (2010) AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59:554–563
Vetterli L, Brun T, Giovannoni L, Bosco D, Maechler P (2011) Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem 286:6049–6060
Wang B, Sun J, Li LN, Zheng J, Shi YH, Le GW (2014) Regulatory effects of resveratrol on glucose metabolism and T-lymphocyte subsets in the development of high-fat diet-induced obesity in C57BL/6 mice. Food Funct 5:1452–1463
Wang GL, Fu YC, Xu WC, Feng YQ, Fang SR, Zhou XH (2009) Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem Biophys Res Commun 380:644–649
Yang SJ, Lim Y (2014) Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metab-Clin Exp 63:693–701
Yonamine CY, Pinheiro-Machado E, Michalani ML, Freitas HS, Okamoto MM, Correa-Giannella ML, Machado UF (2016) Resveratrol improves glycemic control in insulin-treated diabetic rats: participation of the hepatic territory. Nutr Metab 13:44
Zhang JY et al (2012) The protective effect of resveratrol on islet insulin secretion and morphology in mice on a high-fat diet. Diabetes Res Clin Pract 97:474–482
Zimmermann R et al (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386
Funding
This work was supported by the China Agriculture Research System (grant number CARS-46-20).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Table S1.
Primers used to detect mRNAs involved in lipid and glucose metabolism (DOCX 17 kb).
Rights and permissions
About this article
Cite this article
Zhang, D., Yan, Y., Tian, H. et al. Resveratrol supplementation improves lipid and glucose metabolism in high-fat diet-fed blunt snout bream. Fish Physiol Biochem 44, 163–173 (2018). https://doi.org/10.1007/s10695-017-0421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0421-9