Abstract
In order to identify effective hepatoprotective herbs for clinical application in fish farming, 200 mg/kg olaquindox (OLA) was added to a basal diet (group 1, control) to form OLA diet (group 2), then 1.35, 2.7 and 5.4 % (w/w) of a Chinese herbal formulation, Yingchen decoction (YCD), were added to the OLA diet to form three additional diets for groups 3, 4 and 5, respectively. A total of 375 juvenile Jian carp (Cyprinus carpio var. Jian) (52.12 ± 2.95 g/tail) were divided into five groups (triplicates per group) and fed the five diets mentioned above, respectively, for 6 weeks. At the termination of feeding experiment, serum biochemical indexes, viability of hepatocytes and the hepatopancreas microstructure for each group were detected and observed. The results showed that serum ALT and AST in group 2 were significantly higher than the control (P < 0.05). Plasma membranes hepatocyte nuclei in group 2 were found to be mostly indistinct, compared to group 1, and gradually recovered with the increasing supplementation of YCD in group 3, 4 and 5. The viability of isolated hepatocytes in group 2 was the lowest and gradually recovered with the increasing supplementation of YCD in group 3, 4 and 5. The results suggest that YCD protected the Jian carp hepatopancreas against injury from OLA, and that 5.4 % YCD would be the optimum dosage in a Jian carp diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the expansion of intensive fish farming industry, factors including nutrition, parasites and other diseases and poisoning have become increasingly serious. The liver or hepatopancreas of fish, which plays an important role in nutrient and energy metabolism, is sensitive, and diseases of the liver or hepatopancreas are common in fish farming. A primary pathologic symptom of many liver disease or injury is swelling, degeneration, necrosis and apoptosis of hepatic cells (Xue et al. 2009), and liver injury in fish can lead to apositia, low growth performance and even 50–60 % mortality (Jiang 1997), which causes a great economic loss in fish farming. Because Chinese herbal medicines have many benefits, such as improving the immune system and disease resistance in fish (Jian and Wu 2003, 2004; Choi et al. 2013), few side-effects, low cost and are unlikely to lead to resistance, they represent a new opportunity for aquaculture industry (Citarasu 2010).
Yingchen decoction (YCD), composed of herba artemisiae (Artemisia capillaris Thumb), gardenia (Fructus Gradeniae) and rhubarb (Radix et Rhizoma Rhei), is a popular Chinese medicine. Yin chen hao (Artemisiacapillaris Thunb), known as Yin Chen, Capillary or Oriental Wormwood, belonging to Asteracea family, is reported to have many beneficial effects and has been traditionally used to treat hepatic disorders (Reviewed by Hung and Kuo 2013; Lee et al. 2007). Most research related to YCD and its protective effects on the liver were conducted with hamsters (Hong and Lee 2009) or rats (Wang et al. 2012; Zhou et al. 2013; Zhang et al. 2011), while little is known regarding its effects on the liver of fish.
Olaquindox (OLA) previously was used as a feed additive to improve growth rate, feed conversion and animal health by stabilizing the gastro-intestinal microflora (Wang et al. 1992). However, more and more experimental results showed that OLA could weaken the antioxidant system (Zhang et al. 2010), induce autophagic processes and oxidative damage of mitochondria in human hepatoma G2 cells (Zou et al. 2009; Zhao et al. 2013) and be genotoxic in mice cells (Ihsan et al. 2013). It has also been reported that OLA showed cumulative toxicity in common carp, inducing fatty or vacuolar degeneration of liver cells (Wang et al. 2004a, 2005).
Mice and other mammals are often used as experimental animals to establish liver injury models in the fields of human and veterinary medicine (Mondal et al. 2012; Xu et al. 2011; Zhao et al. 2013). With the rapid development of the fish farming industry and taking into account the physiological characteristics of fish, it is urgent to establish an OLA-induced liver damage model for the target fish. This will more accurately reflect the changes of metabolism, function and histological structures in fish.
In the present study, 200 mg/kg OLA (referred to Wang et al. 2005) was added to the basal diet of Jian Carp (Cyprinus carpio var. Jian) to establish the fish liver injury model induced by OLA to provide a basis for the practical applications of fish pathology and clinical diagnosis. In addition, 1.35, 2.7 and 5.4 % of YCD were added to the OLA diets to study the hepatoprotective effects of YCD. The dosage of OLA and the possible hepatoprotective mechanism of YCD were investigated in order to provide a theoretical basis for research and development of hepatoprotective herbal treatments in fish.
Materials and methods
Experimental diets
OLA (Beijing Pharmaceutical Co., Ltd) was added to the basal diets of Jian carp to form the OLA diet (OLA 200 mg/kg, group 2). A Chinese herbal formulation, Ying Chen decoction (YCD), composed of herba artemisiae, gardenia and rhubarb, was powdered and mixed in the ratio of 3:1:1(w/w), then the mixture was extracted using 90 % ethyl alcohol. After the ethyl alcohol evaporated, the extract was incorporated into the OLA diets at 1.35, 2.7 and 5.4 % (w/w), respectively, to prepare three YCD diets (groups 3, 4 and 5). The basal diet was group 1 (control). These diets were pelletized using a hand-machine, at 2.5 mm in diameter and were stored at −20 °C before feeding. The formulation and nutrient levels of five the experimental diets are shown in Table 1.
Feeding experiment
A total of 375 healthy Jian carp (52.12 ± 2.95 g) were randomly divided into five groups (triplicates per group) and fed in fibreglass tanks with the five diets mentioned above. The tanks were operated at a volume of 160 L and received continuous aeration. Over the duration of the feeding experiment, water temperature was 16–23 °C, dissolved oxygen concentration was 10–13 mg/L, pH was 6.9–7.2 and total ammonia nitrogen concentration was below 0.5 mg/L. After 6 weeks, blood and hepatopancreas samples were collected and the following analyses were conducted.
Serum biochemical indexes analysis
The fish blood was first stored at 4 °C for 8 h, then centrifuged (2,000 rpm, 10 min, 4 °C) to obtain the upper layer of serum. Alanine amino transferase (ALT), asparagine amino transferase (AST), total protein (TP), glucose (GLU), cholesterol (Chol), triacylglycerols (TG), total bilirubin (TB), alkaline phosphatase (AKP), K+, Cl− and Na+ serum levels were determined using an automatic biochemical analyser (Hitachi 7180, Tokyo, Japan).
Histological observation of hepatopancreas with optical microscope and electron microscope
The fish hepatopancreas samples were firstly fixed in 2.5 % glutaraldehyde and then dehydrated in alcohol. Following dehydration, they were imbedded in ethoxyline, and finally, they were cut into 5- to 6-μm-thick slices. The pieces were stained with H × E for examination by optical microscope (Motic, America) stained with lead citrate for examination by electron microscope.
Viability of hepatocytes assayed by flow cytometry
The hepatopancreas of six fish per tank were sampled and washed with sterilized phosphate-buffered saline (PBS) (pH 7.4), then were sliced into small pieces (1 mm3) and digested with 0.25 % trypsin for 30 min. After filtration and centrifugation (1,000 rpm for 10 min) three times, the hepatocytes were isolated and the cell suspension was obtained by adding 10 mL medium to the cell pellets. The cell was further diluted (1 × 106 cell/mL) with PI staining solution (4 °C, 15 min) and then the cell viability was measured using an Epics Elite ESP flow cytometer (Coulter, Miami, FL, USA).
Statistical analysis
Data are presented as mean ± S.D. Significant differences among the five groups were determined by one-way ANOVA, followed by the Duncan’s multiple range tests. All statistical analyses were performed using SPSS 11.5. Results were regarded as significant at P < 0.05.
Results
Effect of YCD on serum biochemical indexes in aloquindox-induced hepatopancreas injury in Jian carp
Table 2 shows that serum ALT and AST in group 2, being 265.50 ± 216.58 and 539.9 ± 253.98 U/L, respectively, were significantly higher than those in group 1 (control), being 86.3 ± 24.23 and 319.6 ± 109.97 U/L, respectively (P < 0.05). With increasing supplementation of YCD, serum ALT and AST gradually decreased in groups 3, 4 and 5, and recovered, compared with group 1.
Serum AKP and TP in groups 2, 3, 4 and 5 were not significantly different from those in group 1 (P > 0.05).
Serum TB in group 2 was not significantly different from that in group 1 (P > 0.05), being 0.38 ± 0.29 and 0.48 ± 0.46 μmol/L, respectively. With the supplementation of YCD, serum TB in groups 3 and 4 increased significantly (P < 0.05), being 1.00 ± 0.28 and 1.02 ± 0.35 μmol/L, respectively. With the highest supplementation of YCD, in group 5, serum TB decreased and remained close to the same level (0.44 ± 0.54 μmol/L) as that in group 1.
Serum Na+ in groups 2 and 3, being around 132.5 μmol/L, was significantly lower than that in group 1, being 134.32 ± 1.54 μmol/L (P < 0.05). With increasing supplementation of YCD, in groups 4 and 5, serum Na+ increased and recovered, being around 134.5 μmol/L, and was not significantly different from that in group 1 (P > 0.05). Serum K+ in groups 2, 3, 4 and 5 was not significantly different from those in group 1 (P > 0.05).
Serum Cl− in group 2 (98.18 ± 1.08 μmol/L) was significantly lower than that in group 1, (101.93 ± 4.14 μmol/L) (P < 0.05). With the supplementation of YCD in group 3, 4 and 5, serum Cl−, being around 108 μmol/L, recovered and was not significantly different from that in group 1 (P < 0.05).
Serum Chol, TG and GLU showed no significant differences among the groups (P > 0.05).
Effect of YCD on histopathology of hepatopancreas in Jian carp
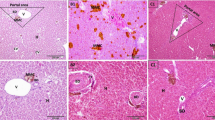
Figure 1 shows that the size of hepatocytes in group 1 (Fig. 1a) was uniform, but in group 2 (Fig. 1b) shows that the cells were wrinkled and huddled, and the size of the cells was smaller than that in group 1. With the supplementation of YCD, the cell size recovered and became more and more uniform.
HE staining of Jian carp hepatopancreas. a group 1; b group 2; c group 3; d group 4; e group 5. a The size of hepatocytes was uniform. b The hepatocytes were wrinkled, huddled, and the size of the cells was smaller than that in a, c, d, e With the increasing supplementation of YCD, the size of hepatocytes recovered was increasingly uniform. The photographs were taken with inverted microscope (×400) (bar = 50 µm)
Effect of YCD on microstructure of hepatocytes in Jian carp
The hepatocyte microstructure of Jian carp exhibited clear and intact plasma and nuclear membranes in group 1 (Fig. 2a, 1–3). While in group 2, the cell was found to be smaller and contracted, and the plasma and nuclear membranes were found to exhibit disaggregation and degradation (Fig. 2b, 1–3). With increasing supplementation of YCD, the cell size increasingly recovered and the plasma membrane, nuclear membrane and endoplasmic reticulum were found to be increasingly clearer and intact (Fig. 2c, d, e).
The state of hepatocytes observation with electron microscope in aloquindox-induced hepatopancreas injury in juvenile Jian carp. a-1, a-2, a-3: group 1; b-1, b-2, b-3: group 2; c-1, c-2, c-3: group 3; d-1, d-2, d-3: group 4; e-1, e-2, e-3: group 5. a Plasma membrane and the nuclear membrane were clear and intact. b The hepatocytes were smaller and contracted, and the plasma membrane and the nuclear membrane were almost disaggregation and degradation. c, d, e With the increasing supplementation of YCD, the cell size increasingly recovered and the plasma membrane, the nuclear membrane and the endoplasmic reticulum were increasingly clearer and intact. The photographs were taken under JEM-1230 transmission electron microscope (TEM). In a-1, a-3; b-1, b-3; c-1, c-3; d-1, d-3; e-1, e-3; f-1, f-3, bar = 1 µm; in a-2, b-2, c-2, d-2, e-2, bar = 10 µm)
The effect of YCD on viability of hepatocytes
The viability of hepatocytes in Jian carp showed that the living cell rate (LC) in group 2, being 70.5 ± 11.89 %, was significantly lower than that in group 1, being 93.9 ± 0.87 % (P < 0.05). With the addition of YCD, the LC rates, being 91.30–92.13 % in group 3, 4 and 5, recovered and were not significantly different from that in group 1 (P > 0.05).
The dead cell rate (DC) and non-viable apoptotic cell rate (NVAC) in group 2, being 7.27 ± 1.66 and 19.30 ± 10.95 %, respectively, were significantly higher than that in group 1, being 2.30 ± 0.44 and 1.53 ± 0.55 %, respectively. With the increasing addition of YCD in the diets (in group 3, 4 and 5), the DC and NVAC rate significantly decreased (P < 0.05) and remained around the same levels with that in group 1. The viable apoptotic cell ratios (VAC) were not significantly different among these five groups (P > 0.05), remaining within the range of 2.33–3.50 % (Table 3 and Fig. 3).
Discussion
Damage effects of OLA on hepatopancreas
OLA is an important member of the quinoxaline family, which were previously used as feed additives in China. As we mentioned above, the use of OLA is restricted due to its undesirable effects (WHO (World Health Organization) 1991). To develop the fish liver injury model induced by OLA in the present study, OLA was mixed into the Jian carp diet, then hepatocytes necrosis was observed (Figs. 1b, 2b), and significantly lower viability of hepatocytes was also observed in group 2 (Table 3). This is in agreement with a previous report that olaquindox induced apoptosis of hepatocytes in common carp (Wang et al. 2004b) and degeneration and necrosis of internal organs (heart, liver, etc.) of carp (Ye et al. 2003, 2005).
The destruction of the liver cell membrane structure and functional integrity would cause an increase in soluble enzyme (ALT and AST) activities; these soluble enzymes are released into the blood from the liver cells and resulted in an increase in serum enzyme activity in group 2 (Table 2). Therefore, the activities of ALT and AST in the serum are a good indicator of liver damage and can reflect the degree of liver damage and necrosis, to a certain extent (Liu et al. 2008).
Protective effects of YCD on hepatopancreas injury of Jian carp
According to the concept of traditional Chinese Veterinary medicine, liver injury exhibits a Qi stagnation pattern. The pathogenesis is damp-heat stagnation causing the difficulties of Qi moving in the liver, and the treatment principle should be to dispel damp-heat and pacify the liver (Liu et al. 2007a).
In the simultaneous OLA + YCD-treated fish, OLA-mediated hepatic AST and ALT activities were restored to near-normal, which is in accordance with previous results in poultry with Longyin decoction, including Capillaries (Yin chen), Gentian (Long dan), Gardenia, Bupleurum root and Licorice (Wang 1955), which reduced liver necrosis and decreased serum transaminase activity (ALT and AST) (Wang et al. 2013). Capillarisin, a flavonoid constituent of Yin Chen, was also reported to decrease serum transaminase activities through orally administered Artemisiae Capillaris (AC) (500, 1,000 or 2,000 mg/10 mL/kg) in murine (Mase et al. 2010). The present results showing that the treated group exhibited significantly reduced hepatic damage by lowering the levels of enzyme markers, such as ALT and AST in the plasma, compared with OLA, alone suggest a metabolic regulatory action of YCD, which could be due to its high antioxidant flavonoid content (Wada and Ou 2002).
The serum of OLA-treated fish showed reduced Na+ and Cl− ions when compared with control fish (Table 2), suggesting that the OLA-treated group experienced ionic disturbances. Previous reports showed that in freshwater fish, membrane-bound ATPases (Mg2+ and Na+/K+-ATPases) play a significant role in ionic regulation of cellular components and maintenance of tissue osmolarity against concentration gradients and across membranes (Sancho et al. 2003; Kirschner 2004). ATPases are integral membrane proteins which require thiol groups and phospholipids to maintain their structure and function (Hazarika and Sarkar 2001). Several xenobiotics and oxidative stress were reported to adversely affect Na+/K+-ATPase activities (De la Torre et al. 2007). The present study did not measure membrane-bound ATPase activities in the liver of OLA-treated fish; however, OLA and its serum Na+ and Cl− decreases were observed, which could possibly affect the structure and function of membrane-bound ATPases and thereby alter the hepatic functions in the OLA-treated Jian carp.
The OLA + YCD simultaneously treated fish showed near-normal serum Na+ and Cl+ mineral levels, which reveals the membrane-stabilizing action of YCD against OLA and could possibly restore the hepatic ions in Jian carp.
Histopathological examination of OLA-exposed Jian carp liver tissue showed hepatocellular contraction and degeneration. These histological alterations, linked with OLA exposure, were effectively mitigated in the OLA + YCD simultaneously treated fish. Accordingly, the YCD-treated group exhibited significantly reduced hepatic damage due to increasing viability of hepatocytes, compared with OLA alone. Artemisiae Capillarisin (AC) is a flavonoid constituent of Yin Chen mentioned above and was reported to increase cell viability of Chinese hamster lung fibroblasts (V79) cells by enhancing the antioxidative activity (Hong and Lee 2009), which is in agreement with the present result with Jian carp. Another study also showed that Yin Chen may prevent the EtOH-induced cytotoxicity on human hepatoma cell lines and Hep G2 cells (Koo et al. 2002). Furthermore, AC was found to inhibit the EtOH-induced apoptosis of Hep G2 cells, and Water extract of Artemisia capillaries (ACWE) was found to be capable of ameliorating the 2,2¢-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced hepatic injury by catechin antioxidant activity in vivo (Han et al. 2006). This suggests that treatment with efficient YCD could act in multiple ways (i.e. preserving hepatocyte structure, protecting antioxidant enzymes, scavenging the OLA-generated oxyradicals and promoting antioxidants levels) to preserve the normal liver function from the toxic effects of OLA and that the flavonoid constituent of Yin Chen probably the primary role of hepatoprotection in Jian carp. This is in accordance with previous reports that the benefits of Chinese herbal medicine treatment on liver injury were mainly obtained through the inhibition of lipid peroxidation, stabilization of biological membranes, improving hepatic micro-circulation and inhibiting liver cell apoptosis and inflammatory cytokines (Yang 2008) (Fig. 3).
Numerous of clinical practices and experimental research have confirmed that Chinese herbal medicine has significant protective effects against liver injury, such as jaundice and fatty liver. Many single Chinese herbs, including Scutellaria, Astragalus, Herba Rhodiola, Polygonum cuspidatum, Radix lithospermi, Angelica sinensis, Radix salviae miltiorrhizae, Cordyceps polysaccharide, Polysaccharide of Grifola Frondosa, Silymarin and Conocarpus erectus, as well as their bio-active ingredients, including Geranium flavonoids, Rhizoma Anemarrhenae total flavone, Momordica glycoside, tetramethylpyrazine, Fly thistle element, schizandrin, tetrandrine glycyrrhizin, etc., have been reported to have hepatoprotective effects (Wang 2010; Luo et al. 2001; Lu et al. 2008; Lv et al. 2007; Liu et al. 2007b; Shang et al. 2007; Li et al. 2008; Wang and Xiao 2007; Zhu and Tang 2008; Dvorák et al. 2003; Jia et al. 2013; Abdel-Hameed et al. 2013).
In addition, a great number of studies have shown that the protective effects of compounds in Chinese herbal medicines on the liver are more comprehensive. The commonly used Chinese herbal prescription includes the modified Xiaochaihu Decoction, Longdan Xiegan powder, compound Caiyu decoction, Peony and Licorice decoction, Yinchenhao decoction, Glycyrrhizae decoction (also for curing stomachache) and Si Ni decoction (Xie et al. 2008; Kouadir 2007; Wang and Xiao 2007; Di et al. 2007). Meanwhile, traditional Chinese medicines, such as Yinchenhao Tang, Si-Ni-San and BaoGan Jiedu decoction, were also found to have hepatoprotective effects in common carp (Wang et al. 2001), crucian carp (Chen et al. 2006) and eel (Mei and Huang 2006).
As well, scientific evidence was found which explains Yin Chen’s effect on treating jaundice in mammals. The constitutive androstane receptor (CAR, NR1I3) was identified as a key regulator of bilirubin clearance in the liver, and Yin Chen treatment in WT mice was able to induce components of bilirubin metabolism pathways to clear bilirubin (Elferink 2004; Huang et al. 2004). In the present study, serum total bilirubin in Jian carp was not significantly affected by OLA or YCD, possibly indicating differences in bilirubin metabolism or jaundice treatment mechanisms of YCD between mammals and fish.
All in all, in relation to fish health, when a possibility of OLA toxicity exists, the inclusion of YCD in fish diets is strongly suggested, and 5.4 % YCD would be an appropriate dosage.
References
Abdel-Hameed E-SS, Bazaid SA, Sabra ANA (2013) Protective effect of conocarpus erectus extracts on CCl4-induced chronic liver injury in mice. Glob J Pharmacol 7:52–60
Chen PF, Chen J, Guo ZX (2006) Preliminary research on the effect of Si-Ni-San on carp’s liver protection and recovery. Feed Ind 27(6):28–31 (in Chinese)
Choi WM, Mo WY, Wu SC, Mak NK, Bian ZX, Nie XP, Wong MH (2013) Effects of traditional Chinese medicines (TCM) on the immune response of grass carp (Ctenopharyngodon idellus). Aquac Int. doi:10.1007/s10499-013-9644-7
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int 18:403–414
De la Torre FR, Salibia’n A, Ferrari L (2007) Assessment of the pollution impact on biomarkers of effect of a freshwater fish. Chemosphere 68:1582–1590
Di L, Liu XY, Chang HF, Wang YS, Zhang QG (2007) Protective effect of Peony and Licorice decoction on acute liver injury in mice. Pharmacol Clin Chin Mater Med 23:4–5
Dvorák Z, Kosina P, Walterová D, Simánek V, Bachleda P, Ulrichová J (2003) Primary cultures of human hepatocytes as a tool in cytotoxicity studies: cell protection against model toxins by flavonolignans obtained from Silybum marianum. Toxicol Lett 137(3):201–212
Elferink RO (2004) Yin Zhi Huang and other plant-derived preparations: where herbal and molecular medicine meet. J Hepatol 41:691–693
Han KH, Jeon YJ, Athukorala Y, Choi KD, Kim CJ, Cho JK et al (2006) A water extract of Artemisia capillaries prevents 2,2′-azobis(2-amidinopropane) dihydrochloride-induced liver damage in rats. J Med Food 9:342–347
Hazarika A, Sarkar SN (2001) Effect of isoproturon pretreatment on the biochemical toxicodynamics of anilofos in male rats. Toxicology 165:87–95
Hong JH, Lee IS (2009) Cytoprotective effect of Artemisia capillaries fractions on oxidative stress-induced apoptosis in V79 cells. BioFactors 35:380–388
Huang W, Zhang J, Moore DD (2004) A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest 113:137–143
Hung HY, Kuo SC (2013) Recent studies and progression of Yin Chen Hao (茵陳蒿Yīn Chén Hāo), a long-term used traditional Chinese medicine. J Tradit Complement Med 3(1):2–6
Ihsan A, Wang X, Zhang W, Tu HG, Wang YL, Huang LL, Iqbal Z, Cheng GY, Pan YH, Liu ZL, Tan ZQ, Zhang YY, Yuan ZH (2013) Genotoxicity of quinocetone, cyadox and olaquindox in vitro and in vivo. Food Chem Toxicol 59:207–214
Jia R, Cao L, Du J, Xu P, Jeney G, Yin G (2013) The protective effect of silymarin on the carbon tetrachloride (CCl4)-induced liver injury in common carp (Cyprinus carpio). In Vitro Cell Dev Biol Anim 49:155–161
Jian JC, Wu ZH (2003) Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture 218:1–9
Jian J, Wu Z (2004) Influences of traditional Chinese medicine on non-specific immunity of Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 16:185–191
Jiang LF (1997) Liver diseases in fish and its prevention and treatment. Fish Sci Technol Inf 24(4):188–192
Kirschner LB (2004) The mechanisms of sodium chloride uptake in hyper regulating aquatic animals. J Exp Biol 2004:1439–1452
Koo HN, Hong SH, Jeong HJ, Lee EH, Kim NG, Choi SD et al (2002) Inhibitory effect of Artemisia capillarison ethanol-induced cytokines (TNF-α, IL-1α) secretion in Hep G2 cells. Immunopharmacol Immunotoxicol 24:441–453
Kouadir M (2007) Study of hepatoprotective and anti-inflammatory effects of Long Dan Xie Gan decoction and their mechanisms. [M.S. thesis], Yangzhou University 23–50
Lee TY, Chang HH, Chen JH, Hsueh ML, Kuo JJ (2007) Herb medicine Yin–Chen–Hao–Tang ameliorates hepatic fibrosis in bile duct ligation rats. J Ethnopharmacol 109:318–324
Li MM, Jiang T, Hang JC, Yao XS, Li YB (2008) Protective effect of Rhizoma anemarrhenae total flavone on liver injury in mice induced by acetic acid. Chin Tradit Herb Drug 39:252–255
Liu D, Tang HF, Zhang SQ, Di Y, Meng J, Wang JW (2007a) Protective effect of concentrated tablet of Polygonum cuspidatum on liver injury in mice. Lishizhen Medicine and Materia Medica Research 18:3034–3035
Liu ZD, Zhao XG, Yiu SL (2007b) Study progress in treatment of hepatic injury and prospect. Guide J TCM 10:81–87
Liu J, Liu DJ, Qiao FF, Ni JN, Lv YJ, Bao ED (2008) Establishment of experimental animal model of chicken liver and kidney damages induced by carbon tetrachloride. J Nanjing Agric Univ 31:117–120
Lu JT, Yang Y, Wei W, Chen ZM (2008) Protective effects of compound astragalus extract on chemical and immunological liver injury in mice. Chin J Inf Tradit Chin Med 1:32–34
Luo DS, Zheng HH, Liu Q (2001) Experimental research of Huangqin elixation’s protective effect on liver injury induced by carbon tetrachloride in rats. J Xianning Med Coll 15:92
Lv HZ, Huang SF, Cui JM, Li G, Piao GC (2007) Protective effect of ultra fine powder of Rhodiola sacra on acute injury in mice. J Med Sci Yanbian Univ 30:259–261
Mase A, Makino B, Tsuchiya N, Yamamoto M, Kase Y, Takeda S et al (2010) Active ingredients of traditional Japanese (kampo) medicine, inchinkoto, in murine concanavalin A-induced hepatitis. J Ethnopharmacol 127:742–749
Mei JL, Huang YF (2006) Studies on enzymatic mechanism of the liver-protective effect of BaoGan Jiedu Decoction on experimental hepatosis in anguilla. Acta Veterinaria et Zootechnica Sinica 37(12):1353–1359 (in Chinese)
Mondal A, Karan SK, Singha T, Rajalingam D, Maity TK (2012) Evaluation of hepatoprotective effect of leaves of Cassia sophera linn. Evid Based Complement Alt Med, vol 2012 Article ID 436139, p 5
Sancho E, Ferna’ndez-Vega C, Ferrando MD, Andreu-Molinar E (2003) Eel ATPase activity as biomarker of thiobencarb exposure. Ecotoxicol Environ Saf 56:434–441
Shang YP, Fang SY, Ge JF, Zhang L, Li J (2007) Protective action of cordyceps polysaccharides on immunological liver injury in mice. West China J Pharm Sci 22:654–655
Wada L, Ou B (2002) Antioxidant activity and phenolic content of Oregon cranberries. J Agric Food Chem 50:3495–3500
Wang S (1955) Eleven herbal formulas for jaundice. Waitai Miyao, People’s Medical, Beijing, China 14:6
Wang YZ (2010) The protective effect of polysaccharide of grifola frondosa on carbon tetrachloride induced liver injury and its mechanism. [M.S. thesis], Shandong University
Wang Q, Xiao D (2007) Protective effect of Mogrosides on chronic liver injury in rats. Guangxi J Tradit Chin Med 30:54–56
Wang YC, Yang XL, Zhao RC, Li JS, Xue FQ, Liang JP, Xu ZZ, Du XL (1992) Study on cumulative toxicity of quinocetone in mice. J Tradit Chin Vet Med 5:14
Wang LQ, Miao LC, Wang XJ (2001) The newest researching development and clinic application of Yinchenhao Tang. J Chin Tradit Herb 8(4):23–25 (in Chinese)
Wang KY, Zhao DM, Geng Y, Huang XL, Liu GX (2004a) Study on hepatic cells apoptosis induced by olaquindox in Cyprinus carpio. J Fish China 28(6):733–737 (in Chinese)
Wang XJ, Li TL, Sun H (2004b) Hepatoprotective effects of Yinchenhao Tang and its constituent absorbed into blood after oral administration. Chin Pharmacol Bull 20(2):239–240 (in Chinese)
Wang KY, Geng Y, Huang XL (2005) Apoptosis of hepatic cell in common carp poisoned with olaquindox by flow cytometry (FCM). J Fish China 12(5):648–651 (in Chinese)
Wang JH, Choi MK, Shin JW, Hwang SY, Son CG (2012) Antifibrotic effects of Artemisia capillaries and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J Ethnopharmacol 140:179–185
Wang CG, Zhang T, Cui XM, Li S, Zhao XH, Zhong XH (2013) Hepatoprotective effects of a Chinese herbal formula, Longyin Decoction, on carbon-tetrachloride-induced liver injury in chickens. Hindawi Publishing Corporation. Evid Based Complement Alt Med vol 2013, Article ID 392743, p 9
WHO (World Health Organization) (1991) Toxicological evaluation of certain veterinary drug residues in food. Olaquindox. WHO Food Additives Series, No 27, no 701 on INCHEM
Xie B, Yu DJ, Cheng SM (2008) Protective effect of modified minor Radix bupleuri decoction on liver injury induced by paracetamol. Lishizhen Medicine and Materia Medica Research 19:130–131
Xu L, Gao J, Wang Y, et al (2011) Myrica rubra extracts protect the liver from CCl4-induced damage. Evid Based Complement Altern Med vol. 2011, Article ID 518302, p 8
Xue CS, Yang QH, Liu J (2009) Protective effect of Jiangzhi Ninggan capsule on acute hepatic injury induced by d-galactosamine in mice. Lishizhen Medicine and Materia Medica Research 20:295–296
Yang RF (2008) Establishment of mice liver injury model induced by DMN and protective effect of Chinese medicine on liver. [Doctor thesis], Guangzhou University of Chinese Medicine
Ye JD, Han YW, Yang YH, Liu HB, Lu DY, Zhao JW (2003) Effect of olaquindox on oxygen consumption, red blood cell number, hematocrit and dyskaryosis in erythrocytes in mirror carp Cyprinus carpio L. J Dalian Fish Univ 18(1):14–18 (in Chinese)
Ye JD, Liu HB, Zhao JW, Lu TY, Yang YH (2005) Changes of body composition, plasma biochemical indexes and Na+, K+ -ATPase activity in gill after feeding diets with different doses of olaquindox to Cyprinus carpio L. J Huazhong Agric Univ 24(2):197–202 (in Chinese)
Zhang T, Chen Q, Tang SS, Jin X, Zou JJ, Liu FY, Zhang S, Xiao XL (2010) Olaquindox weakened the antioxidant system in human hepatoma G2 cells. Carcinog Teratog Mutagen 22(5):1–5
Zhang JJ, Du Z, Wang YJ, Nie FH, Zhu ZY (2011) Preparation of modified oriental wormwood decoction containing serum and it’s protective effect on necrotic hepatocyte injury model. Tianjin Pharm 23(1):3–6
Zhao DX, Zhang CM, Tang SS, Wang CC, Zhang S, Xiao XL (2013) Autophagic process induced by olaquindox in HepG2 cells. Carcinog Teratog Mutagen 25(3):1–5
Zhou XH, Zhong ZD, Zhong S, Chen J (2013) Effect of modified Yinchenhao decoction on the expression of TLR4 mRNA and protein in injury liver of rats. Chin J Exp Tradit Med Formul 19(15):239–242
Zhu SM, Tang ZP (2008) Research progress of protective effect of Yinchenhao decoction on liver. Shanghai J Tradit Chin Med 42:73–74
Zou JJ, Zhang T, Tang SS, Chen Q, Jin X, Chen KP, Xiao XL (2009) Oxidative damage of mitochondria by olaqindox in HepG2 cells. Carcinog Teratog Mutagen 21(5):1–5
Acknowledgments
This work was supported by the fund of Ankang Fisheries Experimental, Demonstration Building Project of Northwest A&F University (Z222020020), the Scientific Research Fund of Repatriation Personnel of Nation Education Commission and the Fundamental Research Funds for the Central Universities (Northwest A&F University, QN2011104).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, J., Li, C., Wang, L. et al. Hepatoprotective effects of a Chinese herbal formulation, Yingchen decoction, on olaquindox-induced hepatopancreas injury in Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem 41, 153–163 (2015). https://doi.org/10.1007/s10695-014-0013-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-0013-x