Abstract
Poison frogs are well known for their ability to sequester alkaloids from their diet of leaf-litter arthropods for use in defense against predators and pathogens. Australian frogs in the genus Pseudophryne (Myobatrachidae) represent an understudied lineage of poison frogs, with a unique ability to both sequester dietary alkaloids and synthesize pseudophrynamine alkaloids. Herein, we describe the alkaloid profiles and diet of six species of Pseudophryne (P. guentheri [Boulenger, 1882], P. occidentalis [Parker, 1940], P. semimarmorata [Lucas, 1892], P. dendyi [Lucas 1892], P. bibronii [Günther, 1859], and P. coriacea [Keferstein, 1868]) to gain a better understanding of how alkaloid defenses and diet are related within and among species. We characterized and quantified alkaloids using Gas Chromatography-Mass Spectrometry (GC-MS), and assessed diet by way of dissection and examination of stomach contents using light microscopy. We found that alkaloid profiles varied significantly among species, with pumiliotoxins (dietary-derived) and pseudophrynamines (biosynthesized) being the most abundant alkaloids. Pseudophryne bibronii and P. dendyi contained mostly dietary-derived alkaloids, whereas P. coriacea, P. guentheri, P. semimarmorata, and P. occidentalis possessed mostly biosynthesized alkaloids. The stomachs of all the studied species were largely empty, containing mostly soil and few partially digested insects. Our data suggest that frogs eat minimally during the breeding season. Therefore, a decrease in dietary alkaloids may be compensated by the biosynthesized pseudophrynamines, which could allow Australian poison frogs to remain defended from predators during this vulnerable time period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anurans possess a diversity of defensive chemicals that are aimed at protection against predators and microbes (Toledo and Jared 1995; Conlon et al. 2011a, b; Hovey et al. 2018; Lawrence et al. 2019, 2023). Most of these chemicals appear to be synthesized by frogs and largely include amines, peptides, proteins, steroidal bufadienolides, tetrodotoxins, as well as a variety of volatile organic compounds (Daly et al. 1987, 2004, 2005; Brunetti et al. 2015; Jeckel et al. 2015; Protti-Sánchez et al. 2019; Gonzalez et al. 2021). Alkaloids are a particularly large group of defensive chemicals, most of which are sequestered unchanged from a natural diet of alkaloid-containing arthropods (Daly et al. 1994, 2000, 2003; Saporito et al. 2007a, 2009, 2015; Jeckel et al. 2022; Alvarez-Buylla et al. 2022). Alkaloid sequestration has been described in five anuran lineages that are collectively referred to as poison frogs, which include species of Bufonidae (Melanophryniscus), Dendrobatidae (several genera), Eleutherodactylidae (Eleutherodactylus), Mantellidae (Mantella), and Myobatrachidae (Pseudophryne frogs; Daly et al. 2005; Rodríguez et al. 2011; Saporito et al. 2012). More than 1,200 alkaloids representing about 24 different structural classes have been identified in poison frogs worldwide (Daly et al. 2005, 2008, 2009; Saporito et al. 2012; Garraffo et al. 2012; Hovey et al. 2018; Saporito unpublished data). Bufonid and myobatrachid poison frogs represent two independent lineages that are capable of both sequestering dietary alkaloids from arthropods and biosynthesizing additional types of alkaloids (Smith et al. 2002; Jeckel et al. 2015). Bufonids in the genus Melanophryniscus sequester a diversity of dietary alkaloids (Daly et al. 2007; Garraffo et al. 2012), but also synthesize several other defensive compounds, including the indolealkylamine alkaloid bufotenine (Daly et al. 1987, 2008; Erspamer 1994; Mebs et al. 2007a; Jeckel et al. 2015). Myobatrachids of the genus Pseudophryne largely sequester pumiliotoxin alkaloids from dietary arthropods (Daly et al. 1990; Smith et al. 2002), but also synthesize a unique class of indolic alkaloids known as pseudophrynamines (Daly et al. 1990; Smith et al. 2002). Higher quantities of sequestered alkaloids are associated with lower quantities of biosynthesized alkaloids in Melanophryniscus moreirae and several species of myobatrachids, suggesting that some members of these poison frog lineages are capable of regulating alkaloid synthesis in relation to alkaloid sequestration (Smith et al. 2002; Jeckel et al. 2015). Further investigating the hypothesized relationship between biosynthesized and sequestered alkaloids is needed to gain insight into how alkaloid defenses are developed, maintained, and used by poison frogs.
Australian poison frogs in the genus Pseudophryne are composed of 14 species primarily found in coastal wetland forests of eastern and western Australia, with one species known from central Australia (Donnellan et al. 2012). Pseudophryne are small (20-30 mm in snout-to-vent length), largely nocturnal and fossorial frogs (Mitchell 2001) with highly variable coloration, ranging from blotchy brown to having patches of yellow and red to the distinct black and yellow striped pattern of the Corroboree Frogs (P. corroboree and P. pengilleyi). These conspicuous colors may have a dual function being cryptic from a distance and aposematic from close range (Lawrence et al. 2018; Umbers et al. 2019) and in general follow a common trait in poison frogs in which alkaloid defenses are associated with conspicuous coloration (Williams et al. 2000; Santos et al. 2003; Bonansea and Vaira 2012; Roberts et al. 2022). Notably, while Lawrence et al. 2018 did examine aposematic signaling, further testing is necessary to confirm aposematic function of this conspicuous coloration given the apparent paradox of conspicuous coloration being associated with nocturnal and fossorial frogs. Approximately six dietary-derived pumiliotoxins and more than 35 frog-synthesized pseudophrynamines have been detected in the seven Pseudophryne species previously examined (Daly et al. 1990; Smith et al. 2002). Little has been published on the diet of Pseudophryne, though they are likely ant-specialists (McFadden et al. 2010), which are a known dietary source of defensive alkaloids in poison frogs (Jones et al. 1999; Daly et al. 2000; Saporito et al. 2004). During the breeding season, which typically occurs between January and June (exact timing depends on species), males move to nesting sites and construct breeding chambers in damp environments, usually just under the leaf litter, from which to call for females (Barker et al. 1995; Mitchell 2001; Byrne and Silla 2023). Once nesting sites are established, females arrive to deposit eggs that after fertilization are guarded for up to four weeks by males (Pengilley 1973). Australian autumn and winter (January through June) rains produce runoff that carries the eggs to the nearest creek or river where they hatch and tadpoles complete development (Barker et al. 1995). These normally fossorial frogs are thus likely at highest risk of predation during the breeding season when they move to nesting sites, making defensive alkaloids particularly important to the survival of individuals in the cooler months.
Pseudophrynamines are a unique class of frog-synthesized alkaloids only found in Pseudophryne and exist in individuals in combination with dietary alkaloids (mostly pumiliotoxins; Daly et al. 1990; Smith et al. 2002). Pseudophrynamines appear to have an inverse relationship with pumiliotoxins in several species (Daly et al. 1990; Smith et al. 2002), whereby individuals with high amounts of pseudophrynamines have low amounts of pumiliotoxins. In P. semimarmorata, sequestration of high levels of pumiliotoxins may limit pseudophrynamine synthesis (Smith et al. 2002). Although alkaloids have been described and compared among Pseudophryne species (Daly et al. 1990), the relationship between pseudophrynamines and dietary alkaloids has not been quantitatively examined across the genus. Thus, we sought to further investigate two aspects of Pseudophryne biology by examining five previously studied species (P. guentheri, P. occidentalis, P. semimarmorata, P. bibronii, and P. coriacea) and one species (P. dendyi) that has not been studied for alkaloids. First, do pseudophrynamine alkaloids show an inverse relationship with diet-derived alkaloids? And second, is there any relationship between diet-derived alkaloids and stomach contents (allowing us to identify possible sources of sequestered alkaloids)? In investigating these two questions, we elucidate how frog-synthesized alkaloids vary with the presence of dietary alkaloids and how diet and alkaloids are related across species of Pseudophryne.
Materials and methods
Field collection
We located individuals by listening for calling males during their autumnal breeding season, and therefore our sampling almost certainly consisted only of males. Pseudophryne are not sexually dimorphic, however we avoided collecting noticeably gravid females. From March to May 2016, we collected 36 specimens representing six different species of Pseudophryne (sample size and coordinates in brackets: P. guentheri [n = 7; 116.634, -28.400], P. occidentalis [n = 7; 115.94, -28.460], P. semimarmorata [n = 5; 141.396, -38.004], P. dendyi [n = 7; 146.536, -38.065], P. bibronii [n = 3; 152.171, -32.750], and P. coriacea [n = 7; 153.547, -28.330]; Fig. 1). Notably, two species (P. guentheri and P. occidentalis) are found in coastal western Australia, while the other four species (P. dendyi, P. semimarmorata, P. bibronii, and P. coriacea) are found in coastal eastern and southeastern Australia (Fig. 1).
Images and ranges of the species examined in this study. Western species include A) Pseudophryne guentheri and B) P. occidentalis, while eastern species include C) P. dendyi, D) P. semimarmorata, E) P. coriacea, and F) P. bibronii. Outline color on images matches the range color on the map. Stars represent approximate collection locations. Ranges are reproduced from AmphibiaWeb
Immediately after collection, individuals were euthanized by cervical dissection and pithing (University of Mississippi IACUC #15-012 and Western Sydney University ACEC #A11490). Whole skins were stored in 4mL of 100% methanol in glass vials with PTFE caps (hereafter, referred to as methanol extracts). Stomachs were dissected and stored in 95% ethanol. Upon return from the field, skins were weighed and stored in -20°C and stomachs were stored in -80°C.
Alkaloid analysis
Alkaloids were extracted from each methanol extract using an acid-base fractionation as previously outlined (Saporito et al. 2010; Jeckel et al. 2015; Hovey et al. 2018). In brief, 50 µL of 1N HCl and an internal nicotine standard was added to 1mL of each methanol extract. The sample was then mixed and evaporated to approximately 100 µL using nitrogen gas. The concentrated sample was diluted with 200 µL of deionized water. Samples were then extracted four times, each with 300 µL of hexane. The remaining aqueous layer was basified with saturated sodium bicarbonate, followed by extraction three times, each with 300 µL of ethyl acetate. The organic layer was dried using anhydrous sodium sulfate and carefully evaporated to dryness using nitrogen gas. Final alkaloid fractions were reconstituted in 100 µL of methanol and stored in glass LVI vials with Teflon-lined septa at − 20°C until alkaloid analysis. To determine the identity and quantity of each alkaloid present in the samples, gas chromatography-mass spectrometry (GC-MS) analysis was performed on a Varian Saturn 2100 T ion trap MS coupled to a Varian 3900 GC with a 30 m × 0.25 mm ID Varian FactorFour VF-5 ms fused silica column. GC separation was achieved using a temperature program that increased from 100°C to 280°C at a rate of 10°C per minute with helium as the carrier gas (flow rate of 1 mL/min). Each alkaloid sample was analyzed using both electron impact mass spectrometry (EI-MS) and chemical ionization mass spectrometry (CI-MS), with methanol as the CI reagent.
Most anuran alkaloids have been assigned code names with a boldfaced number corresponding to their molecular weight and a boldfaced letter to distinguish alkaloids with the same nominal mass (see Daly et al. 2005 for discussion on nomenclature). Alkaloid identification was conducted by comparing GC retention times and mass spectral properties (e.g., diagnostic ion fragments) for each alkaloid with previously established data on frog alkaloids (Daly et al. 2005; additional citations in Hovey et al. 2018; Saporito, unpublished data). Each sample was analyzed in three chromatographic replicates, and the average quantity of alkaloid was determined by comparing alkaloid peak area to that of the nicotine internal standard. Following the methods of previous studies (Bolton et al. 2017; Hovey et al. 2018; Lawrence et al. 2019), only alkaloids that were present in quantities ≥ 0.3 µg were included in the analyses.
Statistical analysis
We confirmed our data were normally distributed and that variance was equal using a Shapiro-Wilk and a Levene’s test, respectively (p > 0.05). To determine if there is a relationship between frog body size (measured as wet skin mass) and total alkaloid quantity, dietary alkaloid quantity, and synthesized alkaloid quantity, we used linear regression. To test for differences in alkaloid quantity among species, we used a one-way analysis of variance (ANOVA) followed by Tukey HSD pair-wise comparisons. All univariate statistical analyses and graphs were constructed using GraphPad Prism, version 9.5.1 for MacOS. Non-metric multidimensional scaling (nMDS) was used to visualize and compare alkaloid composition (a combined measure of richness, type, and quantity) within and among species, and a one-way analysis of similarity (ANOSIM) followed by pairwise comparisons was used to test for differences. Both nMDS and ANOSIM were based on Bray-Curtis similarities, and were performed in PRIMER-E, version 6.
Diet analysis
We examined stomach contents from individual frogs using a stereo dissecting microscope (Olympus SZ61) coupled with a digital camera (Olympus EP50). Images were processed using the app EPview, version 1.4 for Windows. Stomachs were dissected from each frog by separating it from the esophagus and small intestine. A long incision was made along one side of the stomach, and the dietary contents were flushed into a petri dish using a disposable pipette and 95% ethanol. The dietary contents were photographed for identification of the type and number of invertebrates present. Grid paper (dimensions: 5.08mm by 5.08mm) was placed under the petri dish to be used as a scale for the size of the invertebrates. Ants and mites are common dietary sources of defensive alkaloids in poison frogs (Saporito et al. 2012), therefore we identified invertebrates as ants, mites, or other. To avoid duplicates, we only counted complete bodies (mostly mites) or heads (ants). Ants, in particular, would break apart in stomachs, so to be conservative in our counts, we would count heads but not other parts of the body. After all stomach contents were photographed, the dietary contents were stored in individually labeled vials containing 95% ethanol.
Results
Alkaloid analysis
A total of 19 unique alkaloids (including isomers), representing five different structural classes, were detected in 36 individual skins of Pseudophryne examined in the present study. The average quantity for each alkaloid, organized by structural class and frog species are reported in Table 1. Based on similarities in mass spectral data to previously characterized alkaloids (Daly et al. 2005), we identified one tentatively new alkaloid of unclassified structure with a molecular weight of 247, and its GC retention time and mass spectral data are reported in Supplementary Fig. 1. The majority of alkaloids detected in Pseudophryne were the biosynthesized pseudophrynamine and dietary derived pumiliotoxin alkaloids. The presence and quantity of each of these classes of alkaloids varied among species (Figs. 2, 3).
Average quantity of dietary and pseudophrynamine alkaloids among the six species of Pseudophryne (+/- 1 S.E.). Note: the y-axis is split due to the large differences in alkaloid quantity among species and in particular, to allow for better visualization of the small alkaloid quantities in P. occidentalis and P. dendyi
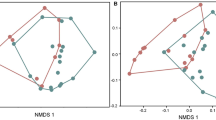
There is no relationship between frog skin mass and total alkaloid quantity (F1,34 = 0.098, p = 0.757), dietary alkaloid quantity (F1,34 = 1.93, p = 0.174), and synthesized alkaloid quantity (F1,34 = 3.62, p = 0.066). Average total alkaloid quantity varied significantly among species (F5,30 = 6.63, p < 0.001), with P. dendyi and P. coriacea possessing an average of 12.9x more alkaloid than P. guentheri and P. occidentalis (p < 0.01 in all comparisons; Fig. 4). Alkaloid composition was significantly different among species (Global R = 0.632; p = 0.001; Fig. 5). However, pairwise comparisons among species found no difference in alkaloid composition between P. guentheri and P. occidentalis (Global R = 0.011; p = 0.325) and P. occidentalis and P. bibronii (Global R = 0.241; p = 0.143).
Diet analysis
We dissected stomachs of the same individuals used in the alkaloid analyses. We observed that the stomachs were largely empty, containing mostly soil and few partially digested invertebrate parts such as heads and limbs (Supplementary Fig. 2). Despite this, there were some intact, identifiable invertebrates including mites, albeit in low quantities. Among the identifiable invertebrates, ants and mites were among the most common (Table 2). There were several invertebrates which were apparently intact, but not known to be important in alkaloid sequestration for frogs, such as worms (e.g., nematodes; Supplementary Fig. 2). Notably, we only had three individuals of P. bibronii in our sampling, none of which had identifiable stomach contents (Table 2). Whether this is an artifact of sampling or a real phenomenon is difficult to ascertain, so we would encourage caution in interpretation of results with regard to this species.
Discussion
Poison frogs in the genus Pseudophryne are chemically defended by dietarily-derived (mostly pumiliotoxins) and biosynthesized pseudophrynamine alkaloids. In the present study, we found that P. dendyi and P. bibronii contained larger quantities of dietary alkaloids as compared to pseudophrynamines, whereas P. guentheri, P. occidentalis, P. semimarmorata, and P. coriacea possessed larger quantities of pseudophrynamines as compared to dietary alkaloids (Figs. 2 and 3). Our findings are consistent with previous studies (Daly et al. 1990; Smith et al. 2002) and provide further evidence of a reciprocal relationship between dietary and frog-synthesized alkaloids in Pseudophryne. The direction of the relationship is identical to previous studies for P. guentheri, P. occidentalis, and P. coriacea (Daly et al. 1990; Smith et al. 2002). However, the higher abundance of dietary alkaloids in P. bibroni is opposite to that of Daly et al. (1990), and the greater quantity of pseudophrynamines in wild-caught P. semimarmorata is opposite to that of Smith et al. (2002). The observed differences in directionality between the present and previous studies are likely due to spatial and temporal variation in the availability of alkaloid-containing arthropods (see further discussion below), and remain consistent with the existence of a trade-off between dietary and synthesized defense types in Pseudophryne.
Previous studies have found that species containing larger quantities of dietary alkaloids typically contain lower quantities of pseudophrynamines and vice versa (Daly et al. 1990; Smith et al. 2002). The results of the present study align with these findings (Figs. 2 and 3), and interestingly, seem to show a tradeoff in diversity as well (though, this pattern is less clear; Table1). Moreover, Smith et al. (2002) provided evidence that accumulation of high quantities of dietary pumiliotoxins may turn-off the biosynthesis of pseudophrynamines in P. semimarmorata. Collectively, these findings suggest that the abundance of biosynthesized pseudophrynamines are dependent on the availability of dietary alkaloids. Variation in dietary alkaloids is common among poison frogs and is thought largely to result from spatial and temporal differences in arthropods (Saporito et al. 2007b; McGugan et al. 2016; Moskowitz et al. 2020; Basham et al. 2021). It is therefore plausible that changes in availability of dietary alkaloids could lead to differences in the production of pseudophrynamines, something that would be expected to change over the course of a lifetime. For example, in the present study, P. dendyi contained significantly higher quantities of dietary alkaloids compared to pseudophrynamines, suggesting the availability of alkaloid-containing arthropods may have limited the production of pseudophrynamines (Fig. 3). Alternatively, P. coriacea had greater quantities of pseudophrynamines than dietary alkaloids, possibly as a result of limited arthropod availability (Fig. 3). Our dietary results suggest that feeding is reduced during the breeding season, which would limit the availability of dietary alkaloids during this time period, at least among likely males. The feeding habits of females warrants further inquiry. Upregulation of pseudophrynamines in the absence of dietary alkaloids may have evolved as an adaptation to maintain defenses during a particularly vulnerable period. Jeckel et al. (2015) found correlative evidence that the bufonid poison frog, Melanophryniscus moreirae, may regulate bufotenine production in relation to sequestered dietary alkaloids. It is interesting to note that both bufonid and myobatrachid poison frogs have punctuated seasonal reproductive (breeding) events (Vaira 2005; Dos Santos et al. 2011; Santos and Grant 2011), which could be associated with the ability to both produce and sequester alkaloids defenses. Our study suggests that a reciprocal relationship between dietary and biosynthesized alkaloids exists across Pseudophryne. However, experimental studies will be needed to understand whether these patterns exist across the lifecycle of these frogs.
Prior studies have reported numerous chemicals other than alkaloids in the skin secretions of poison frogs, including Pseudophryne. Although the function of these compounds are largely unknown, it is possible that some of them serve a defensive role and may exhibit tradeoffs with dietary alkaloid defenses. For example, bufonid poison frogs in the genus Melanophryniscus synthesize bufadienolide-like compounds (Flier et al. 1980; Daly et al. 2008 but see Mebs et al. 2007b), several indolealkylamines, including bufotenine (Cei et al. 1968; Daly et al. 1987; Erspamer 1994; Mebs et al. 2007a; Jeckel et al. 2015), and the phenol hydroquinone (Mebs et al. 2005, 2007a). And in fact, a potential tradeoff between bufotenine synthesis and dietary alkaloids has been suggested (Jeckel et al. 2015), but will require further study. Further, the biosynthesized indolealkylamine, 5-hydroxytryptamine, has been reported in the skin of Pseudophryne (Erspamer 1994), but was not detected in the present study. Amidines, the peptide carnosine, and trace levels of bufadienolide-like compounds have been detected in the skin of some dendrobatids (Daly et al. 1987). More recently, several deltorphin peptides, bufagenins, bufotenines, and bufadienolides have been putatively identified in the dendrobatid Phyllobates vittatus (Protti-Sánchez et al. 2019), and several volatiles, most notably, methylpyridines, benzothiazoles, N-alkylpyrrolidines, pyrazines, and sesquiterpenoids have been identified in the dendrobatid Silverstoneia punctiventris (Gonzalez et al. 2021). Despite numerous examples of these biologically active chemicals present in skin secretions, our understanding of their role in poison frog biology (e.g., anti-predator defenses, pheromones) is lacking and warrants further investigation, and in particular, how they relate to the presence of dietary alkaloids.
Alkaloid composition is known to have important consequences for predator response, particularly when examining among-population variation (Lawrence et al. 2023). When examining total alkaloid quantities among species, we observed that eastern species (P. semimarmorata, P. bibronii, P. dendyi, and P. coriacea) had relatively high alkaloid quantities, while western species (P. guentheri and P. occidentalis) had relatively low alkaloid quantities (Fig. 4). Overall, this is consistent with Daly et al. (1990), who reported similar findings. Interestingly, eastern and western clades are monophyletic (Donnellan et al. 2012), possibly suggesting that the observed differences in quantity of alkaloids among species might have phylogenetic origins. Furthermore, eastern species are comparatively more conspicuous in color than western species (Fig. 1). Whether there is a relationship between color and alkaloid quantity has yet to be determined, but our data suggest that there may be a color-alkaloid relationship in which more conspicuous species possess larger quantities of alkaloid (Blount et al. 2009; Summers et al. 2015). Additional studies will be necessary to further understand the potential importance of phylogeny and color on the evolution of dietary and biosynthesized defenses in Pseudophryne.
We sought to examine the stomach contents among Pseudophryne species in an attempt to determine whether the presence or absence of pumiliotoxins (and other dietary alkaloids) could be explained by what the frogs were eating. The stomachs were largely empty, probably because they were caught close to the breeding season, particularly when compared to other species of poison frogs which derive defensive alkaloids from diet (e.g., dendrobatids; Valderrama-Vernaza et al. 2009; McGugan et al. 2016; Martínez et al. 2019; Pacheco et al. 2021). When frogs had contents in their stomachs, they were often highly digested and not identifiable via morphological means (Supplementary Fig. 2). However, among the most distinguishable invertebrates in the stomachs were ants and mites, both of which are known sources of dietary alkaloids in dendrobatids (Saporito et al. 2004, 2007a; Takada et al. 2005). We caution, though, against inferences about mite/ant specialization from Table 2 given the high degree of digestion across all Pseudophryne in our study. Given this, it is unsurprising that high ant and mite quantities (known alkaloid sources) identified from our Pseudophryne sampled in their breeding system (Table 2) do not appear to predict dietary alkaloid quantities or diversities. For instance, while P. coriacea has the highest mite and ant quantity and highest alkaloid quantities (Figure 4), this species mostly deploys the synthesized pseudophrynamines (Table 1). Furthermore, the species with the second highest ant quantity (P. guentheri) appears to rely exclusively on pseudophrynamines during the breeding season. These results suggest that Pseudophryne males eat minimally during the breeding season, but still remain chemically defended. However, it is important to note that diet is a single measure at one point in time and may not be representative of what a frog regularly consumes, whereas chemical defenses are the result of accumulation/production over the course of a lifetime, thereby reducing our ability to relate frog diet and alkaloids (Saporito et al. 2007b). Collectively, our results suggest that Pseudophryne males eat minimally during the breeding season, but still remain chemically defended with both dietary and synthesized alkaloids.
Taken together, our findings of alkaloids and diet in Pseudophryne suggest how natural history may have shaped their evolution of defenses. Namely, a dearth in diet-derived defenses could leave frogs vulnerable to predation during the breeding season and that, perhaps, this genus evolved the ability to produce alkaloids de novo (or never lost this ability) so that they remain defended through periods of scarcity. How Pseudophryne produce alkaloids de novo remains to be tested, but our results suggest that there may be interplay between defensive traits and breeding. Eastern species display putative aposematic coloration with most species showing axillary color patches (Lawrence et al. 2018), though some species also display conspicuous coloration on their heads (Fig. 1). The most extreme coloration is found in the Northern and Southern Corroboree Frogs (P. pengilleyi and P. corroboree, respectively). All species show some degree of conspicuous black-and-white ventral coloration, which may serve aposematic function as well (Lawrence et al. 2018). While frogs are secretive much of the year, during the breeding season, males will migrate to low lying areas that will flood (J. Lawrence, pers. obs.). Males will construct subterranean breeding chambers from which to call and attract females (Mitchell 2001). This period of breeding represents increased risk of predation to frogs. As aposematism requires a secondary defense to function (Mappes et al. 2005), this period of migration would require that individuals be chemically defended, otherwise, their conspicuous coloration could result in increased loss due to predation. The function of the coloration remains to be poorly understood as there is an apparent paradox of nocturnal, fossorial species having conspicuous coloration. Gallinaceous fowl, such as Australian Brushturkeys (Alectura lathami) or Lyrebirds (Menura novaehollandiae), were regularly sighted on our surveys. Such species forage by scratching up leaf litter, and it is plausible that they could unearth frogs in the process. In these instances, conspicuous coloration coupled with alkaloid defenses, could be to the frog’s benefit, though this needs to be tested. Our diet findings suggest that this is a period of reduced consumption of invertebrates that could provide defensive alkaloids. Thus, there is significant risk of their aposematic signals no longer being effective. We postulate that, perhaps, the ability to create alkaloids de novo evolved in response to this period of reduced invertebrate consumption. By being able to produce alkaloids when dietary sources are scarce or frogs are not eating, they can maintain chemical defenses that enable their putative aposematic signals to be effective.
Our results also have implications for conservation. Aposematic species which derive alkaloid defenses from their diet are at risk when they do not feed on alkaloid-containing invertebrates (such as in ex situ breeding programs). Many at-risk species are bred in captivity with the intention of reintroduction in the wild. These aposematic species pose the challenge that individuals may not be chemically defended for reintroduction, and with a naive predator community, may be quickly preyed upon. Some of the most endangered amphibians in the world are the Corroboree frogs of Australia (P. pengilleyi and P. corroboree). Considerable efforts have been made to breed these frogs in captivity for eventual reintroduction into the highlands of eastern Australia (Hunter et al. 2009; OEH NSW 2012; Skerratt et al. 2016; Rojahn et al. 2018). The conspicuous yellow-and-black coloration of these frogs could be a beacon to predators, warning them of alkaloid defenses (but see Umbers et al. 2019 which posits that this coloration could be cryptic). Our research, coupled with previous work (Daly et al. 1990; Smith et al. 2002), suggests that these frogs may be defended despite not being raised on diets containing alkaloid-producing invertebrates. Predators have the ability to quickly learn avoidance of conspicuous signals when coupled with unpalatable defenses (Lindström et al. 2001; Lawrence et al. 2019). Our results suggest that species in the genus Pseudophryne, such as the Corroboree frogs, will maintain alkaloid defenses regardless of presence of dietary sources of alkaloids. Thus, the reintroduced Corroboree frogs should not be at risk due to lack of defenses.
Notably, however, our research does not address unpalatability of pumiliotoxins or pseudophrynamines. Quantity of alkaloids is not always a good indicator of efficacy of defense (Lawrence et al. 2019), and thus we do not know whether pumiliotoxins and pseudophrynamines contained in these frogs are equivalently unpalatable (or toxic) to potential predators or effective against microbial infection (Hovey et al. 2018; Lawrence et al. 2019, 2023; Protti-Sánchez et al. 2019). The relative unpalatability of these alkaloids would provide important context for these results to understand how defenses change over the lifecycle of these frogs, and how those changes are perceived by predators or microbes. This research builds on what was previously known about defenses in Pseudophryne. We provide a possible explanation for why the ability to synthesize alkaloids evolved in this group of frogs, though this needs further testing. Our data suggest that there is a trade-off between diet-dependent and diet-independent chemical defenses produced by these poison frogs which may have evolved in response to reduced feeding.
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
References
Alvarez-Buylla A, Payne CY, Vidoudez C et al (2022) Molecular physiology of pumiliotoxin sequestration in a poison frog. PLOS ONE 17:e0264540. https://doi.org/10.1371/journal.pone.0264540
Barker J, Grigg G, Tyler M (1995) A field guide to Australian frogs. Surrey Beatty 5:69
Basham EW, Saporito RA, González-Pinzón M et al (2021) Chemical defenses shift with the seasonal vertical migration of a Panamanian poison frog. Biotropica 53:28–37. https://doi.org/10.1111/btp.12842
Blount JD, Speed MP, Ruxton GD, Stephens PA (2009) Warning displays may function as honest signals of toxicity. Proc Biol Sci R Soc 276:871–7. https://doi.org/10.1098/rspb.2008.1407
Bolton SK, Dickerson K, Saporito RA (2017) Variable alkaloid defenses in the dendrobatid poison frog Oophaga pumilio are perceived as differences in palatability to arthropods. J Chem Ecol 43:273. https://doi.org/10.1007/s10886-017-0827-y
Bonansea M, Vaira M (2012) Geographic and intrapopulational variation in colour and patterns of an aposematic toad, Melanophryniscus rubriventris (Amphibia, Anura, Bufonidae). Amphibia-Reptilia 33:11–24. https://doi.org/10.1163/156853811X619754
Brunetti AE, Merib J, Carasek E et al (2015) Frog volatile compounds: application of in vivo SPME for the characterization of the odorous Secretions from two species of Hypsiboas treefrogs. J Chem Ecol 41:360–372. https://doi.org/10.1007/s10886-015-0564-z
Byrne PG, Silla AJ (2023) Lifetime breeding-site and nest-site fidelity in a declining terrestrial toadlet: evidence for a win-stay/lose-shift strategy. Front Conserv Sci 4:52
Cei JM, Erspamer V, Roseghini M (1968) Taxonomic and evolutionary significance of biogenic amines and polypeptides in amphibian skin. II. Toads of the genera Bufo and Melanophryniscus. Systematic Zoology 17:232–245. https://doi.org/10.2307/2412002
Conlon JM, Mechkarska M, Ahmed E et al (2011) Purification and properties of antimicrobial peptides from skin secretions of the Eritrea clawed frog Xenopus clivii (Pipidae). Comp Biochem Physiol Part C Toxicol Pharmacol 153:350–354. https://doi.org/10.1016/j.cbpc.2010.12.007
Conlon JM, Mechkarska M, Ahmed E et al (2011) Host defense peptides in skin secretions of the Oregon spotted frog Rana pretiosa: implications for species resistance to chytridiomycosis. Dev Comp Immunol 35:644–649. https://doi.org/10.1016/j.dci.2011.01.017
Daly JW, Myers CW, Whittaker N (1987) Further classification of skin alkaloids from Neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the Amphibia. Toxicon 25:1023–1095
Daly JW, Garraffo HM, Pannell LK et al (1990) Alkaloids from Australian frogs (Myobatrachidae): Pseudophrynamines and Pumiliotoxins. J Nat Prod 53:407–421. https://doi.org/10.1021/np50068a020
Daly JW, Secunda SI, Garraffo HM et al (1994) An uptake system for dietary alkaloids in poison frogs (Dendrobatidae). Toxicon 32:657–663
Daly JW, Garraffo HM, Jain P et al (2000) Arthropod-frog connection: decahydroquinoline and pyrrolizidine alkaloids common to microsympatric myrmicine ants and dendrobatid frogs. J Chem Ecol 26:73–85. https://doi.org/10.1023/A:1005437427326
Daly JW, Garraffo HM, Spande TF et al (2003) Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus Dendrobates. Proc Natl Acad Sci USA 100:11092–7. https://doi.org/10.1073/pnas.1834430100
Daly JW, Noimai N, Kongkathip B et al (2004) Biologically active substances from amphibians: preliminary studies on anurans from twenty-one genera of Thailand. Toxicon 44:805–815. https://doi.org/10.1016/j.toxicon.2004.08.016
Daly JW, Spande TF, Garraffo HM (2005) Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J Nat Prod 68:1556–1575
Daly JW, Wilham JM, Spande TF et al (2007) Alkaloids in bufonid toads (Melanophryniscus): temporal and geographic determinants for two Argentinian species. J Chem Ecol 33:871–887. https://doi.org/10.1007/s10886-007-9261-x
Daly JW, Garraffo HM, Spande TF et al (2008) Individual and geographic variation of skin alkaloids in three swamp-forest species of Madagascan poison frogs (Mantella). J Chem Ecol 34:252–279. https://doi.org/10.1007/s10886-015-0616-4
Daly JW, Ware N, Saporito RA et al (2009) N-Methyldecahydroquinolines: an unexpected class of alkaloids from Amazonian poison frogs (Dendrobatidae). J Nat Prod 72:1110–1114. https://doi.org/10.1021/np900094v
Donnellan SC, Mahony MJ, Bertozzi T (2012) A new species of Pseudophryne (Anura: Myobatrachidae) from the central Australian ranges. Zootaxa 3476:69–85
Dos Santos TG, Maneyro R, Cechin SZ, Haddad CFB (2011) Breeding habitat and natural history notes of the toad Melanophryniscus pachyrhynus (Miranda-Ribeiro, 1920) (Anura, Bufonidae) in southern Brazil. Herpetol Bull 116:15–18
Erspamer V (1994) Bioactive secretions of the amphibian integument. In: Amphibian Biology. pp 178–350.
Flier J, Edwards MW, Daly JW, Myers CW (1980) Widespread occurrence in frogs and toads of skin compounds interacting with the Ouabain site of Na+, K+-ATPase. Science 208:503–505. https://doi.org/10.1126/science.6245447
Garraffo HM, Andriamaharavo NR, Vaira M et al (2012) Alkaloids from single skins of the Argentinian toad Melanophryniscus rubriventris (ANURA, BUFONIDAE): An unexpected variability in alkaloid profiles and a profusion of new structures. SpringerPlus 1:51. https://doi.org/10.1186/2193-1801-1-51
Gonzalez M, Palacios-Rodriguez P, Hernandez-Restrepo J et al (2021) First characterization of toxic alkaloids and volatile organic compounds (VOCs) in the cryptic dendrobatid Silverstoneia punctiventris. Front Zool 18:39. https://doi.org/10.1186/s12983-021-00420-1
Hovey KJ, Seiter EM, Johnson EE, Saporito RA (2018) Sequestered alkaloid defenses in the dendrobatid poison frog Oophaga pumilio provide variable protection from microbial pathogens. J Chem Ecol 44:312–325. https://doi.org/10.1007/s10886-018-0930-8
Hunter D, Osborne W, Smith M, McDougall K (2009) Breeding habitat use and the future management of the critically endangered Southern Corroboree Frog. Ecol Manag Restor 10:S103–S109. https://doi.org/10.1111/j.1442-8903.2009.00461.x
Jeckel AM, Bolton SK, Waters KR et al (2022) Dose-dependent alkaloid sequestration and N-methylation of decahydroquinoline in poison frogs. J Exp Zool Part A Ecol Integr Physiol 337:537–546. https://doi.org/10.1002/jez.2587
Jeckel AM, Saporito RA, Grant T (2015) The relationship between poison frog chemical defenses and age, body size, and sex. Front Zool 12:27. https://doi.org/10.1186/s12983-015-0120-2
Jones TH, Gorman JST, Snelling RR et al (1999) Further alkaloids common to ants and frogs: Decahydroquinolines and a Quinolizidine. J Chem Ecol 25:1179–1193
Lawrence J, Rojas B, Blanchette A et al (2023) Linking predator responses to alkaloid variability in poison frogs. J Chem Ecol 5:49. https://doi.org/10.1007/s10886-023-01412-7
Lawrence JP, Mahony M, Noonan BP (2018) Differential responses of avian and mammalian predators to phenotypic variation in Australian Brood Frogs. PLoS ONE 13:e0195446
Lawrence JP, Rojas B, Fouquet A et al (2019) Weak warning signals can persist in the absense of gene flow. Proc Natl Acad Sci 116:19037–19045. https://doi.org/10.1073/pnas.1901872116
Lindström L, Alatalo RV, Lyytinen A, Mappes J (2001) Strong antiapostatic selection against novel rare aposematic prey. Proc Natl Acad Sci USA 98:9181–9184. https://doi.org/10.1073/pnas.161071598
Mappes J, Marples N, Endler JA (2005) The complex business of survival by aposematism. Trends Ecol Evol 20:598–603. https://doi.org/10.1016/j.tree.2005.07.011
Martínez MM, Ortega MSC, Lopera JMH, Morales JAR (2019) Diet of the yellow striped poison frog, Dendrobates truncatus (Cope, 1861) (Anura: Dendrobatidae) from the Middle Magdalena river valley, Colombia. Herpetol Notes 12:1185–1191
McFadden M, Harlow P, Kozlowski S, Purcell D (2010) Toe-twitching during feeding in the Australian Myobatrachid frog, Pseudophryne corroboree. Herpetol Rev 41:153–154
McGugan JR, Byrd GD, Roland AB et al (2016) Ant and mite diversity drives toxin variation in the Little Devil Poison Frog. J Chem Ecol 42:537–551. https://doi.org/10.1007/s10886-016-0715-x
Mebs D, Maneyro R, Pogoda W (2007) Further studies on pumiliotoxin 251D and hydroquinone content of the skin secretion of Melanophryniscus species (Anura, Bufonidae) from Uruguay. Toxicon 50:166–169. https://doi.org/10.1016/j.toxicon.2007.02.017
Mebs D, Pogoda W, Maneyro R, Kwet A (2005) Studies on the poisonous skin secretion of individual red bellied toads, Melanophryniscus montevidensis (Anura, Bufonidae), from Uruguay. Toxicon 46:641–650. https://doi.org/10.1016/j.toxicon.2005.07.004
Mebs D, Wagner MG, Pogoda W et al (2007) Lack of bufadienolides in the skin secretion of red bellied toads, Melanophryniscus spp. (Anura, Bufonidae), from Uruguay. Compar Biochem Physiol Part C Toxicol Pharmacol 144:398–402. https://doi.org/10.1016/j.cbpc.2006.11.009
Mitchell NJ (2001) Males call more from wetter nests: effects of substrate water potential on reproductive behaviours of terrestrial toadlets. Proc R Soc Lond B 268:87–93. https://doi.org/10.1098/rspb.2000.1334
Moskowitz NA, Dorritie B, Fay T et al (2020) Land use impacts poison frog chemical defenses through changes in leaf litter ant communities. Neotrop Biodiver 6:75–87
OEH NSW (2012) National recovery plan for the Southern Corroboree Frog Pseudophryne corroboree and Northern Corroboree Frog Pseudophryne pengilleyi. Office of Environment and Heritage (NSW)
Pacheco EO, Ceron K, Akieda PS, Santana DJ (2021) Diet and morphometry of two poison frog species (Anura, Dendrobatidae) from the plateaus surrounding the Pantanal of Mato Grosso do Sul state, Brazil. Stud Neotrop Fauna Environ 56:99–107. https://doi.org/10.1080/01650521.2020.1746098
Pengilley RK (1973) Breeding biology of some species of Pseudophryne (Anura: Leptodactylidae) of the Southern Highlands. New South Wales Aust Zool 18:15–30
Protti-Sánchez F, Quirós-Guerrero L, Vásquez V et al (2019) Toxicity and alkaloid profiling of the skin of the Golfo Dulcean Poison Frog Phyllobates vittatus (Dendrobatidae). J Chem Ecol 45:914–925. https://doi.org/10.1007/s10886-019-01116-x
Roberts SM, Stuart-Fox D, Medina I (2022) The evolution of conspicuousness in frogs: When to signal toxicity? J Evol Biol 35:1455–1464. https://doi.org/10.1111/jeb.14092
Rodríguez A, Poth D, Schulz S, Vences M (2011) Discovery of skin alkaloids in a miniaturized eleutherodactylid frog from Cuba. Biol Lett 7:414–418. https://doi.org/10.1098/rsbl.2010.0844
Rojahn J, Gleeson D, Furlan EM et al (2018) Monitoring post-release survival of the northern corroboree frog, Pseudophryne pengilleyi, using environmental DNA. Wildlife Res 45:620–626. https://doi.org/10.1071/WR17179
Santos JC, Coloma LA, Cannatella DC (2003) Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc Natl Acad Sci USA 100:12792–12797. https://doi.org/10.1073/pnas.2133521100
Santos RR, Grant T (2011) Diel pattern of migration in a poisonous toad from Brazil and the evolution of chemical defenses in diurnal amphibians. Evolut Ecol 25:249–258. https://doi.org/10.1007/s10682-010-9407-0
Saporito RA, Donnelly MA, Jain P et al (2007) Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon 50:757–778
Saporito RA, Donnelly MA, Madden AA et al (2010) Sex-related differences in alkaloid chemical defenses of the dendrobatid frog Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. J Nat Prod 73:317–21. https://doi.org/10.1021/np900702d
Saporito RA, Donnelly MA, Norton RA et al (2007) Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc Nat Acad Sci USA 104:8885–90. https://doi.org/10.1073/pnas.0702851104
Saporito RA, Donnelly MA, Spande TF, Garraffo HM (2012) A review of chemical ecology in poison frogs. Chemoecology 22:159–168. https://doi.org/10.1007/s00049-011-0088-0
Saporito RA, Spande TF, Martin GH, A. Donnelly M, (2009) Arthropod alkaloids in poison frogs: a review of the ‘Dietary Hypothesis.’ Heterocycles 79:277. https://doi.org/10.3987/REV-08-SR(D)11
Saporito RA, Garraffo HM, Donnelly MA et al (2004) Formicine ants: an arthropod source for the pumiliotoxin alkaloids of dendrobatid poison frogs. Proc Natl Acad Sci USA 101:8045–50. https://doi.org/10.1073/pnas.0402365101
Saporito RA, Norton RA, Garraffo MH, Spande TF (2015) Taxonomic distribution of defensive alkaloids in Nearctic oribatid mites (Acari, Oribatida). Exp Appl Acarol 67:317–333. https://doi.org/10.1007/s10493-015-9962-8
Skerratt LF, Berger L, Clemann N et al (2016) Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildlife Res 43:105. https://doi.org/10.1071/WR15071
Smith BP, Tyler MJ, Kaneko T et al (2002) Evidence for biosynthesis of pseudophrynamine alkaloids by an Australian myobatrachid frog (Pseudophryne) and for sequestration of dietary pumiliotoxins. J Nat Prod 65:439–447
Summers K, Speed MP, Blount JD, Stuckert AMM (2015) Are aposematic signals honest? A review. J Evol Biol 28:1583–1599. https://doi.org/10.1111/jeb.12676
Takada W, Sakata T, Shimano S et al (2005) Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J Chem Ecol 31:2403–2415. https://doi.org/10.1007/s10886-005-7109-9
Toledo RC, Jared C (1995) Cutaneous granular glands and amphibian venoms. Comp Biochem Physiol Part A Physiol 111:1–29. https://doi.org/10.1016/0300-9629(95)98515-I
Umbers KDL, Riley JL, Kelly MBJ et al (2019) Educating the enemy: Harnessing learned avoidance behavior in wild predators to increase survival of reintroduced southern corroboree frogs. Conserv Sci Pract 5:139. https://doi.org/10.1111/csp2.139
Vaira M (2005) Annual variation of breeding patterns of the toad, Melanophryniscus rubriventris (Vellard, 1947). Amphibia-Reptilia 26:193–199. https://doi.org/10.1163/1568538054253519
Valderrama-Vernaza M, Ramírez-Pinilla MP, VíH Serrano-Cardozo (2009) Diet of the Andean Frog Ranitomeya virolinensis (Athesphatanura: Dendrobatidae). J Herpetol 43:114–123. https://doi.org/10.1670/07-247R1.1
Williams CR, Brodie ED, Tyler MJ, Walker SJ (2000) Antipredator mechanisms of Australian frogs. J Herpetol 34:431–443
Acknowledgements
We value DEI efforts and took this into account in completing this work. Our team includes representatives from three universities from two countries, as well as established researchers and undergraduate authors. We would like to thank P. Byrne and S. Keogh for discussions about this research, N. Mitchell and S. Macdonald for their assistance in Western Australia, as well as B. Symula and N. Clemann for assistance in eastern Australia. This work was supported by the Endeavour Fellowship to JPL offered by the Australian Department of Education and Training (ERF RDDH 151726 2015). Animals were collected under permits for New South Wales (SL101733), Victoria (10007622), and Western Australia (SF010838) with IACUC Approval 15-012 from the University of Mississippi and ACEC (A11490) from Western Sydney University.
Funding
JPL was supported by the Endeavour Fellowship offered by the Australian Department of Education and Training (ERF RDDH 151726 2015).
Author information
Authors and Affiliations
Contributions
MS and JPL wrote the first draft of the manuscript, and all authors participated in the revision of this draft. JPL and RAS worked on the final version of the manuscript. MS and LP developed the diet analysis protocol, and MS dissected and documented stomach contents. VD and RAS performed the alkaloid analyses among frogs and analyzed patterns. JPL and KDLU developed the project. JPL collected the samples. All authors contributed to the final manuscript and approved of its publication.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable
Consent to participate
All co-authors have consented to participate in the research and manuscript publication.
Consent for publication
All co-authors have approved the manuscript for publication.
Ethics approval
Animals were collected under permits for New South Wales (SL101733), Victoria (10007622), and Western Australia (SF010838) with IACUC Approval 15-012 from the University of Mississippi and ACEC (A11490) from Western Sydney University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sague, M., Dudaitis, V., Plumert, L. et al. Alkaloid-based chemical defenses and diet in six species of Australian poison frogs in the genus Pseudophryne (Myobatrachidae). Evol Ecol (2023). https://doi.org/10.1007/s10682-023-10269-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10682-023-10269-x