Abstract
Dendrobatid poison frogs sequester alkaloids from an arthropod diet and use them in chemical defense. Alkaloid defenses vary considerably within and among species, with important consequences for the protection they can and do provide against microorganisms and predators. Most of this variation is attributed to differences in frog diet and prey availability, but emerging evidence also suggests that frogs differ in their physiological ability to sequester alkaloids. Epibatidines are one of the most geographically and phylogenetically restricted alkaloid classes in poison frogs, having been found naturally only in two genera of dendrobatids (Epipedobates and Ameerega) from Ecuador and northern Peru. To test the hypothesis that the ability to sequester epibatidine is confined to the lineages Epipedobates and Ameerega, we experimentally administered epibatidine to individuals of five species, representing three different lineages of dendrobatid poison frogs, including those known to possess (Epipedobates anthonyi) and lack (Ranitomeya variabilis, Ranitomeya imitator, Phyllobates vittatus, Dendrobates tinctorius) epibatidines in nature. All five species sequestered epibatidine; however, the percentage sequestered varied significantly across species with Epipedobates and Ranitomeya accumulating about 2.4× more than Phyllobates or Dendrobates. Our results suggest that the absence of epibatidine in certain dendrobatids is not due to the inability of these frogs to sequester epibatidine, but may instead result from differences in prey availability and/or dietary preference. Our finding of differences in the percentage of epibatidine sequestered among species points to the importance that physiological differences in sequestration play in explaining some of the alkaloid variation (including epibatidine) observed among dendrobatid poison frogs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkaloids are a diverse group of organic chemicals that vary in structure, the biosynthetic pathway by which they are produced, and pharmacological activity (Blum 1995; Cordell 1998). Often by virtue of their diverse biological effects, alkaloids afford protection against grazers, herbivores, parasites, pathogens, and predators, and their production and use has evolved in microorganisms, fungi, plants, and animals (Roberts and Wink 1998). The presence of these defensive alkaloids in biological communities drives myriad ecological and evolutionary patterns (Wink 1988; Ruxton et al. 2004; Adler et al. 2006; Opitz and Müller 2009; Trigo 2011). Most animals avoid consuming alkaloids and/or evolve mechanisms to prevent or counter their negative physiological effects (Blum 1995; Roberts and Wink 1998). Fewer, but taxonomically diverse, animals have evolved the ability to sequester alkaloids present in their diet and then use these chemicals to protect themselves (e.g., beetles, butterflies, frogs; Brückmann et al. 2000; Nishida 2002; Pasteels and Hartmann 2004; Saporito et al. 2012). The resulting alkaloid defenses of these consumers vary considerably among and even within species, and this variation can lead to differences in protection against predators and pathogens (Hartmann et al. 2001; Hovey et al. 2018; Lawrence et al. 2019). Two non-exclusive mechanisms might explain such among- and within-species variation: (i) consumers may vary in their ability to sequester alkaloids and (ii) variation in alkaloid defenses might reflect differences in diet and/or prey availability.
A well-studied group of animals that sequesters dietary alkaloids is the dendrobatid poison frogs. More than 600 alkaloids from about 24 unique structural classes have been identified in this lineage (Daly et al. 2005; Saporito et al. 2012; Santos et al. 2016; Hovey et al. 2018; Saporito unpub data), most of which are sequestered unchanged from the mites and ants the frogs consume (Daly et al. 2000a; Takada et al. 2005; Saporito et al. 2007a, 2009, 2015). The conspicuous coloration of these charismatic frogs is hypothesized to have co-evolved with the alkaloid-based chemical defenses (Santos et al. 2003) that provide protection against predators and microorganisms (Mina et al. 2015, Murray et al. 2016; Schulte et al. 2016; Bolton et al. 2017; Hovey et al. 2018; Lawrence et al. 2019, 2023). This pairing of chemical defense with visual signals is hypothesized to have, in turn, driven and/or facilitated the evolution of traits like complex parental care (Carvajal-Castro et al. 2021) and perhaps even contributed to reproductive isolation among newly diverged lineages (Summers and Tumulty 2014; but see Yang et al. 2016). However, alkaloid defenses differ significantly across lineages of poison frogs, which is a major hurdle to understanding the causes and consequences of variation in chemical defense. In particular, the proximate mechanisms underlying among-lineage differences remain largely unclear (Basham et al. 2020; Moskowitz et al. 2020; Alvarez-Buylla et al. 2022; Jeckel et al. 2022). Differences in sequestration ability and diet are both viable hypotheses with different implications for how we might expect defense (and its physiological underpinnings) to evolve and co-evolve with predators and other selective pressures.
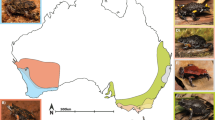
Within Dendrobatidae, alkaloid defenses are present in frogs of the tribe Dendrobatini as well as the genera Ameerega, Epipedobates, Paruwrobates, Phyllobates, and Silverstoneia (Santos et al. 2003; Grant et al. 2006, 2017; Santos and Cannatella 2011; Gonzalez et al. 2021; see Fig. 1). Alkaloid richness and quantity vary considerably within and among species, and most of this variation has been attributed to differences in frog diet and/or prey availability (Daly et al. 1992; Saporito et al. 2007b; McGugan et al. 2016; Moskowitz et al. 2020; Basham et al. 2020). Despite this substantial variation, alkaloids from most of the 24 described classes are present in all lineages of alkaloid-containing dendrobatids (see summary tables in Saporito et al. 2009, 2012; Santos et al. 2016; Grant et al. 2017), suggesting that the physiological ability to sequester different lipophilic alkaloids is shared among lineages and that alkaloid-containing arthropods are geographically widespread. However, several alkaloid classes have only been detected in a few species, raising the possibility that differences in ability to sequester these alkaloids is highly restricted phylogenetically and/or that some dietary sources are highly restricted geographically.
Phylogenetic distribution of alkaloid sequestration in Dendrobatidae. Summary phylogeny based on Grant et al. (2017) and Marin et al. (2018). Red indicates genera in which at least one species is known to possess lipophilic alkaloids. Asterisks indicate the genera sampled in the current study. Photo of Paruwrobates erythromos by Santiago R. Ron, courtesy of FaunaWebEcuador, creative commons license CC BY-NC 4.0; all other photos by TG
One of the most geographically and phylogenetically limited structural classes are the epibatidines, a class of pyridinic alkaloids that act as agonists of nicotinic acetylcholine receptors (nAChRs), one of which, referred to generally as epibatidine, is a potent non-opioid analgesic 200 times stronger than morphine (Badio and Daly 1994; Spande et al. 1992). Epibatidine has only been found naturally in Epipedobates and Ameerega (both in Colostethinae; Grant et al. 2006, 2017) and a dietary arthropod source is yet to be identified. Specifically, Daly et al. (1978) found that skin extracts from three species of poison frogs contained trace amounts of epibatidine, including the two trans-Andean species E. espinosai from northern Ecuador and E. anthonyi from southern Ecuador, and a cis-Andean species reported as Dendrobates pictus from central Peru (possibly A. petersi or an undescribed species; for locality see Daly et al. 1987; for current taxonomy of Ameerega see Guillory et al. 2020). Later, in 1974 and 1979, Daly et al. (1980) reported epibatidine (cited as trace alkaloids from frog skins) in two populations of E. anthonyi (reported as Dendrobates tricolor) from Santa Isabella, Ecuador (alkaloid identification described in Spande et al. 1992, footnotes 1, 14; for localities, see Daly et al. 1987, 1998). On the basis of these studies, epibatidine appears to be restricted to these two genera of colostethine frogs in Ecuador and northern Peru, which suggests either that the ability to sequester epibatidine is confined to these lineages or that the dietary source for epibatidine is unavailable or not consumed by frogs that lack them in nature.
Among dendrobatids, the ability to sequester alkaloids appears to have evolved either once with multiple independent losses or multiple times independently (see Fig. 1; Santos et al. 2003; Santos and Cannatella 2011; Grant et al. 2017; Gonzalez et al. 2021). To test the hypothesis that the ability to sequester epibatidine is confined to the lineages Epipedobates and Ameerega, we experimentally administered epibatidine via diet manipulation to individuals of five dendrobatid species. These species represented three lineages of dendrobatid poison frogs and included species known to either possess or lack epibatidines in nature. Specifically, we tested if epibatidine can be sequestered by E. anthonyi, known to sequester epibatidine in nature, and R. variabilis, R. imitator, P. vittatus, and D. tinctorius, none of which have been shown to contain epibatidine in nature (Daly 1998; Saporito et al. 2012; Santos et al. 2016; Grant et al. 2017). By offering animals a known quantity of epibatidine, we were also able to ask whether accumulation efficiency of epibatidine differs among these species.
Methods
Study animals
We compared epibatidine sequestration across dendrobatid lineages (Fig. 1; Grant et al. 2017) using individuals of five species—Epipedobates anthonyi (n = 3 experimental + 1 control), Ranitomeya variabilis (n = 4 experimental + 1 control), R. imitator (n = 5 experimental + 1 control), Phyllobates vittatus (n = 5 experimental + 1 control), and Dendrobates tinctorius (n = 5 experimental + 1 control). Experimental frogs were administered an ethanol/epibatidine solution, whereas control frogs were administered an ethanol solution without epibatidine (see details below). All frogs were captive bred and either obtained from Josh’s Frogs (Owosso, Michigan, USA) (E. anthonyi and D. tinctorius) or drawn from a research colony at Illinois State University, Normal, IL, USA (R. variabilis, R. imitator, and P. vittatus). For at least two months prior to starting the feeding experiment, we maintained all frogs at John Carroll University (University Heights, OH, USA). Throughout, we kept frogs under a 12 h light/dark cycle and at humidity ≥ 80% and temperature 18.3–26.7 ℃ meant to mimic natural conditions. During the two month acclimation period, we held all individuals of each species together in a single glass terrarium (51 cm × 25 cm × 30 cm), and fed frogs Drosophila melanogaster dusted with vitamin powder (Rep-Cal, Los Gatos, CA, USA) every other day. During the 14 day feeding experiment (see below for details), we moved each frog to an individual 14.5 cm × 14.5 cm × 8 cm plastic terraria with damp paper towels as substrate (replaced every other day) and a small plastic cup for cover.
Alkaloid dosage
We used estimates from the natural diet of wild-caught frogs to define the daily dosage of epibatidine administered to each frog (sensu Jeckel et al. 2022). We first used published data from stomach content analyses to estimate the mean daily intake for an individual of each species of ants and mites, the major dietary source of alkaloids in dendrobatid frogs (Caldwell 1996; Saporito et al. 2012). Diet data were only available for two populations of Dendrobates tinctorius (mean n ants/mites: 41.2, 26.2/3.2, 2.1). As proxy for the Phyllobates and Ranitomeya species we used in the present study, we used dietary data from Phyllobates lugubris (mean n ants/mites: 12.1/8.1) and Ranitomeya ventrimaculata (mean n ants/mites: 23.2/28.8) (data from Caldwell 1996; Born et al. 2010), respectively. No dietary data are available for Epipedobates. Using these diet data, we estimated a mean daily intake of 26 ants and 11 mites for the species examined. We then used published alkaloid quantities for ants and mites (data from Jones et al. 1982, 1996; Takada et al. 2005; Saporito et al. 2011) to estimate the quantity of alkaloids in this number of ants and mites. Our final estimate of average alkaloid intake was 8.3 μg/day per frog, which we rounded to 10 μg/day per frog to allow us to directly compare our results to a previous study that experimentally examined sequestration of decahydroquinoline and histrionicotoxin alkaloids in Adelphobates galactonotus (Jeckel et al. 2022).
Alkaloid administration
We administered alkaloids orally to study animals by delivering a prepared epibatidine solution via micropipette (sensu Jeckel et al. 2022). Alkaloid sequestration occurs relatively quickly across the mucosa of the oral cavity and other places in the digestive tract (Jeckel et al. 2020; O’Connell et al. 2021). We built our liquid solution using (+/−)-epibatidine dihydrochloride hydrate (Sigma-Aldrich, Germany) diluted in 50% ethanol to a final concentration of 2 µg/µl, and we administered 5 µl of the ethanol/alkaloid solution daily to each frog in the epibatidine treatment. We administered 5 µl of 50% ethanol with no epibatidine daily to each frog in the control treatment. We administered the treatment daily for 7 days. To ensure that only sequestered alkaloids were included in our chemical analyses, we ceased alkaloid feeding for 7 days before euthanizing frogs for alkaloid analysis (sensu Jeckel et al. 2022). During the entire 14 days experiment (7 days alkaloid feeding + 7 days of latency), we fed frogs with vitamin-dusted D. melanogaster every other day.
Immediately following the first oral administration of treatments, the E. anthonyi in the epibatidine treatment group (but not the control animal) displayed signs of distress, including approximately 30 min of lethargy and immobility. So, on the following 6 days of the experiment, we fed the three epibatidine-treatment E. anthonyi a solution diluted to 1 µg/µl. Thus, these E. anthonyi consumed 5 μg of epibatidine/day on days 2–7 rather than the 10 μg/day they consumed on day 1 and other frogs consumed throughout the experiment. In total, our E. anthonyi consumed 40 µg of epibatidine over the course of the experiment, and all other frogs consumed 70 µg. The E. anthonyi did not show signs of additional distress following feeding with the diluted epibatidine solution.
We euthanized frogs via freezing at − 20 ℃ and then removed skins, which we stored individually in 4 ml glass vials with Teflon-lined caps containing 1 ml of ≥ 99% methanol (GC Resolv™) at − 20 ℃ (sensu Jeckel et al. 2020, 2022). Prior to alkaloid extraction, we transferred each methanol extract to a new glass vial and added 100 μl of a 0.1 μg/μl solution of nicotine ((−)-nicotine ≥ 99%, Sigma-Aldrich) as an internal standard for later epibatidine quantification. Each methanol extract was evaporated with N2 to a volume of 100 μl prior to analysis with Gas Chromatography–Mass Spectrometry (see GC–MS methods below). Finally, we dried each frog skin at 60 ℃ for 24 h using a vacuum oven and then weighed each to the nearest 0.01 mg using an Explorer® Pro balance.
Alkaloid characterization
We identified and quantified epibatidine in each methanol extract using Gas Chromatography–Mass Spectrometry (GC–MS). We performed GC–MS on a Varian Saturn 2100 T ion trap MS instrument coupled to a Varian 3900 GC with a 30 m × 0.25 mm i.d. Varian Factor Four VF-5 ms fused silica column. GC separation of alkaloids was achieved using a temperature program from 100 to 280 ℃ at a rate of 10 ℃ per minute with helium as the carrier gas (1 ml/min). We analyzed each sample with electron impact and chemical ionization mass spectrometry using an injection volume of 2 μl and split mode (20:1), and identified epibatidine by comparing retention times and mass spectral properties to that of the epibatidine used in the feeding experiment (Rt: 13.00 min, base peak: 68 m/z, major peak: 69 m/z) and other publications (Spande et al. 1992; Daly et al. 2005). To quantify the amount of epibatidine in each sample, we compared the peak area of epibatidine to the peak area of the nicotine internal standard using a Varian MS Workstation v.6.9 SPI. We analyzed each sample five times using electron impact spectrometry and calculated an average quantity of epibatidine for each sample.
Statistical analyses
Because we administered different total quantities of epibatidine to E. anthonyi and individuals of the other four species, we normalized the data and compared the percentage of alkaloid accumulated/mg skin weight among species. Prior to our statistical analysis, we first calculated the percentage of epibatidine accumulated per individual [(total quantity of epibatidine fed/total quantity of epibatidine detected) × 100] and then used the dry weight of each frog skin to calculate the percentage of epibatidine accumulated per unit weight for each sample (percentage of epibatidine accumulated/mg frog skin). We confirmed our data were normally distributed and that variance was equal using a Shapiro–Wilk and a Levene’s test, respectively (p > 0.05). To test for differences in the percentage of epibatidine accumulated/mg skin weight among frog species, we used a one-way analysis of variance (ANOVA) followed by Tukey HSD pair-wise comparisons. All statistical analyses were conducted in R (v.4.1.2; R core Team 2021) and boxplots used to visualize differences in epibatidine accumulation were constructed using PRISM 9 (v.9.4.1).
Results
All five species (E. anthonyi, R. variabilis, R. imitator, P. vittatus, and D. tinctorius) sequestered epibatidine; however, none of the control frogs contained epibatidine (Fig. 2). The percentage of epibatidine sequestered/mg skin weight varied significantly across species (F4,17 = 19.45, p < 0.001), with E. anthonyi, R. variabilis, and R. imitator accumulating about 2.4× more epibatidine than P. vittatus or D. tinctorius (p < 0.002 in all comparisons; Fig. 3). There was no difference in the percentage of epibatidine sequestered/mg skin weight among E. anthonyi, R. variabilis, and R. imitator (p > 0.836 in all comparisons), or between P. vittatus and D. tinctorius (p = 0.557). The average snout–vent length and frog mass, total quantity of epibatidine fed, average amount of epibatidine sequestered (independent of skin weight), and percentage of epibatidine sequestered (independent of skin weight) for each species are presented in Table 1.
a The presence of epibatidine (Rt: 13.00 min) in a GC trace of an experimental Ranitomeya variabilis; and b the absence of epibatidine in a GC trace of a control R. variabilis. Large peaks labelled 1–3 are fatty acid methyl esters that were present in all samples. Smaller unlabeled peaks represent other fatty acid methyl esters and fatty acids that were variably present
Discussion
Although the alkaloid epibatidine has been observed to occur naturally in only one of the five dendrobatid poison frog species we studied, it was sequestered by individuals of all five species in the present study. Epibatidine has been identified in natural populations of Epipedobates and Ameerega (Daly et al. 1980; Spande et al. 1992; Daly 1998), so the ability of E. anthonyi to sequester it was unsurprising. Unexpectedly, our study provides evidence that species of Ranitomeya, Phyllobates, and Dendrobates are also able to sequester epibatidine, suggesting that the absence of this alkaloid among at least some dendrobatids (see Daly et al. 2005; Saporito et al. 2012; Santos et al. 2016; Grant et al. 2017) owes to the lack of an environmental source and not taxonomically restricted sequestration ability.
The ability of Dendrobates to sequester epibatidine is further indicated by Sanchez et al. (2019), who found that one individual of Dendrobates auratus sequestered the alkaloid in a feeding experiment, while one individual of D. tinctorius did not. Although the finding that D. tinctorius does not sequester epibatidine contrasts with the results of our study, the methods used by Sanchez et al. (2019) differed significantly from ours. Sanchez et al. (2019) provided frogs with fruit flies that were dusted with a 1% mixture of vitamin powder and the alkaloids epibatidine and sparteine, but the actual quantity of epibatidine consumed by each frog was not (and could not) be determined. In contrast, we orally administered a known quantity of epibatidine to individual frogs. We also administered more epibatidine than did Sanchez et al. (2019), and therefore, the apparent differences in ability to sequester in D. tinctorius could be due to differences in the methodology and quantity of epibatidine consumed by the frogs. Additional feeding experiments are necessary to resolve this question. Regardless, it is clear that at least part of the Dendrobates clade is capable of sequestering this alkaloid.
All of the species we studied share the physiological ability to sequester epibatidine; however, their accumulation efficiency (measured as percentage of epibatidine accumulated/mg skin weight) differed. Epipedobates anthonyi and both Ranitomeya species were more efficient at sequestering epibatidine than were P. vittatus and D. tinctorius (Fig. 2). Although the ability to sequester an alkaloid is either present or absent in a species (e.g., D. auratus is incapable of sequestering certain piperidine alkaloids; Davison et al. 2021), the efficiency at which different alkaloids are accumulated varies within and among species (e.g., Daly et al. 2000a, 2003; Saporito et al. 2019; present study). More specifically, Hantak et al. (2013) found that the bufonid poison frog Melanophryniscus stelzneri sequestered the alkaloid decahydroquinoline (DHQ) more efficiently than a 5,8-disubstituted indolizidine, and a 3,5-disubstituted indolizidine more efficiently than DHQ. In a feeding experiment similar to those performed in the present study, Jeckel et al. (2022) administered increasing amounts of the alkaloids DHQ and histrionicotoxin (HTX) 235A to Adelphobates galactonotus and found that accumulation efficiency in the skin increased at higher doses for HTX 235A while DHQ accumulation efficiency remained constant, demonstrating different efficiencies for these two structurally different alkaloids.
Jeckel et al. (2022) suggested that some of these differences in accumulation efficiency could be related to differences in lipophilicity between alkaloids, which can affect their absorption, movement across plasma membranes, metabolism, and excretion (Leo et al. 1971; Lipinski et al. 1997; Lapins et al. 2018). Poison frogs absorb alkaloids through the mucosa of the digestive tract, where they are then presumably transported through the circulatory system until storage (Santos et al. 2016; Caty et al. 2019; Jeckel et al. 2020, 2022; Alvarez-Buylla et al. 2022). Bile acid and several protein-based mechanisms have been proposed for the absorption and transport of alkaloids (Clark et al. 2012; Caty et al. 2019; O’Connell et al. 2021; Alvarez-Buylla et al. 2022), and differences in both the ability and efficiency to sequester alkaloids by frogs (including epibatidine) are likely related to such transporters. Most recently, Alvarez-Buylla et al. (2023) discovered a plasma protein of the serpin family, referred to as alkaloid-binding globulin (ABG), that is capable of binding multiple alkaloid classes in Dendrobates tinctorius, Epipedobates tricolor (a close relative of E. anthonyi, studied here), and Oophaga sylvatica. They also found that ABG binding specificity varies among species and alkaloids, potentially accounting for variability in sequestration efficiency observed in other studies (including the present study). Although the shared occurrence of ABG in these species, which represent two independent origins of alkaloid sequestration within Dendrobatidae, is suggestive of a single mechanism of alkaloid transport, Alvarez-Buylla et al. (2022) reported that ABG is absent in the distantly related mantellid poison frog Mantella auratiaca, suggesting that additional alkaloid transporters might have evolved as well.
The present study demonstrates that dendrobatid species other than Epipedobates and Ameerega are capable of sequestering epibatidine, and there is reason to predict that other lineages not yet examined might also have this ability. Tarvin et al. (2017) found that species of Epipedobates, Ameerega, and Oophaga have a key substitution, S108C, in the β subunits of nicotinic acetylcholine receptors (nAChRs) that, when expressed experimentally in human nAChRs, appears to reduce sensitivity to epibatidine. This substitution in frogs might confer resistance to autotoxicity from epibatidine (Tarvin et al. 2017), which could favor and/or constrain the evolution of the ability to sequester this alkaloid. A good way to test the hypothesis that this trait is key to the evolution of epibatidine sequestration would be to test for epibatidine uptake in Oophaga. This genus is part of Dendrobatini and nested within the clade that includes D. tinctorius and the two Ranitomeya species we examined (Grant et al. 2017), suggesting it should be able to sequester epibatidine. Further, Alvarez-Buylla et al. (2023) demonstrated that alkaloid-binding globulin in Oophaga sylvatica binds epibatidine (possibly for transport), providing additional evidence that Oophaga is likely capable of sequestering epibatidine. Nevertheless, Tarvin et al. (2017) reported the absence of the S108C substitution in Ranitomeya, Phyllobates, and Dendrobates, species of which sequestered epibatidine in the present study. Our findings therefore provide evidence that epibatidine sequestration is not dependent on the presence of the S108C substitution, and is more widespread than previously thought. This finding is consistent with the apparent broad range of binding affinities of the presumed transporters.
Given that five species from diverse dendrobatid lineages can sequester epibatidine, a more likely explanation for its restricted occurrence in natural populations might be related to the geographic distribution of the arthropod(s) from which this alkaloid is sequestered. Differences in the availability of arthropods appear to contribute to both small and large-scale differences in alkaloid defenses among dendrobatids (e.g., Saporito et al. 2007b; McGugan et al. 2016; Basham et al. 2020; Moskowitz et al. 2020). Epibatidine has only been found naturally in some Epipedobates and Ameerega (Daly et al. 1980; Spande et al. 1992; Daly 1998), which range from Ecuador to northern Peru, and it is probable that the dietary arthropod source(s) of this alkaloid shares a similar distribution. Daly (1998) reported that a population of E. anthonyi from Ecuador contained trace amounts of epibatidine in 1974, yet no epibatidine was detected in the same species from a nearby site in 1976, which suggests that the dietary source (or frog diet) varies on similar spatial and temporal scales. Of equal importance is the fact that epibatidine has only ever been detected in trace amounts of approximately 1 µg/frog or less in natural populations (Daly et al. 1980; Spande et al. 1992), which is much less than most other major alkaloids (> 50 µg/frog) in dendrobatid frogs (e.g., Daly et al. 1987; Saporito et al. 2007b). The quantity of epibatidine in E. anthonyi is more than 20 times less than the co-occurring pumiliotoxin 251D (Spande et al. 1992; footnotes 1, 14). In fact, if it were not for the presence of a conspicuous Straub-tail reaction in mice indicating the presence of an extremely potent analgesic in skin extracts of E. anthonyi (Daly et al. 1978, 2000b), it is possible that epibatidine would have been overlooked in early studies. It should be noted, however, that epibatidine is highly toxic, with an LD50 of approximately 0.4 μg per mouse (Badio and Daly 1994; Fitch et al. 2018), and even trace amounts probably function as an effective defense. Regardless, the exceptionally small quantities of epibatidine in natural frogs suggests that the arthropod source is rare and/or infrequently consumed by poison frogs. It is also possible that the source itself contains small or variable quantities of epibatidine, independent of its abundance or consumption by frogs. Collectively, this could help explain the apparent absence of epibatidine in other dendrobatids that share similar geographic distributions with known epibatidine-containing frogs (e.g., species of Ranitomeya), which, based on the present study, are presumably capable of sequestering epibatidine. Nevertheless, we caution that not all dendrobatid species (or populations) in this range (and outside this range) have been analyzed for alkaloids, and it remains possible that epibatidine has yet to be detected in some of these unstudied species. The dietary source(s) of epibatidine is unknown, but its discovery will be essential to further understanding these open questions regarding epibatidine sequestration.
Although all five species examined in the present study are clearly resistant to epibatidine, possibly due to the S108C substitutions in nAChRs (Tarvin et al. 2017), resistance appears dose dependent and related to body size. On average, the specimens of E. anthonyi were approximately 10% shorter and their skins were 27% lighter than the next smallest species (Table 1), and E. anthonyi was the only species to exhibit ill effects from the initial dose, requiring a reduction for the remainder of the experiment. Epibatidine is highly toxic and has only been detected naturally in trace quantities, and it is possible the quantity of epibatidine used in the present study exceeded the amount a frog would consume naturally. Additional studies will be necessary to further understand this relationship, but resistance is likely a multifaceted physiological adaptation, involving more than just ion channel modification (e.g., Tarvin et al. 2016, 2017; Marquez et al. 2019).
The findings of the present study demonstrate that the ability to sequester epibatidine is not restricted to species of Epipedobates and Ameerega but is in fact present in other dendrobatid species, including R. variabilis, R. imitator, D. tinctorius, and P. vittatus. These results further strengthen the hypothesis that alkaloid variation among natural populations largely arises due to differences in alkaloid intake, which, in turn, likely develops from differences in the availability of dietary sources and/or the propensity of frogs to consume them (Saporito et al. 2007b; Jeckel et al. 2015; McGugan et al. 2016; Basham et al. 2020; Moskowitz et al. 2020). The observed differences in sequestration efficiency of epibatidine in the present study, coupled with several other feeding experiments (Daly et al. 1994, 2003; Hantak et al. 2013; Mebs et al. 2014; Davison et al. 2021; O’Connell et al. 2021; Alvarez-Buylla et al. 2022; Jeckel et al. 2022), also illustrate the importance of physiological differences in sequestration to explaining some of the alkaloid variation within and among poison frog species, as well as the potential pitfalls of only using alkaloid profiles of wild-caught animals to make predictions about such differences among lineages (Grant et al. 2006). Given the importance of alkaloid defenses to taxonomically diverse consumers and consumed alike, unraveling these sources of natural variation will be critical to understanding the ecological and evolutionary causes and consequences of chemical defense.
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Adler LS, Wink M, Distl M, Lentz AJ (2006) Leaf herbivory and nutrients increase nectar alkaloids. Ecology Letters 9:960–967
Alvarez-Buylla A, Payne CY, Vidoudez C, Trauger SA, O’Connell LA (2022) Molecular physiology of pumiliotoxin sequestration in a poison frog. PLoS ONE 17(3):e0264540
Alvarez-Buylla A, Moya-Garzon MD, Rangel AE, Tapia EE, Tanzo J, Tom Soh T, Coloma LA, Long JZ, O’Connell LA (2023) Binding and sequestration of poison frog alkaloids by a plasma globulin. bioRxiv. https://doi.org/10.1101/2022.11.22.517437
Badio B, Daly JW (1994) Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol 45:563–569
Basham EW, Saporito RA, González-Pinzón M, Romero-Marcucci A, Scheffers BR (2020) Chemical defenses shift with the seasonal vertical migration of a panamanian poison frog. Biotropica 53:28–37
Blum MS (ed) (1995) The toxic action of marine and terrestrial alkaloids. Alaken, Colorado
Bolton SK, Dickerson K, Saporito RA (2017) Variable alkaloid defense in the dendrobatid poison frog (Oophaga pumilio) are perceived as differences in palatability to arthropods. J Chem Ecol 43:273–289
Born M, Bongers F, Poelman EH, Sterck FJ (2010) Dry-season retreat and dietary shift of the dart-poison frog Dendrobates tinctorius (Anura: Dendrobatidae). Phyllomedusa 9:37–52
Brückmann M, Trigo JR, Foglio MA, Hartmann T (2000) Storage and metabolism of radioactively labeled pyrrolizidine alkaloids by butterflies and larvae of Mechanitis polymnia (Lepidoptera: Nymphalidae, Ithomiinae). Chemoecology 10:25–32
Caldwell JP (1996) The evolution of myrmecophagy and its correlates in poison frogs (Family Dendrobatidae). J Zool 240:75–101
Carvajal-Castro JD, Vargas-Salinas F, Casas-Cardona S, Rojas B, Santos JC (2021) Aposematism facilitates the diversification of parental care strategies in poison frogs. Sci Rep 11:19047
Caty SN, Alvarez-Buylla A, Byrd GD, Vidoudez C, Roland AB, Tapia EE, Budnik B, Trauger SA, Coloma LA, O’Connell LA (2019) Molecular physiology of chemical defenses in a poison frog. J Exp Biol 222:1–12
Clark VC, Harinantenaina L, Zeller M, Ronto W, Rocca J, Dossey AT, Rakotondravony D, Kingston DGI, Shaw C (2012) An endogenous bile acid and dietary sucrose from skin secretions of alkaloid-sequestering poison frogs. J Nat Prod 75:473–478
Cordell GA (ed) (1998) The alkaloids: chemistry and biology, vol 50. Academic Press, New York
Daly JW, Brown GB, Mensah-Dwumah M (1978) Classification of skin alkaloids from neotropical poison-dart frogs (Dendrobatidae). Toxicon 16:163–188
Daly JW, Tokuyama T, Fujiwara T, Highet RJ, Karle IL (1980) A new class of indolizidine alkaloids from the poison frog, dendrobates tricolor. X-ray analysis of 8-Hydroxy-8methyl-6-(2′-methylhexylidene)-1-azabicyclo[4.3.0]nonane. J Am Chem Soc 102:830–836
Daly JW, Myers CW, Whittaker N (1987) Further classification of skin alkaloids from neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the amphibia. Toxicon 25:1023–1095
Daly JW, Secunda SI, Garraffo HM, Spande TF, Wisnieski A, Nishihira C, Cover JF (1992) Variability in alkaloid profiles in neotropical poison frogs (Dendrobatidae): genetic versus environmental determinants. Toxicon 30:887–898
Daly JW, Secunda S, Garraffo HM, Spande TF, Wisnieski A, Cover JF (1994) An uptake system for dietary alkaloids in poison frogs (Dendrobatidae). Toxicon 32:657–663
Daly JW (1998) Thirty years of discovering arthropod alkaloids in amphibian skin. J Nat Prod 61:162–172
Daly JW, Garraffo HM, Jian P, Spande TF, Snelling RR, Jaramillo C, Rand AS (2000a) Arthropod-frog connection: decahydroquinoline and pyrrolizidine alkaloids common to microsympatric myrmicine ants and dendrobatid frogs. J Chem Ecol 26:73–85
Daly JW, Garraffo HM, Spande TF, Decker MW, Sullivan JP, Williams M (2000b) Alkaloids from frog skin: the discovery of epibatidine and the potential for developing novel non-opioid analgesics. Nat Prod Rep 17:131–135
Daly JW, Garraffo HM, Spande TF, Clark VC, Ma J, Ziffer H, Cover JF (2003) Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus dendrobates. Proc Natl Acad Sci 100:11092–11097
Daly JW, Spande TF, Garraffo HM (2005) Alkaloids from the amphibian skin: a tabulation of over eight-hundred compounds. J Nat Prod 68:1556–1575
Davison I, Saporito RA, Schulte LM, Summers K (2021) Piperidine alkaloids from fire ants are not sequestered by the green and black poison frog (Dendrobates auratus). Chemoecology 31:391–396
Fitch RW, Snider BB, Zhou Q, Foxman BM, Pandya AA, Yakel JL, Olson TT, Al-Muhtasib N, Xiao Y, Welch KD, Panter KE (2018) Absolute configuration and pharmacology of the poison frog alkaloid phantasmidine. J Nat Prod 81:1029–1035
Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, Means DB, Noonan BP, Schargel WE, Wheeler WC (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bull Am Mus Nat Hist 299:1–262
Grant T, Rada M, Anganoy-Criollo M, Batista A, Dias PH, Jeckel AM, Machado DJ, Rueda-Almonacid JV (2017) Phylogenetic systematics of dart-poison frogs and their relatives revisited (Anura: Dendrobatoidea). S Am J Herpetol 12:1–90
Gonzalez M, Palacios-Rodriguez P, Hernandez-Restrepo J, González-Santoro M, Amézquita A, Brunetti AE, Carazzone C (2021) First characterization of toxic alkaloids and volatile organic compounds (VOCs) in the cryptic dendrobatid Silverstoneia punctiventris. Front Zool 18:1–15
Guillory WX, French CM, Twomey EM, Chávez G, Prates I, von May R, De la Riva I, Lötters S, Reichle S, Serrano-Rojas SJ, Whitworth A, Brown JL (2020) Phylogenetic relationships and systematics of the Amazonian poison frog genus Ameerega using ultraconserved genomic elements. Mol Phylogenet Evol 142:106638
Hantak MM, Grant T, Reinsch S, McGinnity D, Loring M, Toyooka N, Saporito RA (2013) Dietary alkaloid sequestration in a poison frog: an experimental test of alkaloid uptake in Melanophryniscus stelzneri (Bufonidae). J Chem Ecol 39(1):1400–1406
Hartmann T, Theuring C, Witte L, Pasteels JM (2001) Sequestration, metabolism and partial synthesis of tertiary pyrrolizidine alkaloids by the neotropical leaf-beetle Platyphora boucardi. Insect Biochem Mol Biol 31:1041–1056
Hovey KJ, Seiter EM, Johnson EE, Saporito RA (2018) Sequestered alkaloid defenses in the dendrobatid poison frog Oophaga pumilio provide variable protection from microbial pathogens. J Chem Ecol 44:312–345
Jeckel AM, Saporito RA, Grant T (2015) The relationship between poison frog chemical defenses and age, body size, and sex. Front Zool 12:27–27
Jeckel AM, Matsumura K, Nishikawa K, Morimoto Y, Saporito RA, Grant T, Ifa DR (2020) Use of whole-body cryosectioning and desorption electro spray ionization mass spectrometry imaging (DESI-MSI) to visualize alkaloid distribution in poison frogs. J Mass Spectrom 55:e4520
Jeckel AM, Bolton SK, Waters KR, Antoniazzi MM, Jared C, Matsumura K, Nishikawa K, Morimoto Y, Grant T, Saporito RA (2022) Dose-dependent alkaloid sequestration and N-methylation of decahydroquinoline in poison frogs. J Exp Zool 2022:1–10
Jones TH, Blum MS, Howard RW, McDaniel CA, Fales HM, DuBois MB, Torres J (1982) Venom chemistry of ants in the genus Monomorium. J Chem Ecol 8:285–300
Jones TH, Torres JA, Spande TF, Garraffo HM, Blum MS, Snelling RR (1996) Chemistry of venom alkaloids in some Solenopsis (Diplorhoptrum) species from Puerto Rico. J Chem Ecol 22:1221–1236
Lapins M, Arvidsson S, Lampa S, Berg A, Schaal W, Alvarsson J, Spiuth O (2018) A confidence predictor for logD using conformal regression and a support-vector machine. J Cheminform 10:1–10
Lawrence JP, Rojas B, Fouquet A, Mappes J, Blanchette A, Saporito RA, Bosque RJ, Courtois EA, Noonan B (2019) Weak warning signals can exist in the absence of gene flow. Proc Natl Acad Sci USA 116(38):19037–19045
Lawrence JP, Rojas B, Blanchette A, Saporito RA, Mappes J, Fouquet A, Noonan BP (2023) Linking predator responses to alkaloid variability in poison frogs. J Chem Ecol 49:195–204
Leo A, Hansch C, Elkins D (1971) Partition coefficients and their uses. Chem Rev 71:525–616
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25
Márquez R, Ramírez-Castañeda V, Amézquita A (2019) Does batrachotoxin autoresistance coevolve with toxicity in Phyllobates poison-dart frogs? Evolution 73:390–400
Marin CM, Molina-Zuluaga C, Restrepo A, Cano E, Daza JM (2018) A new species of Leucostethus (Anura: Dendrobatidae) from the eastern versant of the Central Cordillera of Colombia and the phylogenetic status of Colostethus fraterdanieli. Zootaxa 4461:359
McGugan JR, Byrd GD, Roland AB, Caty SN, Kabir N, Tapia EE, Trauger SA, Coloma LA, O’Connell LA (2016) Ant and mite diversity drives toxin variation in the little devil poison frog. J Chem Ecol 42:537–551
Mebs D, Alvarez JV, Pogoda W, Toennes SW, Köhler G (2014) Poor alkaloid sequestration by arrow poison frogs of the genus Phyllobates from Costa Rica. Toxicon 80:73–77
Mina AE, Ponti AK, Woodcraft NL, Johnson EE, Saporito RA (2015) Variation in alkaloid-based microbial defenses of the dendrobatid poison frog Oophaga pumilo. Chemoecology 25:169–178
Moskowitz NA, Dorritie B, Fay T, Nieves OC, Vidoudez C, Rindge C, Latin 2017 Biology Class, Masconomet 2017 Biotechnology Class, Fischer EK, Trauger SA, Coloma LA, Donoso DA, O’Connell LA (2020) Land use impacts poison frog chemical defenses through changes in leaf litter ant communities. Neotrop Biodivers 6:75–87
Murray EM, Bolton SK, Berg T, Saporito RA (2016) Arthropod predation in a dendrobatid poison frog: does frog life stage matter? Zoology 119:169–174
Nishida R (2002) Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 47:57–92
O’Connell LA, LS50: Integrated Science Laboratory Course, O’Connell JD, Paulo JA, Trauger SA, Gygi SP, Murray AW (2021) Rapid toxin sequestration modifies poison frog physiology. J Exp Biol 224:1–8
Opitz SEW, Müller C (2009) Plant chemistry and insect sequestration. Chemoecology 19:117–154
Pasteels JM, Hartmann T (2004) Sequestration of pyrrolizidine alkaloids in Oreina and Platyphora leaf beetles: physiological, ecological and evolutionary aspects. In: Jolivet P, Santiago-Blay J, Schmitt M (eds) New developments in the biology of chrysomelidae. Brill, Leiden, pp 677–691
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Roberts MF, Wink M (eds) (1998) Alkaloids: Biochemistry, ecology, and medicinal applications. Plenum Press, New York
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford University Press, Oxford
Sanchez E, Rodriguez A, Grau JH, Lötters S, Künzel S, Saporito RA, Ringler E, Schulz S, Valero KCW, Vences M (2019) Transcriptomic signatures of experimental alkaloid consumption in a poison frog. Genes 10:1–14
Santos JC, Coloma LA, Cannatella DC (2003) Multiple recurring origins of aposematism and diet specialization in poison frogs. Proc Natl Acad Sci 100:12792–12797
Santos JC, Cannatella DC (2011) Phenotypic integration emerges from aposematism and scale in poison frogs. Proc Natl Acad Sci 108:6175–6180
Santos JC, Tarvin RD, O’Connell LA (2016) A review of chemical defense in poison frogs (Dendrobatidae): ecology, pharmacokinetics, and autoresistance. Chem Signal Vertebr 13:305–337
Saporito RA, Donnelly MA, Norton R, Garraffo HM, Spande TF, Daly JW (2007a) Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc Natl Acad Sci 104:8885–8890
Saporito RA, Donnelly MA, Jain P, Garraffo HM, Spande TF, Daly JW (2007b) Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon 50:757–778
Saporito RA, Spande TF, Garraffo HM, Donnelly MA (2009) Arthropod alkaloids in poison frogs: a review of the ‘dietary hypothesis.’ Heterocycles 79:277–297
Saporito RA, Norton RA, Andriamaharavo NR, Garraffo HM, Spande TF (2011) Alkaloids in the mite Scheloribates laevigatus: Further alkaloids common to oribatid mites and poison frogs. J Chem Ecol 37:213–218
Saporito RA, Donnelly MA, Spande TF, Garraffo HM (2012) A review of chemical ecology in poison frogs. Chemoecology 22:159–168
Saporito RA, Norton RA, Garraffo HM, Spande TF (2015) Taxonomic distribution of defensive alkaloids in Nearctic oribatid mites (Acari, Oribatida). Exp Appl Acarol 67:317–333
Saporito RA, Russell MW, Richards-Zawacki CL, Dugas MB (2019) Experimental evidence of maternal provisioning of alkaloid defenses in a dendrobatid frog. Toxicon 161:40–43
Schulte LM, Saporito RA, Davison I, Summers K (2016) The palatability of poison frogs: do alkaloids make the difference? Biotropica 49(1):23–26
Spande TF, Garraffo HM, Edwards MW, Yeh HJC, Pannell L, Daly JW (1992) Epibatidine: a novel (Chloropyridyl) azabicycloheptane with potent analgesic activity from an Ecuadoran poison frog. J Am Chem Soc 114:3475–3478
Summers K, Tumulty J (2014) Parental care, sexual selection, and mating systems in neotropical poison frogs. In: Machado G, Macedo RD (eds) Sexual selection. Perspectives and models from the neotropics. Academic Press, London, pp 289–320
Takada W, Sakata T, Shimano S, Enami Y, Mori N, Nishida R, Kuwahara Y (2005) Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J Chem Ecol 31:2403–2415
Tarvin RD, Santos JC, O’Connell LA, Zakon HH, Cannatella DC (2016) Convergent substitutions in a sodium channel suggest multiple origins of toxin resistance in poison frogs. Mol Biol Evol 33:1068–1080
Tarvin RD, Borghese CM, Sachs W, Santos JC, Lu Y, O’Connell LA, Cannatella DC, Harris RA, Zakon HH (2017) Interacting amino acid replacements allow poison frogs to evolve epibatidine resistance. Science 357:1261–1266
Trigo JR (2011) Effects of pyrrolizidine alkaloids through different trophic levels. Phytochem Rev 10:83–98
Wink M (1988) Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor Appl Genet 75:225–233
Yang Y, Richards-Zawacki CL, Devar A, Dugas MB (2016) Poison frog color morphs express assortative mate preferences in allopatry but not sympatry. Evolution 70(12):2778–2788
Acknowledgements
We thank N. Savastano for her care of the frogs used in the study, and V. DelSignore for his assistance with the feeding experiments. We also thank the editor and two anonymous reviewers for helping to improve the quality of this manuscript. All of the research protocols were approved by the Institutional Animal Care and Use Committees of John Carroll University (#2102 for “Sequestered chemical defenses in poison frogs”). TG was supported by the São Paulo Research Foundation (FAPESP Proc. 2018/15425-0) and Brazilian National Council for Scientific and Technological Development (CNPq Proc. 314480/2021-8).
Funding
TG was supported by the São Paulo Research Foundation (FAPESP Proc. 2018/15425-0) and Brazilian National Council for Scientific and Technological Development (CNPq Proc. 314480/2021-8).
Author information
Authors and Affiliations
Contributions
KRW and RAS conceived, designed, and carried out the experiments and performed the chemical analyses. RAS and MBD performed the statistical analysis. MBD bred and raised the R. variabilis, R. imitator, and P. vittatus. KRW wrote the first draft of the manuscript, and all authors participated in the revision of this draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval
All of the research protocols were approved by the Institutional Animal Care and Use Committees of John Carroll University (#2102 for “Sequestered chemical defenses in poison frogs”).
Consent to participate
All co-authors have consented to participate in the research and manuscript publication.
Consent for publication
All co-authors have approved the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Waters, K.R., Dugas, M.B., Grant, T. et al. The ability to sequester the alkaloid epibatidine is widespread among dendrobatid poison frogs. Evol Ecol (2023). https://doi.org/10.1007/s10682-023-10260-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10682-023-10260-6