Abstract
Bufonid toads of the genus Melanophryniscus represent one of several lineages of anurans with the ability to sequester alkaloids from dietary arthropods for chemical defense. The alkaloid profile for Melanophryniscus stelzneri from a location in the province of Córdoba, Argentina, changed significantly over a 10-year period, probably indicating changes in availability of alkaloid-containing arthropods. A total of 29 alkaloids were identified in two collections of this population. Eight alkaloids were identified in M. stelzneri from another location in the province of Córdoba. The alkaloid profiles of Melanophryniscus rubriventris collected from four locations in the provinces of Salta and Jujuy, Argentina, contained 44 compounds and differed considerably between locations. Furthermore, alkaloid profiles of M. stelzneri and M. rubriventris strongly differed, probably reflecting differences in the ecosystem and hence in availability of alkaloid-containing arthropods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphibians produce bioactive substances and in some cases sequester bioactive substances from dietary sources (Daly et al., 1987; Daly, 1995). Such substances appear to be stored in so-called granular or poison glands of the skin and are used as chemical defenses against predators and/or microbial infection. Substances produced by amphibians include a variety of antibiotic peptides (Bevins and Zasloff, 1990), distasteful biogenic amines and congeners (Roseghini et al., 1976, 1986), toxic steroidal bufadienolides (Flier et al., 1980; Daly et al., 2004), toxic samandarine alkaloids (Schöpf, 1961; Mebs and Pogoda, 2005), and the indolic pseudophrynamine alkaloids (Daly et al., 1990; Smith et al., 2002). The role of symbiotic microorganisms in biosynthesis of tetrodotoxins in amphibians remains unresolved. Evidence both for a role in symbiotic organisms (Daly et al., 1997b) and in production by an amphibian (Cardall et al., 2004) have been presented. The remarkable sequestration of a wide structural range of lipophilic alkaloids into skin glands of so-called poison frogs/toads of the neotropical dendrobatid genera Dendrobates, Epipedobates, Minyobates, and Phyllobates (Daly et al., 1994a,b; Jones et al., 1999 and unpublished) the Madagascan mantellid genus Mantella (Daly et al., 1997a), and undoubtedly the South American bufonid genus Melanophryniscus (Garraffo et al., 1993) suggests that these taxa either have overexpressed a primitive alkaloid-transport system or have independently evolved such a system. Myobatrachid frogs of the Australian genus Pseudophryne apparently have a different transport system that can transport pumiliotoxins, but not simple decahydroquinoline and izidine alkaloids that would occur in their ant diet (Smith et al., 2002).
Analysis of sequestered arthropod alkaloids has led to discovery and characterization of over 800 alkaloids during 40 year of investigation of skin extracts of poison frogs/toads (Daly et al., 2005). Fifty of such frog skin alkaloids have been detected in extracts of ants (Jones et al., 1999; Daly et al., 2000; Saporito et al., 2004; Clark et al., 2005), beetles (Dumbacher et al., 2004), millipedes (Saporito et al., 2003), mites (Takada et al., 2005), and various other arthropods (Daly et al., 2002).
The occurrence of alkaloids in different populations and species of poison frogs/toads provides information as to the distribution and availability of the arthropods that provide alkaloids to them. Thus, if species of the bufonid genus Melanophryniscus at a particular site and/or collection time do not have alkaloids known or presumed to be from a certain group of arthropods, then that mite, ant, beetle, or millipede is either not present or not targeted by the toad, perhaps, in the latter case because of more readily available arthropod prey that do not contain alkaloids. This question has been recently investigated and discussed both from a temporal and spatial standpoint for seven populations of the poison frog Dendrobates pumilio on a small island in the Bocas archipelago of Panamá (Saporito et al., 2006). Early studies of the alkaloid profile of D. pumilio extracts from some of the same populations also had indicated that distance between sites and the nature of habitat greatly influenced the profile (Daly et al., 2002). Similarly, the number of shared alkaloids in populations of the Colombian/Ecuadorian poison frog Dendrobates histrionicus was inversely related to the distance between collection sites, with frogs from sites close to each other sharing a greater number of alkaloids (Myers and Daly, 1976).
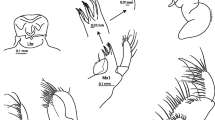
We report in this study both temporal changes in alkaloid profiles for one population of the bufonid toad Melanophryniscus stelzneri and spatial differences in alkaloid profiles for four populations of the bufonid toad Melanophryniscus rubriventris. The results are discussed in terms of possible arthropod food sources of the 72 alkaloids identified in extracts of these two toad species. Additional trace alkaloids were detected, but could not be adequately characterized. Structures of most of the major and minor alkaloids detected are shown (Fig. 1). Preliminary evidence for the postulated structures (Daly et al., 2005) of one pumiliotoxin, a dehydro-5,8-disubstituted indolizidine, and seven izidine alkaloids are presented.
Structures of major and minor alkaloids in collections of Melanophryniscus stelzneri and Melanophryniscus rubriventris. Postulated tentative structures of izidines 207S, 207T, and 221R (Daly et al., 2005) are shown in Fig. 5. Structures of proposed tricyclics 203B, 205H, 221S, 235I, and 237O and unclassified alkaloid 223T are not known (see Daly et al., 2005)
Methods and Materials
Specimens of Melanophryniscus stelzneri were collected in May, 1989 (23 skins), and again in October, 1999 (eight skins), in fields with marshy streams and ponds during periods of heavy rain near the small town of Tanti, Córdoba, Argentina. Two males (combined skins) and two females (combined skins) were collected as amplexing pairs at the same site in November, 2001. M. stelzneri (31 skins) from another site in Córdoba were provided by Dr. Eduardo Gros.
Specimens from four populations of M. rubriventris were collected in 2002 in Argentina from forested sites at Cedral de Baritú (22° 29′ S, 64° 45′ W) (13 skins) and Canto del Monte (22° 22′ S, 64° 43′ W) (nine skins) in Salta and at Abra Colorada (23° 40′ S, 64° 53′ W) (11 skins) and Tiraxi (24° 01′ S, 65° 23′ W) (two skins) in Jujuy.
Skins were placed in methanol and extracts were subjected to acid-base partitioning as described (Garraffo et al., 1993). Compounds in the resultant alkaloid fraction were characterized by GC-MS and GC-FTIR spectral analysis (Garraffo et al., 1999). Arthropod stomach contents of M. rubriventris from the four sites were assessed as described (Bonanseal and Vaira, 2007).
Mass spectral data (EIMS, EI-MS/MS, CIMS (NH3) and CI-MS/MS (NH3)) were obtained with a Finnigan Thermoquest GCQ instrument, having a Restek RTX-5MS capillary column (30 m, 0.25 mm i.d.) programmed from 100° to 280° at 10° per min. GC-FTIR and EIMS spectra were obtained with a Hewlett–Packard model 5890 gas chromatograph, having an HP-5 fused silica-bonded capillary column (30 m, 0.32 mm i.d.) programmed from 100 to 280° at a rate of 10° per min and interfaced with an HP model 5971 Mass Selective Detector and a Model 5965B IRD with a narrow band (4,000–750 cm−1) detector. An HP ChemStation was used to generate EIMS and FTIR spectra.
Results and Discussion
Analysis of alkaloids in skin of the toad Melanophryniscus stelzneri collected in Tanti, Córdoba, Argentina, in 1989 was reported in 1993 (Garraffo et al., 1993) and has been updated in the present report with additional data (Table 1). Alkaloid profiles for two males and two females collected in 2001 were similar (Table 2). Profiles from another population of M. stelzneri from Las Alpacas, Córdoba were reported in 1993 (Garraffo et al., 1993). An updated profile is reported (Table 3).
The profile of alkaloids reported in 1993 (Garraffo et al., 1993) for M. stelzneri montevidensis from La Coronilla, Rocha, Uruguay, consists of pumiliotoxin 251D and 3,5-disubstituted indolizidine 5Z,9E-195B as major alkaloids. Six trace alkaloids were also reported. This frog is now considered to be Melanophryniscus montevidensis, populations of which were recently reported to contain mainly pumiliotoxin 251D (Mebs et al., 2005). However, pumiliotoxin 251D in the La Coronilla population was present in only trace amounts (see below).
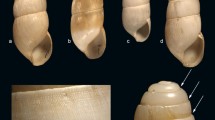
GC-MS chromatograms for the alkaloid fractions from the 1989 and 1999 collections of M. stelzneri are shown (Fig. 2). The toad and the collection site near Tanti are shown in Fig. 3. The site reportedly had undergone little obvious change from 1989 to 1999. Some major and minor alkaloids were present in both the 1989 and 1999 extracts, namely pumiliotoxin 251D, both cis- and trans-isomers of the 3,5-disubstituted pyrrolizidine 251K, the 5,8-disubstituted indolizidine 259B, the dehydro-5,8-disubstituted indolizidine 219G, and the 5,6,8-trisubstituted indolizidine 235E (Table 1). The pumiliotoxins and the 5,8-disubstituted- and 5,6,8-trisubstituted-indolizidines are likely of mite origin (Takada et al., 2005; Saporito et al., 2006), while the 3,5-disubstituted pyrrolizidines are likely of ant origin (Jones et al., 1999). However, all of the decahydroquinolines, likely of ant origin (Jones et al., 1999), present in abundance in the 1989 extract, were completely absent from the 1999 extract (Table 1). The 3,5-disubstituted indolizidines, a class likely of ant origin (Jones et al., 1999), present in the 1989 extract, were also absent from the 1999 extract. Finally, the beetle alkaloid precoccinelline (193C), and the millipede spiropyrrolizidine alkaloid 236 were absent in the 1999 extract. The most likely explanation is that the ants, beetles, and millipedes had come to represent a less available source of alkaloids during the 10-year period at this site, while perhaps mites had become a more dominant source of alkaloids for M. stelzneri. The arthropod stomach contents of M. stelzneri from “El Trapiche”, San Luis, Argentina, have been reported (Filipello and Crespo, 1994). The three most common categories of prey items were ants, mites, and Collembola.
The proposed structure for the dehydro-5,8-disubstituted indolizidine 219G (Daly et al., 2005) present as a minor alkaloid in the 1989 extract and as a major one in the 1999 extract, is based on chemical and spectral properties. The structure is shown (Fig. 1), as are the EIMS and FTIR spectra (Fig. 4). The FTIR band at 963 cm−1 indicates a trans-double bond. About 30 skin alkaloids are now assigned to the dehydro-5,8-disubstituted indolizidine class (Daly et al., 2005).
Two alkaloids (193I and 207S) in the 1999 extract have been proposed to be “izidines” (Daly et al., 2005), based on GC-MS spectral properties, and for 207S also on GC-FTIR spectral properties. The spectra of 207S are shown (Fig. 4). The proposed structures (Fig. 5) are tentative, and isolation and NMR spectral analysis will be required to establish absolute structures.
Tentative structures for izidines and a pumiliotoxin in collections of Melanophryniscus stelzneri and Melanophryniscus rubriventris. Structures for those indicated by an apostrophe (*) were postulated (Daly et al., 2005), based on GC-MS and in some cases GC-FTIR data. ‡High resolution MS data now obtained
The males/females from two amplexing pairs collected in 2001 from the site near Tanti had similar profiles (Table 2). Twenty-eight alkaloids, including isomers, were identified. All major/minor alkaloids except the unclassified alkaloids 233K and 267Y had been detected in the 1989 and/or 1999 collections. Nine of the 14 trace alkaloids (six in males, three in females) were detected in only one sex. Seven of the trace alkaloids were detected for the first time in four decades of analysis of anuran skin alkaloids. Tentative structures for the two new indolizidines 193M and 251EE are depicted (Fig. 5). The properties of the seven trace alkaloids (in the format of the supporting information of Daly et al., 2005) that were previously unreported are as follows: 193M. 5,8-I. ‘C13H23N’. Rt 7.26. MS: 193(1), 178(3), 151(21), 138(100), 96(30). 0D. Bufonid; 247L. Unclass. ‘C17H29N’. Rt 12.18. MS: 247(100), 218(6), 204(48), 190(17), 176(87), 162(13), 134(11), 120(33), 94(14). 0D. Bufonid; 247M. Unclass. ‘C17H29N’. Rt 11.65. MS: 247(14), 232(39), 206(100), 180(8), 84(3). 0D. Bufonid; 251EE. 5,6,8-I. ‘C16H29NO’. Rt 11.37. MS: 251(5), 168(100), 150(31), 110(5), 70(5). 1D. Bufonid; 255F. Unclass. ‘C15H29NO2’. Rt 11.47. MS: 255(3), 226(100), 196(8), 168(36), 166(81), 126(13), 86(5), 84(7). 1D. All major fragments have 1D. Bufonid; 265BB. Unclass. C16H27NO2. Rt 13.67. MS: 265(4), 250(2), 208(10), 180(76), 152(25), 140(15), 112(43), 98(36), 96(15), 86(100). 0D. Bufonid; 267Y. Unclass. C16H29NO2. Rt 12.40. MS: 267(21), 252(2), 210(100), 182(9), 138(10). 0D. Bufonid.
An updated alkaloid profile for a 1987 collection of M. stelzneri from Las Alpacas, Córdoba, Argentina (Garraffo et al., 1993) is shown (Table 3). The exact location of this site is uncertain. Decahydroquinolines, pyrrolizidines, and indolizidines comprise 18 of the 21 alkaloids detected. All major alkaloids in this extract are likely of ant origin (cf., Jones et al., 1999).
The profile of alkaloids in a 1987 collection of M. montevidensis from La Coronilla, Rocha, Uruguay, was quite different. Pumiliotoxin 251D and 3,5-disubstituted indolizidine 5Z,9Z-195B were the only major alkaloids (Garraffo et al., 1993). There were six others, all in trace amounts. These consist of three homopumiliotoxins 319A, 319B, and 321B, the 5E,9Z isomer of indolizidine 195B, and two 3,5-disubstituted pyrrolizidines, cis-223H and 237G. Structures for the homopumiliotoxins have been proposed (Garraffo et al., 1993; Daly et al., 2005). The indolizidines and pyrrolizidines are undoubtedly of ant origin (cf., Jones et al., 1999), while pumiliotoxin 251D appears likely to be of mite origin (cf., Takada et al., 2005; Saporito et al., 2006) as are probably the structurally similar homopumiliotoxins.
Recently, alkaloids detected in whole body methanolic extracts of 81 M. montevidensis collected from six sites in Uruguay from October, 2003, to February, 2004, were reported (Mebs et al., 2005). La Coronilla was one of the six sites. Pumiliotoxin 251D and indolizidine 5Z,9E-195B were major alkaloids (>50 μg/toad skin) in extracts from 39 skins of M. montevidensis collected in 1987 (see above) and supplied to us by Vittorio Erspamer (Garraffo et al., 1993). None of the whole body extracts from 20 individual toads collected in 2003–2004 had more than trace amounts (1 g/toad or less) of pumiliotoxin 251D or of an unidentified indolizidine (perhaps 195B, which was a major alkaloid in the 1987 collection). This seems to represent a remarkable temporal change in alkaloid content, although it is uncertain whether the locations of the La Coronilla sites are exactly the same. Toads from two of the more southern sites had variable, but in some cases very high levels (>400 g/toad) of pumiliotoxin 251D (Mebs et al., 2005).
The profile of alkaloids in the four different populations of M. rubriventris differed markedly (Table 4). GC-MS chromatograms are shown (Fig. 6) and toads from each site illustrate the variation in color and patterns (Fig. 7). The known distribution of the species in Argentina is confined to the upland portion of northwestern subtropical montane forests (Andean Yungas) and limited to six disjunct areas. Three populations are known in the north and central regions of Salta, and three are in the southeast of Jujuy. All are between 1,000 to 2,000 m elevation (Vaira, 2002). A map shows the four collection sites (Fig. 8). The collection site near Tanti, Córdoba, for M. stelzneri is also included in this map. The sites at which M. rubriventris are found, usually are well-structured cloud forests with richness in vegetation species and high vegetation density. Such steep, forested slopes are dominated by an evergreen-shaded forest, with a lush epiphytic growth mainly of bromeliads, ferns, mosses, and liverworts. Moderately steep portions of the forest allow temporary ponds that M. rubriventris use as breeding sites. The toads also are relatively common and easily seen in secondary forests, even on partly altered habitats, such as along montane trails or roads (Lavilla and Vaira, 1997). Toads choose small shallow ponds with rooted herbs for egg deposition. These little ponds are filled only during spring and summer rainstorms (Vaira, 2005). Further detailed studies on the botanical composition and arthropod occurrence at sites of the Melanophryniscus collections are needed. The spectrum of arthropods in the stomachs of toads from each of the collection sites are reported (Table 5). The spectra differed in terms of proportions of the two major prey items, ants, and mites.
Pumiliotoxins were present as major alkaloids at each site. Pumiliotoxin 307G was a major alkaloid in extracts of toads from Abra Colorada, Cedral de Baritú, and Canto del Monte, and a minor one in extracts from Tiraxi. Other major pumiliotoxins were 323A from Abra Colorada and Tiraxi, 291G from Cedral de Baritú and Canto del Monte, and 251D from Tiraxi. If mites are the source of the pumiliotoxins, different species might be used as dietary sources at the 4 sites. Only in the extract from Tiraxi, a trace of the allopumiliotoxin 267A was detected, while a trace of the deoxyhomopumiliotoxin 207O was detected only in the extract from Canto del Monte. One of the trace pumiliotoxins (319C) was detected for the first and, as yet, only time in these M. rubriventris extracts. This alkaloid was reported as unclassified with an unknown structure (Daly et al., 2005). Further analysis of the data indicates that it is a pumiliotoxin. A tentative structure is depicted (Fig. 5).
Decahydroquinolines, presumably of ant origin, were absent from the extracts of M. rubriventris, as were the ant-derived 3,5-disubstituted pyrrolizidines and 3,5-disubstituted indolizidines, with the sole exception being a trace amount of the 3,5-disubstituted indolizidine 223AB, in the extract from Canto del Monte. In contrast, the 5,8-disubstituted indolizidines 261D and 273C occurred as minor alkaloids, each in one extract, while the 5,6,8-trisubstituted indolizidine 195G occurred as a trace alkaloid in all four extracts. Two other 5,6,8-trisubstituted indolizidines (207C and 221Q) also occurred in trace amounts in one or two extracts. Such indolizidines with branch points in the carbon skeleton are suspected to be of mite origin (Takada et al., 2005; Saporito et al., 2006). Several izidines (191D, 191E, 205F, 205G, 207T, and 221R) occurred in minor or trace amounts usually in only one extract with the exception of 221R, which occurred in two. Possible izidine structures have been proposed for some (Daly et al., 2005) and are shown (Fig. 5). All except 205F and 205G had not been detected previously and, thus, like pumiliotoxin 319C, are as yet known only from M. rubriventris.
Tricyclics were present as major or minor alkaloids in all extracts of M. rubriventris except the one from Abra Colorada, where tricyclic 221W was present as a trace alkaloid. Presumably, the tricyclics, in particular precoccinelline (193C), a major alkaloid in the Tiraxi extract, are of beetle origin (Ayer and Browne, 1977). However, precoccinelline and another tricyclic alkaloid were recently reported from an oribatid mite (Takada et al., 2005). Except for 193C, structures for all proposed tricyclics from Melanophryniscus species are not known (Daly et al., 2005).
The results suggest that the nature and/or availability of dietary sources of alkaloids found in skin of Melanophryniscus toads is strongly dependent on the site of collection and can change markedly with time. Alkaloid profiles from other sites and species of Melanophryniscus have been determined (Garraffo et al., 1993; Mebs et al., 2005; and for Melanophryniscus klappenbachi and Melanophryniscus cupreuscapularis, unpublished data with J. Faivovich and P. Cacivio). Such profiles differ markedly from the alkaloid profiles in the two species of the present report.
Individual variability in alkaloid profiles within a population does occur in Melanophryniscus (Mebs et al., 2005) and also in dendrobatid (Saporito et al., 2006) and mantellid frogs (Clark et al., 2005). Remarkable variability in levels of pumiliotoxin 251D has been reported for 81 individual M. montevidensis from Uruguay (Mebs et al., 2005). The occurrence in trace amounts or nondetection of six other pumiliotoxins was also reported. An undefined indolizidine x was detected in all extracts, but apparently only in trace amounts. Pumiliotoxin 251D varied from undetectable in certain individuals and present in trace amounts in others (<1 g/toad) from the two northern populations to levels of over 200 g/toad in individuals of three of the four southern populations. Mean values for the southern populations ranged from 29 ± 16 g/toad (only two individuals) and 44 ± 21 g/toad (17 individuals) to very high levels of 198 ± 51 g/toad (11 individuals) and 307 ± 34 g/toad (29 individuals). Obviously, both site and individual variations were considerable for pumiliotoxin 251D in that study on Melanophryniscus. Pumiliotoxins were not detected in small arthropods, mainly ants collected in “various areas of Uruguay”. Mites are the probable dietary source of pumiliotoxins (Takada et al., 2005; Saporito et al., 2006). However, only a limited number of mites were analyzed. Further studies on the factors responsible for temporal, individual, and site variation are needed.
The arthropod stomach contents of M. rubriventris are summarized (Table 5). Arthropod stomach contents were essentially similar in number of prey categories, but with a noticeable interpopulation diet variation with respect to the proportions of each prey item consumed. The high proportion of ants and low proportion of mites in the diet of toads from Cedral de Baritú was striking. However, pumiliotoxins, thought to be of mite origin, were the dominant alkaloids (Table 4). Perhaps, no simple correlation exists between the number of prey items and the alkaloid content in toad skin because some of the minor prey items might have very high alkaloid content and major prey items might be devoid of alkaloids. Toads from Tiraxi had a more generalized diet than the other populations. Ants represented a minor component of diet. Instead Acari (mites), hemipterans, and collembolans were the main prey categories. Pumiliotoxins thought to be of mite origin were the dominant alkaloids. Such differences in decreased consumption of ants may reflect changes in the composition of the arthropod assemblage or species availability.
Consumption of a different array of prey items could also be a consequence of changes in foraging habits in the individuals from the Tiraxi population. Prey-searching behavior is unknown for M. rubriventris, but adults are mainly diurnal and mobile with most of their reproductive activity taking place during daylight over the entire breeding season (Vaira, 2005). However, one of us repeatedly registered events of breeding activity at night in the population of Tiraxi (M.V. personal observation). Differences in diet for this population might reflect foraging adaptations to nocturnal habits, and consequently different prey communities, rather than prey selectivity or differential availability of arthropod species.
Further studies on the dietary origin of the arthropod alkaloids sequestered into anuran skin are needed. The factors that govern availability of such alkaloid-containing arthropod prey may help to understand better the ecology of Melanophryniscus and other anurans that depend either in part or exclusively on a diet of alkaloid-containing arthropods for protection against predators. Dendrobatid and mantellid poison frogs apparently have no other noxious substances beyond the alkaloids sequestered from their diet. However, toads of the genus Melanophryniscus, like other bufonids (Daly et al., 2004), have high levels of toxic bufadienolides or bufadienolide-like compounds (Flier et al., 1980), which would protect populations even where alkaloid-containing prey are not available. Myobatrachid frogs of the genus Pseudophryne produce their own pseudophrynamine alkaloids, in addition to sequestering pumiliotoxins from dietary arthropods (Smith et al., 2002). The evolutionary forces that led in four different anuran lineages to sequestration and use of dietary alkaloids in chemical defense remains intriguing and enigmatic.
References
Ayer, W. A. and Browne, L. M. 1977. The ladybug alkaloids including synthesis and biosynthesis. Heterocycles 7:685–707.
Bevins, C. L. and Zasloff, M. 1990. Peptides from frog skin. Annu. Rev. Biochem. 59:395–414.
Bonanseal, M. I. and Vaira, M. 2007. Geographic variation of the diet of Melanophryniscus rubriventris (Anura: Bufonidae) in northwestern Argentina. J. Herpetol., in press.
Cardall, B. L., Brodie, E. D. Jr., Brodie, E. D. III, and Hanifin, C. T. 2004. Secretion and regeneration of tetrodotoxin in the rough-skin newt (Taricha granulosa). Toxicon 44:933–938.
Clark, V. C., Raxworthy, C. J., Rakotomalala, V., Sierwald, P., and Fisher, B. L. 2005. Convergent evolution of chemical defense in poison frogs and arthropod prey between Madagascar and the neotropics. Proc. Natl. Acad. Sci. U.S.A. 102:11617–11622.
Daly, J. W. 1995.The chemistry of poisons in amphibian skin. Proc. Natl. Acad. Sci. U.S.A. 92:9–13.
Daly, J. W., Myers, C. W., and Whittaker, N. 1987. Further classification of skin alkaloids from neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the amphibian. Toxicon 25:1023–1095.
Daly, J. W., Garraffo, H. M, Pannell, L. K., and Spande, T. F. 1990. Alkaloids from Australian frogs (Myobatrachidae): pseudophrynamines and pumiliotoxins. J. Nat. Prod. 53:407–421.
Daly, J. W., Secunda, S., Garraffo, H. M., Spande, T. F., Wisnieski, A., and Cover, J. F. Jr. 1994a. An uptake system for dietary alkaloids in poison frogs (Dendrobatidae). Toxicon 32:657–663.
Daly, J. W., Garraffo, H. M., Spande, T. F., Jaramillo, C., and Rand, S. A. 1994b. Dietary source for skin alkaloids of poison frogs (Dendrobatidae)? J. Chem. Ecol. 20:943–955.
Daly, J. W., Garraffo, H. M., Hall, G. S. E., and Cover, J. F. Jr. 1997a. Absence of skin alkaloids in captive-raised Madagascan mantelline frogs (Mantella) and sequestration of dietary alkaloids. Toxicon 35:1131–1135.
Daly, J. W., Padgett, W. L., Saunders, R. L., and Cover, J. F. Jr. 1997b. Absence of tetrodotoxins in a captive-raised riparian frog, Atelopus varius. Toxicon 35:705–709.
Daly, J. W., Garraffo, H. M., Jain, P., Spande, T. F., Snelling, R. R., Jaramillo, C., and Rand, A. S. 2000. Arthropod-frog connection: Decahydroquinoline and pyrrolizidine alkaloids common to microsympatric myrmicine ants and dendrobatid frogs. J. Chem. Ecol. 26:73–85.
Daly, J. W., Kaneko, T., Wilham, J., Garraffo, H. M., Spande, T. F., Espinosa, A., and Donnelly, M. A. 2002. Bioactive alkaloids of frog skin: Combinatorial bioprospecting reveals that pumiliotoxins have an arthropod source. Proc. Natl. Acad. Sci. U.S.A. 99:13996–14001.
Daly, J. W., Noimai, N., Kongkathip, B., Kongkathip, N., Wilham, J. M., Garraffo, H. M., Kaneko, T., Spande, T. F., Nimit, Y., Nabhitabhata, J., and Chan-ard, T. 2004. Biologically active substances from amphibians: Preliminary studies on anurans from twenty-one genera of Thailand. Toxicon 44: 805–815.
Daly, J. W., Spande, T. F., and Garraffo, H. M. 2005. Alkaloids from amphibian skin: a tabulation of over eight-hundred alkaloids. J. Nat. Prod. 68:1556–1575.
Dumbacher, J. P., Wako, A., Derrickson, S. R., Samuelson, A., Spande, T. F., and Daly, J. W. 2004. Melyrid beetles (Choresine): A putative source for the batrachotoxin alkaloids found in poison-dart frogs and passerine birds. Proc. Nat. Acad. Sci. 101:15857–15860.
Filipello, A. N. and Crespo, F. A. 1994. Alimentacion en Melanophryniscus stelzneri (Anura: Bufonidae). Cuad. Herpetol. 8:18–24.
Flier, J., Edwards, M. W., Daly, J. W., and Myers, C. W. 1980. Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+, K+-ATPase. Science 208:503–505.
Garraffo, H. M., Spande, T. F., Daly, J. W., Baldessari, A., and Gros, E. G. 1993. Alkaloids from bufonid toads (Melanophryniscus): decahydroquinolines, pumiliotoxins and homopumiliotoxins, indolizidines, pyrrolizidines, and quinolizidines. J. Natl. Prod. 56:357–373.
Garraffo, H. M., Spande, T. F., Jones, T. H., and Daly, J. W. 1999. Ammonia chemical ionization tandem mass spectrometry in structure determination of alkaloids. I. Pyrrolidines, piperidines, decahydroquinolines, pyrrolizidines, indolizidines, quinolizidines and an azabicyclo[5.3.0]decane. Rapid Commun. Mass Spectrom. 13:1553–1563.
Jones, T. H., Gorman, J. S. T., Snelling, R. R., Delabie, J. H. C., Blum, M. S., Garraffo, H. M., Jain, P., Daly, J. W., and Spande, T. F. 1999. Further alkaloids common to ants and frogs: decahydroquinolines and a quinolizidine. J. Chem. Ecol. 25:1179–1193.
Lavilla, E. O. and Vaira, M. 1997. La larva de Melanophryniscus rubriventris (Vellard, 1947). Alytes 15:19–25.
Mebs, D. and Pogoda, W. 2005. Variability of alkaloids in the skin secretion of the European fire salamander (Salamandra salamandra terrestris). Toxicon 45:603–606.
Mebs, D., Pogoda, W., Maneyro, R., and Kwet, A. 2005. Studies on the poisonous skin secretion of individual red bellied toads, Melanophryniscus montevidensis (Anura, Bufonidae), from Uruguay. Toxicon 46:641–650.
Myers, C. W. and Daly, J. W. 1976. Preliminary evaluation of skin toxins and vocalizations in taxonomic and evolutionary studies of poison-dart frogs (Dendrobatidae). Bull. Am. Mus. Nat. Hist. 157:173–262.
Roseghini, M., Erspamer, V., and Endean, R. 1976. Indole-, imidazole- and phenyl-alkylamines in the skin of one hundred amphibian species from Australia and Papua New Guinea. Comp. Biochem. Physiol. 54C:31–43.
Roseghini, M., Erspamer, V., Erspamer, G. F., and Cei, J. M. 1986. Indole-, imidazole- and phenyl-alkylamines in the skin of one hundred and forty American amphibian species other than bufonids. Comp. Biochem. Physiol. 85C:139–147.
Saporito, R. A., Donnelly, M. A., Hoffman, R. L., Garraffo, H. M., and Daly, J. W. 2003. A siphonotid millipede (Rhinotus) as the source of spiropyrrolizidine oximes of dendrobatid frogs. J. Chem. Ecol. 29:2781–2786.
Saporito, R. A., Garraffo, H. M., Donnelly, M. A., Edwards, A. L., Longino, J. T., and Daly, J. W. 2004. Formicine ants: an arthropod source for the pumiliotoxin alkaloids of dendrobatid poison frogs. Proc. Natl. Acad. Sci. 101:8045–8050.
Saporito, R. A., Donnelly, M. A., Garraffo, H. M., Spande, T. F., and Daly, J. W. 2006. Geographic and seasonal variation in alkaloid-based chemical defenses of Dendrobates pumilio from Bocas del Toro, Panamá. J. Chem. Ecol. 32:795–814.
Schöpf, C. L. 1961. Die Constitution der Salamander-Alkaloide. Experientia 17:285–328.
Smith, B. P., Tyler, M. J., Kaneko, T., Garraffo, H. M., Spande, T. F., and Daly, J. W. 2002. Evidence for biosynthesis of pseudophrynamine alkaloids by an Australian myobatrachid frog (Pseudophryne) and sequestration of dietary pumiliotoxins. J. Nat. Prod. 65:439–447.
Takada, W., Sakata, T., Shimano, S., Enami, Y., Mori, N., Nishida, R., and Kuwahara, Y. 2005. Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J. Chem. Ecol. 31:2403–2415.
Vaira, M. 2002. Variación de la coloración en poblaciones argentinas de Melanophryniscus rubriventris (Vellard, 1947). Cuad. Herpetol. 16:151–163.
Vaira, M. 2005. Annual variation of breeding patterns of Melanophryniscus rubriventris (Vellard, 1947). Amphib-Reptil. 26:193–199.
Acknowledgments
J.M.W. was supported by the NIH Undergraduate Scholarship Program. Fieldwork on M. rubriventris was done as part of a project supported by a grant from CONICET to M.V. Permits for sample collection of the species were provided by Delegación Técnica de Parques Nacionales, Regional Noroeste, Argentina. The research at NIH was supported by the intramural program of NIDDK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daly, J.W., Wilham, J.M., Spande, T.F. et al. Alkaloids in Bufonid Toads (Melanophryniscus): Temporal and Geographic Determinants for Two Argentinian Species. J Chem Ecol 33, 871–887 (2007). https://doi.org/10.1007/s10886-007-9261-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9261-x