Abstract
Soybean (Glycine max L. Merr.) seeds are highly sensitive to the environmental conditions experienced in STORAGE. The objectives of this study were to identify QTLs for soybean seed storability by evaluating seed viability and seed vigor and investigating candidate genes for these traits in detected QTL regions. These objectives will aid in developing soybean cultivars with a high capacity for storability. The seeds of 109 and 90 recombinant inbred lines (RILs) derived from a cross between Misuzudaizu and Moshidou Gong 503 in 2010 and 2019, respectively, were used to evaluate seed viability by a germination test and seed vigor by an accelerated aging test, after storage under two temperature conditions (25 °C and 35 °C) for six months. Seed viability and seed vigor of Moshidou Gong 503 were higher than those of Misuzudaizu in both temperature conditions. The average seed viability and seed vigor of RILs decreased when stored at the higher temperature. A total of five QTLs were found for the two traits, seed viability and seed vigor, located in chromosomes 2, 6 and 8. The Misuzudaizu alleles decreased seed viability and seed vigor at all detected QTLs. Most QTLs in this study were found near loci controlling seed viability, maturity, germination, seed hardness, and seed surface micromorphology, indicating that seed storability is related to these traits. In addition, two new QTLs were found that are associated with seed storability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed storability is of paramount importance in agricultural production. Short longevity due to loss in seed viability and vigor during storage negatively impacts seedling development and crop productivity (Han et al. 2014; Börner et al. 2018). Reduced seed viability and seed vigor due to seed deterioration is influenced by high temperature and humidity in the storage environment that affects biochemical processes in seeds, such as increases in hydrolytic enzyme activity, enhanced respiration, and lipid peroxidation leading to increased levels of free fatty acids (Copeland and McDonald 2001). Lipid peroxidation (Wang et al. 2018) and protein damage (Davies 2005) always occur in oilseed crops during high-temperature storage. In humid tropical and sub-tropical climates, including Southeast Asia, where temperature and relative humidity are high, the rapid loss in seed germination ability during storage is a major problem in soybean production (Nkang and Umlo 1996).
Soybean is the most important legume crop globally because the seeds contain large amounts of storage components, including proteins and lipids, used for food, feed and many industrial materials. Seed proteins and lipids are susceptible to high temperatures and elevated humidity in storage. In unconditioned storage environments, soybean seed deterioration occurs rapidly (TeKrony et al. 1993). Lengthy storage of soybeans causes reduced seed longevity, lower levels of germination, and impaired seedling establishment due to the utilization of glucose, oils and fatty acids during seed respiration. Thus, developing cultivars with significant resistance to high-temperature storage is necessary to improve seed viability and vigor during storage. Nevertheless, understanding the genetic bases for seed storability is insufficient; we must also develop genetic material with resistance to storage conditions. Genetic analyses should be conducted to identify soybean genotypes that are resistant to storage conditions and to develop genetic markers for marker-assisted selection (MAS).
Several genetic analyses of seed storability have been reported in Arabidopsis (Clerkx et al. 2004), rice (Sasaki et al. 2005; Xue et al. 2008; Wang et al. 2010; Li et al. 2012; Miura et al. 2002), rye seed (Chwedorzewska et al. 2002), safflower (Vijay et al. 2009) and soybean (Green and Pinnell 1968; Lanyon 1970; Kueneman 1983; Singh and Ram 1986; Verma and Ram 1987; Cho and Scott 2000; Singh et al. 2008a, b). Verma and Ram (1987) found that two to four genes presumably control seed longevity in soybean. Polymorphisms in SSR profiles of aged soybean seeds were found and four SSR markers were associated with seed coat permeability and electrolyte leaching in soybean (Singh et al. 2008a, b). Four SSR markers related to seed longevity in an F2:3 soybean population derived from a cross between good and poor storage genotypes have been reported (Singh et al. 2008a). Vijay et al. (2009) clustered the genetic variation of safflower and soybean into three different seed age groups, including un-aged, naturally aged, and age-accelerated seeds by RAPD, AFLP, and SSR markers. RAPD markers clustered naturally aged and age-accelerated seeds types in soybean and safflower, whereas the control and naturally aged seed were clustered by SSR and AFLP markers (Vijay et al. 2009).
Quantitative trait loci (QTL) analysis is effective because seed storability is a quantitative trait controlled by several genes. Several QTL analyses of seed storability have been reported in Arabidopsis (Bentsink et al. 2000; Clerkx et al. 2004), rice (Sasaki et al., 2005; Xue et al. 2008; Wang et al. 2010; Li et al. 2012; Miura et al. 2002), barley (Nagel et al. 2016), maize (Han et al. 2014) and oilseed rape (Nagel et al. 2011). In soybean, QTLs associated with seed storability remain to be identified and characterized, and there are no studies of QTL analysis for seed storability based on seed vigor and under high temperature and long term storage condition. Although QTLs controlling seed storability have been reported, genes responsible for seed storability have not yet been identified. Dargahi et al. (2014) reported that there are three QTLs on three linkage groups that are associated with seed storability. However, this report found the QTLs in seeds under ambient condition and accelerated aging.
In this study, a RIL population derived from a cross between Misuzudaizu and Moshidou Gong 503 was stored under two different temperatures to evaluate seed viability and seed vigor. The objectives of this study were to identify QTLs for seed storability at the two different temperatures and analyze the responsible genes by detecting QTL regions.
Materials and methods
Plant materials
Recombinant inbred lines (RILs) derived from a cross between Misuzudaizu and Moshidou gong 503 were obtained from the National BioResource Project (NBRP) for Lotus and Glycine, University of Miyazaki. Misuzudaizu is a yellow soybean seed cultivar developed at the Nagano Prefectural Agricultural Experiment Station, Japan and Moshidou Gong 503 is a brown soybean seed line originating from Jilin province, China. In 2010 and 2019, 109 and 90 RILs, respectively, were used in this study.
Seed storage conditions
Before storage treatments, seeds in 2010 were kept at 4 °C for 8 years and seeds in 2019 were kept at 4 °C for 1 month. During the storage treatment, seeds were kept in paper bags (19.5 cm × 11.9 cm) at 25 ± 1 °C or 35 ± 1 °C at 40–57% relative humidity for six months.
Seed viability
Seed viability was evaluated by the between-paper germination test (Rao et al. 2006). Three replications of 30 seeds from each the RILs were placed between moistened double-folded paper towels (size 40 × 33 cm). After being scarified using sandpapers to break seed dormancy, the seeds were moved to Plant Boxes for Plant Culture (125 mL, size 75 × 75 × 100 mm VWR International, USA) and placed into incubators at 25 °C for eight days (ISTA 2011). At the end of the treatment, the number of normal seedlings was counted. The germination percentage was calculated as the number of normal seedlings/number of all seeds × 100.
Seed vigor
The accelerated aging (AA) test (ISTA 2011) was used to evaluate seed vigor. Seeds were treated at 41 ± 1 °C and 100% relative humidity for 72 h. Subsequently, the between-paper germination test was conducted as described above. After eight days, the number of normal seedlings was counted. Seeds with higher germination percentages indicated greater seed vigor.
QTL analysis
The markers used in this study were a total of 1,131 simple sequence repeat (SSR) markers (Tajuddin et al. 2003; Watanabe et al. 2004) from from the Glycine max/ G. soja database at LegumeBase: (https://legumebase.nbrp.jp/glycine/rilChromosome/rilChromosomeList.jsp) where genotypes and physical maps of all RIL populations are also available. The map had a distance of 3,240.72 cM across 20 linkage groups (LGs), and the average genetic distance between two neighboring marker loci was 2.87 cM. The presence of QTLs was calculated by composite interval mapping using R/qtl software (Broman et al. 2003). The logarithm of odd (LOD) value significance threshold at 5% was determined by a permutation test module (1000 replications).
Data analysis
The effects of genotype and storage temperature on seed viability and seed vigor were indicated by seed germination and AA tests, based on an analysis of variance (ANOVA), calculated by R software (R Core Team, 2013). All treatments were considered as fixed effects, whereas interactions with replication were treated as random effects. Means and interactions were compared by Duncan’s test with least-square means comparisons.
Results
Seed viability
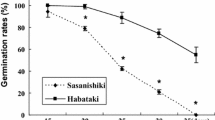
The initial germination percentages of Misuzudaizu in 2010 and 2019 were 80% and 60%, respectively, whereas those of Moshidou Gong 503 were 80% and 100%, respectively (Table 1). The germination percentages of Misuzudaizu in 2010 had fallen to 10% and 0%, respectively after storage at 25 °C and 35 °C for six months while the germination percentages of Moshidou Gong 503 still showed germination percentages 45 and 20, respectively, after storage at the high temperature (Fig. 1). However, the germination percentages of the two parents and RILs in both years decreased after storage in all conditions. The averages germination percentages of RILs after storage at 25 °C for six months was higher than for RILs after storage at 35 °C for six months in both years (Table 1).
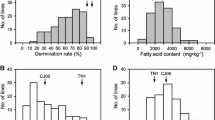
Frequency distribution of seed viability and seed vigor in each storage condition for RIL seeds in 2010 and 2019. a seed viability of 35 °C/6 months storage in 2010, b seed vigor of untreated seed in 2010, c seed vigor of 35 °C/6 months storage in 2010, d seed vigor of untreated seed in 2019 and e seed vigor of 25 °C/6 months storage in 2019. Open inverted triangle symbol: Misuzudaizu, filled inverted triangle symbol : Moshidou Gong 503
Seed vigor
After conducting the accelerated aging test, the germination percentages of Misuzudaizu in 2010 and 2019 were 10% and 20%, respectively, and those of Moshidou Gong 503 were 40%, and 80%, respectively (Table 1). The two parents after storage in all conditions showed decreased germination percentages in both years with Misuzudaizu in 2010 showing 0% in both storage conditions.
However, the germination percentages in both years of Moshidou Gong 503 was higher than Misuzudaizu in all conditions. The averages of RILs decreased with the storage temperature increased in both years (Table 1).
Effect of line and storage temperature on seed viability and seed vigor
An analysis of variance for seed viability and seed vigor after sstorage at each condition is shown in Table 2. Significant two-way interactions among lines X storage temperature (P > 0.0001) were observed for seed viability and seed vigor, except for seed viability in 2019.
QTL analysis
A total of five QTLs were identified for two traits, seed viability and seed vigor (Table 3). For seed viability after storage at 35 °C for six months in 2010, a QTL (qSG-Chr.8) with an LOD value of 4.03 and a phenotypic variance of 6.84% was detected on Chromosome 8. In seeds tested for seed vigor in the untreated condition in 2010, a QTL (qSV-Chr.6.1) was detected on Chromosome 6 with an LOD value of 3.64 and a phenotypic variance of 16.6%. For seed vigor at 35 °C for six months, a QTL (qSV-Chr.6.2) was detected on Chromosome 6 in 2010 with an LOD value of 2.95 and a phenotypic variance of 4.34%. For seed vigor in the untreated condition in 2019, a QTL (qSV-Chr.6.3) was detected on Chromosome 6 with an LOD value of 4.13 and a phenotypic variance of 13.1%. For seed vigor at 25 °C for six months, a QTL (qSV-Chr.2) was detected on Chromosome 2 with an LOD value of 5.02 and a phenotypic variance of 16.6% (Fig. 2).
Discussion
Soybean is categorized in the poor storability group of crop plants (Justice and Bass 1978). The environmental conditions for storage are significant factors influencing seed viability and vigor. This study used soybean recombinant inbred lines derived from a cross between Misuzudaizu (yellow) and Moshidou Gong 503 (brown) harvested in 2010 and 2019 to evaluate seed viability and vigor and to identify QTLs controlling seed storability. In comparing the germination percentage in the parental lines, Moshidou Gong 503 had a higher germination percentage in high temperature and long-term storage than Misuzudaizu (Fig. 1). After conducting the AA test, the germination percentage of Misuzudaizu, Moshidou Gong and the RILs decreased (Table 1). In particular, the germination percentage of Misuzudaizu was as low as 10% in untreated seed, whereas AA treated seeds failed to germinate in 2010 (Table 1, Fig. 1).
Genetic factors play a role in soybean seed storability (Shelar et al. 2008). Seed storability is a complex quantitative trait controlled by multiple genes and is easily influenced by the environment. Genetic loci associated with seed storability should be identified to develop cultivars resistant to storability issues and to characterize ways of controlling seed storability. This study found five QTLs associated with seed storability, which includes four for seed vigor and one for seed viability. A Misuzudaizu allele decreased seed viability at qSG-Chr.8 and showed similar effects at the QTLs qSV-Chr.6.1, qSV-Chr.6.2, qSV-Chr.6.3 and qSV-Chr.2 (Table 3). The QTLs qSV-Chr.6.1, qSV-Chr.6.2 and qSV-Chr.6.3 were located and closely placed on the same chromosome 6. The only seed vigor QTL that map on another chromosome is qSV-Chr.2 on chromosome 2. Watanabe et al. (2004) found sixty-six QTLs associated with three reproductive development traits and four seed quality traits as well as Otobe et al. (2015) could detect five QTLs associated with micromorphology on the seed coat surface in a RIL population derived from a cross between Misuzudaizu and Moshidou Gong 503 using the total of 360 markers. However, the total of 1131 markers were used in this study and five QTLs associated with seed storability were detected. In comparing our QTL results with QTLs for seed quality, we found that the qSV-Chr.6.2 and qSV-Chr.6.3 QTLs are new because they have not been associated with any other QTLs in previous reports. The qSG-Chr.8 QTL was located close to QTLs for maturity (HAV4), seed viability (VIS4) (Watanabe et al. 2004), and seed surface micromorphology (Otobe et al. 2015). The mapped location of qSV-Chr.6.1 at 119 cM was close to QTLs for germination rate (GRS1) at 117.6 cM and seed hardness (RAS1) at 117 cM (Watanabe et al. 2004) by the position of GRS1 located in the middle between RAS1 and qSV-C2.1. The qSV-D1b QTL was located at 127 cM and close to QTLs for seed hardness (RAS2) at 122.7 cM (Watanabe et al. 2004) and a seed surface trait (qSR3) at 114 cM (Otobe et al. 2015) by the position of RAS2 located in the middle between qSR3 and qSV-Chr.2. For QTL associated with seed storability, Daragahi et al. (2004) used the total of 128 SSR makers constructed 38 linkage groups and identified three QTLs on three linkage groups (C1, F and L) associated with seed storability in soybean under ambient condition (28 ± 2 °C) for 5 months and accelerated aging (42 ± 1 °C) for 72 h, and no stable QTL were found in different years. However, this study used 1,131 SSR markers and found five QTLs associated with seed storability on three linkage groups (A2, C2 and D1b) from 20 linkage groups, three QTLs located on same linkage group (C2) and the QTLs for seed vigor of untreated seeds in both 2010 and 2019 were stable QTLs. Moreover, only one QTL for seeds viability under high temperature condition (35 °C for 6 months) were found in this study.
Our study identified for the first time two new QTLs for seed vigor that were found in treated seeds stored at 35 °C for six months in 2010 and untreated seeds in 2019. Seed vigor analyzed by the accelerated aging test (AA Test) imitates stressful conditions for soybean seeds. Seeds with high vigor can tolerate stressful conditions during the AA Test. Therefore, evaluating seed vigor by the AA Test can detect changes in vigor more sensitively than the standard germination test. For seed viability, Watanabe et al. (2004) found five QTLs associated with seed viability; however, only qSG-Chr.8 was found in our study since seeds were stored long term at a high temperature. Moreover, qSG-Chr.8 was found to be located closely positioned on the same chromosome as VIS4, a QTL initially reported by Watanabe et al. (2004).
Seed longevity is thought to be progressively acquired during maturation and reaches its maximum during the seed filling phase or a later maturation phase (Zanakis et al. 1994; Gillen et al. 2012; Marcos-Filho 2016; Lima et al. 2017). The finding that the qSG-Chr.8 QTL was located close to the HAV4 QTL may indicate a relationship between seed longevity and seed maturation.
Pigmented seeds have higher germination rates and vigor than unpigmented seed coats and exhibit higher resistance to deterioration during storage (Singh and Ram 1986; Liu et al. 2017). Most hard seed characteristics in dark-colored soybeans are associated with permeability and protection against abiotic and biotic stresses and function to protect seeds from deterioration and to maintain seed vigor during long storage periods. There are multiple cell layers in the seed coats of dark seeds filled with phenolic compounds that function as antioxidants (Liu et al. 2017) and may act to reduce metabolic activity. Moshidou Gong 503 is a brown accession with a high degree of seed storability due to increased seed hardness and a smooth seed surface (Otobe et al. 2015). Smooth seed surfaces are derived from waxy materials that protect the seed from the environment and confer seed impermeability. Most yellow soybean seeds lose germination ability during storage and aging due to their thin and weak seed coats with wide pores. Although seed dormancy was broken by scarification in our study, some seeds were not viable and could not germinate. Soybean seeds stored long term in high temperature conditions had low seed viability and seed vigor. During storage, the influence of fatty acid metabolism continues to be important. During longer storage periods, the concentrations of fatty acid glucosides and aglycons increase, whereas the levels of acetyl glucoside and malonyl glucoside decreased after three years (Liu et al. 2017). Thus, deterioration at the biochemical, physiological and molecular levels may occur during storage, especially lipid peroxidation and membrane lipid hydrolysis.
In conclusion, seed storability is related to several seed quality traits. Most QTLs controlling seed storability are related to other QTLs for seed quality. The two new QTLs found in this study will provide useful information for identifying genes responsible for seed storability and for developing soybean cultivars with higher degrees of storability in the future. Although QTLs that regulate seed storage have been discovered, seed storability depends on the biochemical and physiological processes occurring in stored seeds. Changes in seed components that occur during seed storage are dynamic, and the relationship between the degrading enzymes and other factors involved in these changes should be analyzed in the future.
Data availability
The dataset in this study is available on the on the Glycine max / G. soja database in LegumeBase: (https://legumebase.nbrp.jp/glycine/rilChromosome/rilChromosomeList.jsp).
References
Bentsink L, Alonso-Blanco C, Vreugdenhil D, Tesnier K, Groot SPC, Koornneef M (2000) Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol 124:1595–1604. https://doi.org/10.1104/pp.124.4.1595
Börner A, Nagel M, Agacka-Mołdoch M, Gierke PU, Oberforster M, Albrecht T, Mohler V (2018) QTL analysis of falling number and seed longevity in wheat (Triticum aestivum L.). J Appl Genet 59(1):35–42. https://doi.org/10.1007/s13353-017-0422-5
Chwedorzewska KJ, Bednarek PT, Puchalski J (2002) AFLP profiling long term stored and regenerated rye gene bank sample. Cell Mol Biol Lett 7:569–576 (PMID: 12378250)
Clerkx EJM, Vries BD, Ruys GJ, Groot SPC, Koornneef M (2004) Genetic differences in seed longevity of various Arabidopsis mutants. Physiol Plant 121:448–461. https://doi.org/10.1111/j.0031-9317.2004.00339.x
Copeland LC, McDonald MB (2001) Principles of seed science and technology. 4th edition, USA. https://doi.org/10.1007/978-1-4615-1619-4
Dargahi H, Tanya P, Srinives P (2014) Mapping of the genomic regions controlling seed storability in soybean (Glycine max L.). J Genet 93:365–370. https://doi.org/10.1007/s12041-014-0381-0
Davies MJ (2005) The oxidative environment and protein damage. Biochimica Et Biphysica Acta 1703:93–109. https://doi.org/10.1016/j.bbapap.2004.08.007
Green DE, Pinnell EL (1968) Interitance of soybean seed quality I: Heritability of laboratory germination and field emergence. Crop Sci 8:5–11. https://doi.org/10.2135/cropsci1968.0011183X000800010003x
Gillen AM, Smith JR, Mengistu A, Bellaloui N (2012) Effects of maturity and Phomopsis longicolla on germination and vigor of soybean seed of near-isogenic lines. Crop Sci 52:2757–2766. https://doi.org/10.2135/cropsci2011.10.0566
Han Z, Ku L, Zhang Z, Zhang J, Guo S, Liu H, Zhao R, Ren Z, Zhang L, Su H, Dong L, Chen Y (2014) QTLs for seed vigor-related traits identified in maize seeds germinated under artificial aging conditions. PLoS ONE 9(3):e92535. https://doi.org/10.1371/journal.pone.0092535
International Seed Testing Association [ISTA] (2011) Seed vigour testing. international rule for seed testing, Zurich, Switzerland.
Justice OL, Bass LN (1978) Principles and practices of seed storage. Agriculture Handbook 506. Science and Education Administration, USDA, Washington D.C., USA
Li LF, Lin QY, Liu SY, Liu X, Wang WY, Hang NT, Liu F, Zhao ZG, Jiang L, Wan J (2012) Identification of quantitative trait loci for seed storability in rice (Oryza sativa L.). Plant Breeding 131:739–743. https://doi.org/10.1111/j.1439-0523.2012.02007.x
Lima JJP, Buitink J, Lalanne D, Rossi RF, Pelletier S, Silva EAA et al (2017) Molecular characterization of the acquisition of longevity during seed maturation in soybean. PLoS ONE 12:1–25. https://doi.org/10.1371/journal.pone.0180282
Liu J, Qin WT, Wu HJ, Yang CQ, Deng JC, Iqbal N, Liu WG, Du JB, Shu K (2017) Metabolism variation and better storability of dark-versus light-colored soybean (Glycine Max L. Merr.) seeds. Food Chem 233:104–113. https://doi.org/10.1016/j.foodchem.2016.12.036
Marcos-Filho J (2016) Seed physiology of cultivated plants. 2nd Edition, ABRATES, Londrina
Miura K, Lin SH, Yano M, Nagamine T (2002) Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.) Theor Appl Genet 104:981–986. https://doi.org/10.1007/s00122-002-0872-x
Nagel M, Rosenhauer M, Willner E, Snowdon RJ, Friedt W, Börner A (2011) Seed longevity in oilseed rape (Brassica napus L.) genetic variation and QTL mapping. Plant Genet Resour 9:260–263. https://doi.org/10.1017/S1479262111000372
Nagel M, Kodde J, Pistrick S, Mascher M, Börner A, Groot SP (2016) Barley seed aging: genetics behind the dry elevated pressure of oxygen aging and moist controlled deterioration. Front Plant Sci 7:388. https://doi.org/10.3389/fpls.2016.00388
Nkang A, Umlo EO (1996) Six month storability of five soybean cultivars as influenced by stage of harvest, storage temperature and relative humidity. Seed Sci Technol 25:93–99
Otobe K, Watanabe S, Harada K (2015) Analysis of QTLs for the micromorphology on the seed coat surface of soybean using recombinant inbred lines. Seed Sci Res 25:409–415. https://doi.org/10.1017/S0960258515000318
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/. Accessed 20 June 2021
Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowell D, Larinde M (2006) Between-paper method in the “Manual of seed handling in genebanks. Handbooks for Genebanks No. 8. Bioversity” International, Rome, Italy. pp 61–62
Sasaki K, Fukuta K, Sato T (2005) Mapping of quantitative trait loci controlling seed longevity of rice (Oryza sativa L.) after various periods of seed storage. Plant Breed 124:361–366. https://doi.org/10.1007/s00122-002-0872-x
Shelar VR, Shaikh RS, Nikam AS (2008) Soybean seed quality during storage: a review. Agric Rev 29(2):125–131
Singh RK, Ram HH (1986) Inheritance study of soybean seed storability using an accelerated ageing test. Field Crop Res 13(1):89–98. https://doi.org/10.1016/0378-4290(86)90013-4
Singh RK, Raipurai RK, Bhatia VS, Rani A, Pushpendra T, Husain SM, Satyavathi CT, Chauhan GS, Mohapatra T (2008a) Identification of SSR markers associated with seed coat permeability and electrolyte leaching in soybean. Physiol Mol Biol Plants 14:173–177. https://doi.org/10.1007/s12298-008-0016-0
Singh RK, Raipurai RK, Bhatia VS, Rani A, Pushpendra T, Husain SM, Satyavathi CT, Chauhan GS, Mohapatra T (2008b) SSR markers associated with seed longevity in soybean. Seed Sci Tech 36:162–167. https://doi.org/10.15258/sst.2008b.36.1.17
Tajuddin T, Satoshi W, Naoki Y, Kyuya H (2003) Analysis of quantitative trait loci for protein and lipid contents in soybean seeds using recombinant inbred lines. Breed Sci 53(2):133–140. https://doi.org/10.1270/jsbbs.53.133
TeKrony DM, Nelson C, Egli DB, White GM (1993) Predicting soybean seed germination during warehouse storage. Seed Sci Technol 21:127–137
Verma VD, Ram HH (1987) Genetics of seed longevity in soybean. Crop Improv 14:42–46
Vijay D, Dadlani M, Kumar PA, Panguluri SK (2009) Molecular marker analysis of differentially age seed of soybean and safflower. Plant Mol Report 27:282–291. https://doi.org/10.1007/s11105-008-0085-9
Wang Z, Wang J, Bao J, Wang F, Zhang H (2010) Quantitative trait loci analysis for rice seed vigor during the germination stage. J Zhejiang Univ Sci B 11:958–964. https://doi.org/10.1631/jzus.B1000238
Wang T, Hou L, Jian H, Di F, Li J, Liu L (2018) Combined QTL mapping, physiological and transcriptomic analyses to identify candidate genes involved in Brassica napus seed aging. Mol Genet Genomics 293:1421–1435. https://doi.org/10.1007/s00438-018-1468-8
Watanabe S, Tajuddin T, Yamanaka N, Hayashi M, Harada K (2004) Analysis of QTLs for reproductive development and seed quality traits in soybean using recombinant inbred lines. Breed Sci 54:399–407. https://doi.org/10.1270/jsbbs.54.399
Xue Y, Zhang S, Yano Q, Peng R, Xiong A, Li X, Zhu W, Zhu Y, Zha D (2008) Identification of quantitative trait loci for seed storability in rice (Oryza sativa L.). Euphytica 164:739–744. https://doi.org/10.1007/s10681-008-9696-3
Zanakis GN, Ellis RH, Summerfield RJ (1994) Seed quality in relation to seed development and maturation in 3 genotypes of soyabean (Glycine max). Expl Agric 30:139–156. https://doi.org/10.1017/S0014479700024091
Acknowledgements
This work was supported by the National BioResource Project (NBRP) of the Japan Agency for Medical Research and Development (AMED).
Funding
Japan Agency for Medical Research and Development (Grant No. 17930791).
Author information
Authors and Affiliations
Contributions
For conceptualization was design by MH, KH and PJ. Material preparation and data collection were performed by PJ. Data analysis was performed by PJ and IL. The first draft of the manuscript was written by PJ and MH and all author commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The author declare that there is no conflict of interest.
Ethics approval
This research did not involve research with human participants and/or animals.
Consent to participate
All author have reviewed the manuscript and approved their participation.
Consent for publication
All author have reviewed the manuscript and approved its submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiamtae, P., Hashiguchi, M., Lelapiyamit, I. et al. QTL analysis for soybean (Glycine max L. Merr.) seed storability in high-temperature storage conditions. Euphytica 218, 169 (2022). https://doi.org/10.1007/s10681-022-03122-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-022-03122-9