Abstract
Seed storability is especially important in the tropics due to high temperature and relative humidity of storage environment that cause rapid deterioration of seeds in storage. The objective of this study was to use SSR markers to identify genomic regions associated with quantitative trait loci (QTLs) controlling seed storability based on relative germination rate in the F2:3 population derived from a cross between vegetable soybean line (MJ0004-6) with poor longevity and landrace cultivar from Myanmar (R18500) with good longevity. The F2:4 seeds harvested in 2011 and 2012 were used to investigate seed storability. The F2 population was genotyped with 148 markers and the genetic map consisted of 128 SSR loci which converged into 38 linkage groups covering 1664.3 cM of soybean genome. Single marker analysis revealed that 13 markers from six linkage groups (C1, D2, E, F, J and L) were associated with seed storability. Composite interval mapping identified a total of three QTLs on linkage groups C1, F and L with phenotypic variance explained ranging from 8.79 to 13.43%. The R18500 alleles increased seed storability at all of the detected QTLs. No common QTLs were found for storability of seeds harvested in 2011 and 2012. This study agreed with previous reports in other crops that genotype by environment interaction plays an important role in expression of seed storability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two most important environmental factors that influence seed storage life are relative humidity, which controls seed moisture content; and temperature, which affects the rate of biochemical processes in seeds (Harrington 1972; Copeland and McDonald 2001). The effects of relative humidity and temperature on storage environment are highly interdependent. High relative humidity increases seed moisture content, leading to biochemical events such as increased hydrolytic enzyme activity, enhanced respiration and increase in free fatty acids. High temperature elevates rates of many enzymatic and metabolic reactions, causing more rapid deterioration. High temperatures hasten deterioration of high moisture seeds by increasing metabolic activity of hydrolysed substrates and enzymes, but exert only a minimal deteriorative effect on low moisture seeds (Copeland and McDonald 2001). A humid tropical climate characterized by high temperature and high relative humidity is very conducive to rapid deterioration of soybean seeds in storage, causing reduction in vigour and viability, leading to poor germination and suboptimal plant stand (Singh and Ram 1986). Moreover, soybeans is classified in the least storable group in their relative storability index (Justice and Bass 1978).
Development of cultivars resisting to adverse storage conditions is the most promising solution to the problem of seed deterioration in storage. To provide genetic material for this purpose, soybean genotypes resistant to the conditions should be identified. Then, molecular markers linking to seed storability should be located to accelerate breeding programmes by using marker-assisted selection (MAS).
Seed storability is a complex trait affected by environmental factors during seed formation, harvest and storage, and is usually controlled by several genes (Clerkx et al. 2004b). The use of molecular markers has provided insights into the genomic location and gene action of individual QTLs (Tanksley et al. 1989; Zhang et al. 2004). Genetic analyses of seed storability in soybean have been reported (Green and Pinnell 1968; Lanyon 1970; Kueneman 1983; Singh and Ram 1986; Cho and Scott 2000; Singh et al. 2008). Green and Pinnell (1968) showed low broad-sense heritability estimates for field emergence and laboratory germination characters because of large environmental variances in parents and F2 progenies. Lanyon (1970) reported that soybean varietal differences in field emergence may be related to one major gene and a complex of gene modifiers. Singh and Ram (1986) observed a maternal influence on seed storability in soybean lines and suggested that as few as one major gene may control seed storability. Four reciprocal crosses were made between soybean genotypes with good and poor seed longevity. Significant differences between reciprocal crosses revealed that the maternal plant genome can influence the longevity of seeds (Kueneman 1983). Cho and Scott (2000) reported significant genotype and environment effects for seed vigour in soybean lines, and general combining ability (GCA) effects for seed vigour were larger than specific combining ability (SCA) effects. Singh et al. (2008) tested 153 F2:3 soybean lines collected from replicated trails at two locations by accelerated ageing in laboratory to identify simple sequence repeat (SSR) markers associated with seed longevity. Out of 21 polymorphic markers used for genotyping the population, four independent SSR markers were significantly associated with seed longevity, explaining between 6.3 (satt285) and 7.5% (satt434) of total phenotypic variations for the trait.
Several QTLs for seed storability have been identified in Arabidopsis thaliana (Bentsink et al. 2000; Clerkx et al. 2004a, b), rice (Miura et al. 2002; Zeng et al. 2006; Xue et al. 2008), barley (Nagel et al. 2009), wheat (Rehman et al 2012) and oilseed rape (Nagel et al. 2011). In rice, three major QTLs for seed longevity were identified on different chromosomes (Xue et al. 2008). Clerkx et al. (2004b) detected three QTLs affecting viability in Arabidopsis that totally explained 48.9% of the variation. Several different QTLs have been reported for seed longevity in wheat from two different harvests (Rehman et al. 2012).
The objective of the present study was to determine genome position of QTLs controlling seed storability in soybean.
Materials and methods
Plant materials
One hundred and twenty nine F2:3 lines derived from a cross between R18500 (landrace cultivar with good longevity from Myanmar) and MJ0004-6 (vegetable soybean breeding line with low longevity from Thailand) were used in the experiment. The F2:3 population and their parents were grown in a randomized complete block design with two replications in the field of Kasetsart University, Kamphaeng Saen, Nakhon Pathom, Thailand in 2011 and 2012 growing seasons (November 2011 to March 2012 and November 2012 to March 2013). The harvested seeds (F2:4) were sundried until about 10% seed moisture was attained and then used for evaluation of seed storability.
Trait measurement
Two experiments were conducted to assess storability of parental soybean lines. One is accelerated ageing (AA) test, the other is ambient conditions test. For the AA test, the parental seeds were placed in a stainless metal cage sealed inside a glass jar containing 200 mL deionized water. The jars were kept at 42 ± 1∘C for 72 h, following International Seed Testing Association (ISTA) protocols (ISTA 2003). For ambient conditions test, the seeds were stored under ambient conditions for five months. The room temperature was 28 ± 2∘C and relative humidity was 50–60% during storing period. In each experiment, four replicates of 25 treated seeds from each parental line were tested for germination between moist rolled paper towels and kept at 25∘C in an incubator. On the eighth day after incubation, the number of germinated seeds and normal seedlings were counted as germination percentage. Higher germination percentage revealed higher seed storability.
DNA extraction and molecular marker development
DNA was extracted from fresh leaves of the F2 population at the seedling stage by using modified cetyl trimethylammonium bromide (CTAB) method advocated by Lodhi et al. (1994). The DNA was quantified against lambda DNA on 1.0% agarose gel stained with ethidium bromide and diluted to 10 ng/ μL. SSR markers were synthesized following the sequences published in the SoyBase website (http://soybase.agron.iastate.edu/). Five hundred and six SSR primers were used to survey for polymorphism among the parental lines. DNA amplification was performed using 2 μL of dH 2O, 1 μL of 10 × PCR buffer, 2 μL of 1 mM of each dNTP, 2 μL of each SSR primer, 0.8 μL of 25 mM MgCl 2, 0.2 μL of Taq DNA polymerase and 2 μL of 10 ng/ μL template DNA. PCR reactions were performed with predenaturing at 94∘C for 2 min and denaturing at 94∘C for 30 s. The cycle was repeated 35 times, then annealing at 47∘C for 30 s, extension at 72∘C for 1 min and the final extension at 72∘C for 1 min. The PCR products were separated by electrophoresis on denaturing 5% polyacrylamide gels in 0.5 × TBE stained with silver stain.
Construction of a genetic linkage map and QTL mapping
A linkage map was constructed using the program JoinMap 3.0 (Van Ooijen and Voorrips 2001). Genetic distance between markers was calculated using Kosambi map function (Kosambi 1944). Linkages among the adjacent markers were ensured at a minimum likelihood of odds (LOD) >3.0 and a maximum distance <50 centimorgan (cM). All SSR markers were initially tested for their significance by single marker analysis. QTL analysis was performed using composite interval mapping (CIM) method (Zeng 1994), using software application WinQTL Cartographer 2.5 (Wang et al. 2007). One thousand permutation tests were performed to establish empirical LOD thresholds at a significance level of 0.05 (Churchill and Doerge 1994). A LOD score of 2.5 was set as a threshold for declaring the presence of a QTL.
Results
Seed storability in parents and population
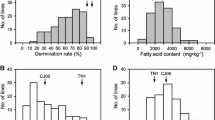
Germination rate of the two parents, showed higher difference when observed in the experiment of ambient conditions as compared to the AA test (table 1); so the ambient conditions were used for population screening. Frequency distribution of relative germination rate (%) (figure 1) indicated a clear difference between the two parental lines in both years. In the population, the relative germination rate ranged from 31% to 92% with a mean of 64.73% in 2011 harvest and from 29% to 87% with a mean of 61.41% in 2012 harvest. Normal distribution of relative germination rate in both years suggested that this trait is a quantitative trait.
Genetic map construction
Of the 506 SSR markers, 232 (46%) were found polymorphic between the parental lines. Of these, 148 markers were used in the initial linkage map construction. Based on the relative positions of these markers on the reference genetic maps (http://soybase.agron.iastate.edu/), 128 markers with clear polymorphic bands were assigned to 38 linkage groups covering 1664.3 cM of the soybean genome with an average density of 13 cM per marker, while 20 markers remained unlinked. Linkage groups were designated by names corresponding to the integrated public soybean genetic map (Song et al. 2004). In this study, five linkage groups (B1, D1a, H, I and N) were consistent with those of Cregan et al. (1999), while A1, A2, B2, C2, D1b, D2, G, J, K, L, M and O linkage groups were split into two sub-groups, and C1, E and F were divided into three sub-groups. Most of the markers mapped in the population showed Mendelian segregation (1:2:1), while 24 markers (16.0%) showed a significant deviation from the expected ratio.
Locating QTLs conditioning seed storability
Both single marker analysis (table 2) and composite interval mapping (table 3) were used to identify SSR markers associated with storability for seeds harvested in 2011 and 2012. Locations of the QTLs are marked on the genetic map (figure 2). The results of single marker analysis showed that there were three markers consisting of satt399 on linkage group C1 and satt598 and satt045 on linkage group E linked to the trait in 2011 harvest. Satt399 was detected in both seasons and showed highest coefficient of determination (R 2) for storability in 2011. CIM analysis located a QTL (qRGR-C1) on linkage group C1 explaining 13.43% of total variation of seed storability in 2011 harvest. Only satt399 on linkage group C1 was found significant from both analytical methods. Ten SSR markers with the R 2 ranged from 3.2% (satt313) to 10.2% (satt423) linking to seed storability in 2012 harvest were located on linkage groups D2, F, J and L by single marker analysis (table 2). Satt423 had the highest R 2. Via CIM, linkage groups F and L harbouring two QTLs (qRGR-F and qRGR-L) accounted for 10.02% and 8.79% of the variation in 2012 harvest, respectively. All of the detected markers on chromosomes F and L were significant from both single marker analysis and CIM. However, markers on linkage groups D2 and J did not reach the significant threshold by CIM.
Discussion
Soybean seeds decline in quality faster than seeds of other crops (Fabrizius et al. 1999). A major problem for soybean production in the tropics is the rapid loss of seed viability and vigour during storage under ambient tropical storage conditions (Nkang and Umho 1996). Moreover, in developing countries, most farmers have poor drying and storage facilities, causing the seed to rapidly lose its quality.
In this study, we used F2:4 seeds harvested in 2011 and 2012 derived from MJ0004-6 (poor longevity) and R18500 (good longevity) to identify QTLs determining seed storability in soybean. By comparing the germination rate of parents after treating with two methods (AA test and ambient condition for five months), less seed deterioration occurred in AA test, while severe deterioration occurred after storing the seeds in ambient conditions (table 1). Two markers on linkage group J (satt414 and satt380) associated significantly with storability in 2012, were within 14 and 19.93 cM of the marker (satt285) identified by Singh et al. (2008), respectively. However, no QTLs were detected on linkage group J through CIM. According to additive effects of all detected QTLs, the alleles contributed by MJ0004-6 decreased seed storability. No stable QTLs were found for storability in this investigation, revealing a strong genotype by environment interaction. The interaction could be created by many factors such as pest and disease infestation and preharvest and postharvest management practices (Lewis et al. 1998). Cho and Scott (2000) found significant genotypes and environment effects for seed vigour in 55 F2 soybean lines. Schwember and Bradford (2010) and Rehman et al. (2012) found strong genotype by environment interactions for seed longevity in lettuce and wheat genotypes, respectively. In both cases, no common QTLs for seed storability were detected from seeds produced in different years. Further investigation on seed storability QTLs is needed to explain this phenomenon. Expansion of our existing linkage map is also required for more comprehensive examination of the genetic basis for this trait.
All QTLs for seed storability detected in this study are mapped to the regions found associated with other traits in soybean. qRGR-C1 mapped closely to yield-related traits QTLs including QTLs for seed filling period (Cheng et al. 2011), days to maturity (Zhang et al. 2004) and seed yield (Guzman et al. 2007). The QTL identified on linkage group F (qRGR-F) mapped to the regions associated with seed oil content (Qi et al. 2011) and resistance to Phytophthora sojae (Burnham et al. 2003). Finally, qRGR-L on chromosome L is near to QTLs for seed weight (Csanadi et al. 2001; Hoeck et al. 2003), seed yield (Guzman et al. 2007), seed sucrose content (Kim et al. 2005) and seed glycine content (Panthee et al. 2006). This leads to the conclusion that superior agronomic performance may lead to good seed storability in soybean.
Information on the genetic basis of seed storability in soybean is still limited. Investigation on variation for seed storability across diverse germplasm in multiple environments and under different storage conditions will help discover stable and validated QTLs for improvement of this trait through marker-assisted selection.
References
Bentsink L., Alonso-Blanco C., Vreugdenhil D., Tesnier K., Groot S. P. C. and Koornneef M. 2000 Genetic analysis of seed soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol. 124, 1595–1604.
Burnham K., Dorrance A., VanToai T. and St. Martin S. 2003 Quantitative trait loci for partial resistance to Phytophthora sojae in soybean. Crop Sci. 43, 1610–1617.
Cheng L., Wang Y., Zhang C., Wu C., Xu J., Zhu H. et al. 2011 Genetic analysis and QTL detection of reproductive period and post-flowering photoperiod responses in soybean. Theor. Appl. Genet. 123, 421–429.
Cho Y. and Scott R. A. 2000 Combining ability of seed vigour and seed yield in soybean. Euphytica 112, 145–150.
Churchill G. A. and Doerge R. W. 1994 Empirical threshold values for quantitative trait mapping. Genetics 138, 963–971.
Clerkx E. J. M., Blankestijn-De V. H., Ruys G. J., Groot S. P. C. and Koornneef M. 2004a Genetic differences in seed longevity of various Arabidopsis mutants. Plant Physiol. 121, 448– 461.
Clerkx E. J. M., El-Lithy M. E., Vierling E., Ruys G. J., Blankestijn-De H., Groot S. P. C. et al. 2004b Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol. 135, 432–444.
Copeland L. C. and McDonald M. B. 2001 Principles of seed science and technology. 4th edition, 467 pp. Kluwer Academic Publishers, Massachusetts, USA.
Cregan P. B., Jarvik T., Bush A. L., Shoemaker R. C., Lark K. G., Kahler A. L. et al. 1999 An integrated genetic linkage map of the soybean genome. Crop Sci. 39, 1464–1490.
Csanadi G., Vollmann J., Stift G. and Lelley T. 2001 Seed quality QTLs identified in a molecular map of early maturing soybean. Theor. Appl. Genet. 103, 912–919.
Fabrizius E., Tekroni D. M., Egli D. B. and Rucker M. 1999 Evaluation of viability model for prediction soybean seed germination during warehouse storage. Crop Sci. 39, 194–201.
Green D. E. and Pinnell E. L. 1968 Inheritance of soybean seed quality I: Heritability of laboratory germination and field emergence. Crop Sci. 8, 5–11.
Guzman P., Diers B., Neece D., St. Martin S., LeRoy A., Grau C. et al. 2007 QTL associated with yield in three backcross-derived populations of soybean. Crop Sci. 47, 111–122.
Harrington J. F. 1972 Seed storage and longevity. In Seed biology, Insects, and seed collection, storage, testing and certification (ed. T. T. Kozlowski) vol. 3, pp. 145–245, Academic Press, New York USA.
Hoeck J. A., Fehr W. R., Shoemaker R. C., Welke G. A., Johnson S. L. and Cianzio S. R. 2003 Molecular marker analysis of seed size in soybean. Crop Sci. 43, 68–74.
International Seed Testing Association 2003 International rules for seed testing, pp. 6–9. International Seed Testing Association, Zurich, Switzerland.
Justice O. L. and Bass L. N. 1978 Principles and practices of seed storage. Handbook 506. pp. 289 Science and Education Administration, USDA, Washington D.C., USA.
Kim H., Kang S., Cho J., Choung M. and Suh D. 2005 Quantitative trait loci associated with oligosaccharide and sucrose contents on soybean (Glycine max L.) J. Plant Biol. 48, 106–112.
Kosambi D. 1944 The estimation of map distances from recombination values. Ann. Eugen. 12, 172–175.
Kueneman E. A. 1983 Genetic control of seed longevity in soybean. Crop Sci. 23, 5–8.
Lanyon L. 1970 Have you had problems with soybean emergence? Crops Soils 23, 13–14.
Lewis D. N., Marshall A. H. and Hides D. H. 1998 Influence of storage conditions on seed germination and vigour of temperate forage species. Seed Sci. Technol. 26, 643–655.
Lodhi M. A., Ye G. N., Weeden N. F. and Reisch B. I. 1994 A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Rep. 12, 6–13.
Miura K., Lyn S. Y., Yano M. and Nagamine T. 2002 Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.). Theor. Appl. Genet. 104, 981–986.
Nagel M., Vogel H., Landjeva S., Buck-Sorlin G., Lohwasser U., Scholz U. et al. 2009 Seed conservation in ex situ genebanks – genetic studies on longevity in barley. Euphytica 170, 5–14.
Nagel M., Rosenhauer M., Willner E., Snowdon R. J., Friedt W. and Borner A. 2011 Seed longevity in oilseed rape (Brassica napus L.) genetic variation and QTL mapping. Plant Genet. Res. 9, 260–263.
Nkang A. and Umho E. O. 1996 Six month storability of five soybean cultivars as influenced by stage of harvest, storage temperature and relative humidity. Seed Sci. Technol. 25, 93–99.
Panthee D., Pantalone V., Saxton A., West D. and Sams C. 2006 Genomic regions associated with amino acid composition in soybean. Mol. Breed. 17, 79–89.
Qi Z., Wu Q., Han X., Sun Y., Du X., Liu C. et al. 2011 Soybean oil content QTL mapping and integrating with meta-analysis method for mining genes. Euphytica 179, 499–514.
Rehman A. M. A., Nagel M., Neumann K., Kobiljski B., Lohwasser U. and Borner A. 2012 Genetic studies of seed longevity in hexaploid wheat using segregation and association mapping approaches. Euphytica 186, 1–13.
Schwember R. A. and Bradford K. J. 2010 Quantitative trait loci associated with longevity of lettuce seeds under conventional and controlled deterioration storage conditions. J. Exp. Bot. 61, 4423–4436.
Singh R. K., Raipuria R. K., Bhatia V. S., Rani A., Pushpendra H. S. M. et al. 2008 SSR markers associated with seed longevity in soybean. Seed Sci. Technol. 36, 162–167.
Singh R. K. and Ram H. H. 1986 Inheritance study of soybean seed storability using an accelerated aging test. Field Crops Res. 13, 89–98.
Song Q. J., Marek L. F., Shoemaker R. C., Lark K. G., Concibido V. C., Delannay X. et al. 2004 A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 109, 122–128.
Tanksley S. D., Young N. D., Paterson A. H. and Bonierbale M. W. 1989 RFLP mapping in plant breeding: new tools for an old science. Nat. Biotech. 7, 257–264.
Van Ooijen J. W. and Voorrips R. E. 2001. JoinMap3, software for the calculation of genetic linkage maps. Plant Research International, Wageningen, The Netherlands.
Wang S., Basten C. J. and Zeng Z. B. 2007 Windows QTL cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, N.C. Available from http://statgen.ncsu.edu/qtlcart/WQTLCart.htm.
Xue Y., Zhang S. Q., Yao Q. H., Peng R. H., Xiong A. S., Li X. et al. 2008 Identification of quantitative trait loci for seed storability in rice (Oryza sativa L.) Euphytica 164, 739–744.
Zeng D. L., Guo L. B., Xu Y. B., Yasukumi K., Zhu L. H. and Qian Q. 2006 QTL analysis of seed storability in rice. Plant Breed. 125, 57–60.
Zeng Z. B. 1994 Precision mapping of quantitative trait loci. Genetics 136, 1457–1468.
Zhang W. K., Wang Y. J., Luo G. Z., Zhang J. S., He C. Y., Wu X. L. et al. 2004 QTL mapping of ten agronomic traits on the soybean (Glycine max L. Merr.) genetic map and their association with EST markers. Theor. Appl. Genet. 108, 1131–1139.
Acknowledgements
This research was supported by the Centre of Advanced Studies in Agriculture and Food, KU Institute for Advanced Studies, Kasetsart University, Thailand.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Dargahi H., Tanya P. and Srinives P. 2014 Mapping of the genomic regions controlling seed storability in soybean (Glycine max L.). J. Genet. 93, xx–xx]
Rights and permissions
About this article
Cite this article
DARGAHI, H., TANYA, P. & SRINIVES, P. Mapping of the genomic regions controlling seed storability in soybean (Glycine max L.). J Genet 93, 365–370 (2014). https://doi.org/10.1007/s12041-014-0381-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-014-0381-0