Abstract
Chemical insecticides comprise the most widely used method of tomato pest control. Genotypes resistant to pests such as the silver leaf whitefly Bemisia tabaci may contribute towards the decreased use of insecticides to protect the environment. Leaf trichomes play a role in imparting resistance to pests, and exploiting intraspecific and interspecific variability for leaf trichomes in the tomato gene pool is an alternative to speed up introgression of resistance into improved lines or cultivars. We assessed the levels of whitefly resistance and trichome morphology/density in an array of accessions of the tomato gene pool and their hybrids with a tomato cultivar. A commercial cultivar (S. lycopersicum cv. Redenção), six wild species accessions, two S. lycopersicum var. cerasiforme genotypes, and eight intra and interspecific F1 hybrids were tested. The accessions LA 1401 (S. galapagense), AF 19684 (S. peruvianum), PI 127826 (S. habrochaites var. hirsutum), PI 134417 (S. habrochaites var. glabratum), LA 716 (S. pennellii), and RVTC 03 (S. lycopersicum var. cerasiforme) genotypes showed a higher density of glandular trichomes and carried the highest levels of whitefly resistance than the commercial cultivar. Segregating generations from their crosses with the cultivar may be promising sources to select plants with both higher density of glandular trichomes and resistance to whiteflies. Populations derived from S. lycopersicum var. cerasiforme’ RVTC 03’, which is part of intraspecific S. lycopersicum variability, maybe a more convenient source of pest resistance than those derived from interspecific crosses to obtain pest-resistant tomato cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tomato crop is plagued by an array of arthropod pests, which unchecked may cause considerable yield and quality losses (Mahmoud et al. 2020; Moerkens et al. 2020). Chemical pesticide sprays are the most often used method of pests/disease control but can lead to increased costs, environmental damages and harm to consumer’s health (Lachhab et al. 2015; Youssef et al. 2019). The use of pest-resistant tomato cultivars may represent a less costly and environmentally safer alternative to chemical control (Zanin et al. 2018; Neiva et al. 2019).
Whitefly (Bemisia tabaci biotype B) is currently one of the major tomato pests worldwide (Firdaus et al. 2012; Yule et al. 2019; Jafarbeigi et al. 2020). Its direct injury occurs by the insect sucking the sap, and triggering physiological disorders in tomato plants (Firdaus et al. 2012; Neiva et al. 2019), while indirect damage occurs through the transmission of begomovirus, as well as secretion of sugars that remain on the surface of the plant epidermis and may lead to the appearance of sooty mold (Capnodium spp.) (Andrade et al. 2017; Gouveia et al. 2018). These damages lead to yield losses, as well as to irregular fruit ripening that renders fruit unsuitable for fresh consumption or processing. The development of whitefly-resistant cultivars can therefore be considered a tomato breeding priority (Firdaus et al. 2012; Neiva et al. 2019). The main physic-chemical plant factors conferring whitefly resistance are reported to be the trichomes (Glas et al. 2012; Andrade et al. 2018).
Trichomes are essential as natural plant defenses especially against phytophagous insects (Silva et al. 2019). They consist of epidermal structures similar to hairs that can influence insect behavior and consequently confer pest resistance (Rakha et al. 2017). The different types of trichomes are classified based on the presence or absence of trichome glandular head, stalk length, cell number and base cell number properties (Luckwill 1943). Tomato trichomes are usually categorized as glandular types I, IV, VI and VII and non-glandular types II, III and V (Luckwill 1943; Channarayappa et al. 1992). Glandular trichomes release sticky and/or toxic exudates, which may cause pest death (Andrade et al. 2018). Additionally, non-glandular trichomes may act by mechanical means and by forming a barrier that prevents pest contact with plant tissue (Simmons and Gurr 2005).
Levels of resistance to whitefly are highly variable among accessions of the tomato gene pool (Firdaus et al. 2012). Genotypes of wild tomato species S. pennellii (Resende et al. 2002) and S. habrochaites (Maluf et al. 2001) are noteworthy concerning the introgression of pest resistance characteristics in cultivated tomatoes. However, many horticultural undesirable genes are involved in the process of introgression of whitefly resistance from wild parents into commercial cultivars (Momotaz et al. 2010). Therefore, exploiting resistance derived from accessions that are taxonomically closer to S.lycopersicon may contribute to a reduction in time and costs necessary to obtain improved resistant cultivars. The species S. pimpinellifolium and S. galapagense are closer to S. lycopersicum in comparison with S. pennellii and S. habrochaites (Peralta et al. 2008). S. lycopersicum var. cerasiforme was also considered to be promising as a donor of resistance genes (Lucini et al. 2016). Considering the wide genetic variability of tomatoes, relatively few studies exploring potential pest resistance are available (Firdaus et al. 2012). Further studies related to the presence of glandular trichomes and their relation with whitefly resistance in specific wild or feral accessions and F1 their hybrids with S. lycopersicum would provide important information for resistance introgression. Further studies are needed on the presence of glandular trichomes in putative whitefly-resistant accessions, and their relationship with resistance and the levels of resistance eventually found in their hybrids with S. lycopersicum.
The objectives of this work are: (a) to assess the comparative levels of whitefly resistance in wild tomato accessions, in their hybrids with a commercial S. lycopersicum, and the possible relationship with the presence of glandular trichomes; (b) to identify putative sources of whitefly resistance in accessions taxonomically close to S. lycopersicum, that could be deployed more efficiently in the process of introgression of resistance to obtain improved cultivars.
Materials and methods
Genotypes and experimental design
Six wild tomato species were assessed (S. pimpinellifolium ‘AF 26970’, S. galapagense ‘LA 1401’, S. peruvianum ‘AF 19684’, S. habrochaites var. hirsutum ‘PI 127826’, S. habrochaites var. glabratum ‘PI134417’ and S. pennellii ‘LA 716’) along with two S. lycopersicum var. cerasiforme genotypes (‘RVTC 03’ and ‘RVTC 66’), one S. lycopersicum line (cultivar Redenção); and eight hybrids [F1(Redenção × AF 26970), F1(Redenção × LA 1401), F1(Redenção × AF 19684), F1(Redenção × PI 127826), F1(Redenção × PI 134417), F1(Redenção × LA 716), F1(Redenção × RVTC 03) and F1(Redenção × RVTC 66)]. A randomized block design was used, with five replications with eight plants each. All interspecific F1 hybrids bore small fruit (ca. 1–2 cm diameter), and the only ones showing mature fruit colors similar (red or orange) to S. lycopersicum were those with the species that are more closely related to the latter, i.e., S. pimpinellifolium and S. galapagense.
Sowing was carried out in expanded polystyrene trays with 200 cells containing a commercial substrate based on bio-stabilized pine bark. Trays were maintained in a greenhouse until the date of transplant. ‘Redenção’, ‘RVTC 03’, ‘RVTC 66’, ‘AF 26970’ and ‘AF 19684’, as well as the F1hybridswere sown in the same date, while genotypes LA 1401, PI 127,826, PI 134,417 and LA 716 were sown ten days before, due to expected differences in germination and emergence. Seedlings were transplanted to 8 dm3 pots at the stage of 4–5 fully expanded leaves. The pots containing a commercial substrate based on bio-stabilized pine bark. Base fertilization with 2 g of N, 3 g of P, 3 g of K, 3 g of Ca, 1.8 g of Mg and 1.5 of S per pot was used. The plants were irrigated as needed with the use of micro-drippers. No phytosanitary sprayings (insecticides or fungicides)were carried out during plant growth and development.
Assessment of resistance to whitefly Bemisia tabaci
At the onset of flowering (30 days after transplanting) plants were assessed for resistance to whitefly in a greenhouse test under free choice conditions. A total of 150 non-sexed B. tabaci biotype B whitefly adults were released per plant. Attractiveness was assessed 24, 48 and 96 h after insect release. With the aid of a mirror, the number of adults present on the abaxial surface of three previously marked leaflets in the upper, middle and lower thirds of each plant was counted (Baldin et al. 2007). Whitefly egg and nymph counts were performed in quadruplicate on the 28th day after infestation. Three fully expanded young leaflets were collected during each evaluation, one from each third part (upper, middle and lower) of each plant. The leaflets were properly identified, placed in a gearbox and sent to the laboratory. The number of live eggs and nymphs present on the abaxial faces of each leaflet was counted with the aid of a stereoscopic binocular microscope (Olympus® bh2), in a 4 cm2 area, excluding the central rib.
Leaf trichome identification and counts
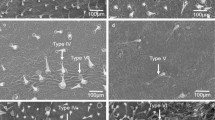
Four young leaflets were removed from the upper third of each plant for trichome enumeration and identification. Slides were mounted in quadruplicate with paradermic cuts on the abaxial and adaxial surfaces for each recently collected leaflet and subsequently fixed with carbon tape on a metallic support. A 5 mm2area was analyzed using a scanning electron microscope (Tescan® Vega3 HV) at 30 kv. Leaflet surfaces were photographed to facilitate length observations and identification of the presence or absence of gland(s) at the apical end of the leaf trichomes, as well as their shape. Based on these characteristics, trichome identification was carried out according to Channarayappa et al. (1992).
Data analyses
The data related to trichomes and whitefly resistance were subject to using the log (x + 1) transformation. Normality and homogeneity of residual variance assumptions were confirmed, the data were subjected to an analysis of variance (ANOVA). Means were subjected to statistical tests when the F test was significant, to differentiate and identify the most promising genotypes. Spearman correlations were calculated between glandular trichomes, non-glandular trichomes, number of adults 96 h after infestation, number of eggs, and number of whitefly nymphs. All analyses were performed using the GENES software (Cruz 2013).
Results
Trichome morphology and density
The most frequently observed glandular trichomes were IV and VI types, with higher numbers on abaxial leaflet surfaces (Table 1). All genotypes, except for ‘AF 26970’, F1(Redenção × RVTC 66) and F1(Redenção × AF 26970), displayed a higher total abaxial density of glandular trichomes (types I + IV + VI + VII) than the commercial S.lycopersicum cultivar Redenção (P < 0.05). The highest abaxial density of type IV and VI trichomes compared to ‘Redenção’ was observed in genotypes PI 127826 (312.89 per 5 mm2) and AF 19684 (327.50 per 5 mm2), respectively. However, ‘AF 19684’ displayed only type IV trichomes, on abaxial leaflet surfaces. The commercial cultivar Redenção presented the highest density of non-glandular trichomes on the same surface (492.44 per 1 mm2) (Table 1).
On the adaxial leaflet surface, the highest glandular trichome densities were observed (types I + IV + VI + VII) in genotypes LA 1401, AF 19684, PI 127826, PI 134417, LA 716, F1(Redenção × LA 1401), F1(Redenção × PI 127826), F1 (Redenção × PI 134417) and F1(Redenção × LA 716) (P < 0.05). The highest densities of type IV and VI trichomes on the adaxial leaflet surface were observed in ‘PI 127826’ (85.00 per 5 mm2) and ‘AF 19684’ (122.33 per 5 mm2), respectively. Non-glandular trichome densities in the commercial cultivar Redenção did not differ those found in ‘RVTC 03’, ‘RVTC 66’, F1(Redenção × RVTC 03), F1(Redenção × RVTC 66) and F1(Redenção × AF 19684) (Table 1). Type VI trichomes were not found in the adaxial surface either in ‘RVTC03’, ‘RVTC66’, ‘AF26970’, ‘LA716’, or in their hybrids with Redenção. Type IV trichomes neither were nor present in ‘AF26970’ and in ‘PI 134417’s adaxial surfaces, and were present in low densities in AF19684, and in the hybrids with these three accessions. Type IV trichomes were not present in the abaxial surfaces of genotypes AF26970, AF19684’, and in F1(Redenção × AF 26970), and Type IV trichomes were not present in’RVTC03’, ‘AF26970’, ‘LA716’, or in the hybrids F1(Redenção × RVTC66), F1(Redenção × AF26970) and F1(Redenção × LA716).
Assessment of resistance to whitefly Bemisia tabaci
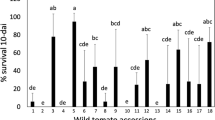
In the insect free-choice trials, RVTC 03, AF 19684, LA 716, LA 1401, PI 127826 and PI 134417 genotypes and their respective hybrids with Redenção presented an adult whitefly infestation of ≤ 1 per 4 cm². No whitefly adults in the LA 716, LA 1401 and PI 127826 genotypes were observed at 24, 48 and 96 hours after infestation. In contrast, ‘Redenção’, ‘RVTC 66’, ‘AF 26970’, F1(Redenção × RVTC 66) and F1(Redenção × AF 26970) grouped as the most susceptible genotypes (P < 0.05), due to the higher presence of whitefly adults (Fig. 1). At 28 days after infestation, ‘LA 716’, ‘LA 1401’, ‘PI 127826’, ‘AF 19684’, ‘PI 134417’, F1(Redenção × LA 1401), F1(Redenção × LA 716) and ‘RVTC 03’ showed the lowest number of eggs and whitefly nymphs (P < 0.05). The highest egg density was observed in the interspecific hybrid F1(Redenção × AF 26,970), while the highest nymph density was detected in AF 26,970 (Fig. 2).
Association between trichomes and whitefly resistance
Table 2 showed that the determined parameters were significantly correlated. In particular, glandular trichome densities were negatively correlated with non-glandular trichomes (r = − 0.56), number of adults (r = − 0.89), number of eggs (r = − 0.72), and number of nymphs (r = − 0.88).In addition, non-glandular trichomes were positive correlated with number of eggs (r = 0.63) and nymphs (r = 0.51). Additionally, positive correlations were detected between the number of whitefly adults, eggs, and nymphs (Table 2).
Discussion
One of the objectives of the present research was to exploit resistance to whitefly observed in a sample of the tomato gene pool and verify its association with leaf trichome morphology and density. The trichome morphology of both cultivated and wild tomato species has been well characterized (Luckwill 1943; Channarayappa et al. 1992; Silva et al. 2019). However, considering the genetic variation among tomato species and their potential use in improving cultivated genotypes, there is still much to be explored regarding the trichome-related parameters (Zeist et al. 2018a). Variations between trichome types and densities in tomato species and hybrids were detected. Trichome characteristics and the potential of glandular trichomes in the breeding of commercial tomato plants aiming resistance for pests have been reported in populations derived from crosses of S. lycopersicum with S. galapagense ‘LA-1401’ (Andrade et al. 2017), with S. habrochaites var. hirsutum ‘PI 127826’ (Oliveira et al. 2018), with S. habrochaites var. glabratum ‘PI 134417’ (Neiva et al. 2013) and with species S. pennellii ‘LA-716’ (Dias et al. 2019). Our results reinforce these previously reported whitefly resistances of ‘LA-1401’, ‘PI 127826’, ‘PI 134417’ and ‘LA-716’, and their associations with glandular trichomes. However, trichome-related characters in ‘AF 26970’ (S. pimpinellifolium), ‘AF 19684’ (S. peruvianum), ‘RVTC 03’ and ‘RVTC 66’ (S. lycopersicum var. cerasiforme) genotypes were not reported previously. While the whitefly resistant accessions LA1401, PI 127826 and PI134417 contain both type IV and type VI trichomes, the also resistant ‘LA-716’ contains type IV glandular trichomes only, and ‘AF 19684’almost exclusively type VI trichomes (Table 1).
Our results showed that the S. lycopersicum var. cerasiforme accessions ‘AF 19684’ and ‘RVTC 03’ are potential sources of genes to obtain improved tomato lines with whitefly resistance. In ‘RVTC 03’, however, resistance may not be explained by higher densities of glandular trichomes, because they are even lower than those found in the whitefly-susceptible ‘RTVC66’.
The use of the interspecific variability of the LA 1401, PI 127826, PI 134417 and LA 716 genotypes in tomato breeding has previously reported (Neiva et al. 2013; Silva et al. 2019), however, only feasible in the long term and a very laborious process (Zeist et al. 2018a). This is due to the non-commercial patterns of these wild genotypes and the morphological differences in S. lycopersicum (Zeist et al. 2020). Thus, in the incorporation of genes related to trichomes and resistance to pests, successive cycles of backcrossing and selection are necessary to obtain advanced lines with good commercial standard (Neiva et al. 2013; Zeist et al. 2018a). The same aspect is valid to the AF 19684 accession from S. peruvianum and its Redenção hybrid (Zeist et al. 2018a, b, 2020), which demonstrated potential in generating whitefly-resistant genotypes with a higher density of type VI glandular trichomes. While testing 26 wild accessions that differed from the ones in our study, Firdaus et al. (2012) identified among them both whitefly susceptible and whitefly resistant accessions, with the greatest resistance potential in Solanum galapagense ‘PRI95004/PY8027’. Resistance in the latter accession was associated with type IV trichomes. Neither S. lycopersicum var cerasiforme nor interspecific hybrids were tested by these authors. In the present work, a different wider array of wild species were tested, and we report that some genotypes bear only type IV or type VI trichomes and that the trichome types present on the abaxial and adaxial leaf surfaces may differ.
Significant negative correlations between glandular trichomes and the number of whitefly adults, eggs and nymphs indicated that trichomes were associated with whitefly resistance, as genotypes with a higher number of granular trichomes presented low incidences of whitefly adults, eggs and nymphs. Whitefly resistance is associated with allelochemical levels present in glandular trichomes (Andrade et al. 2017; Neiva et al. 2019), naturally occurring chemical substances with antagonistic action toward pests. However, this may not be the only mechanism imparting insect resistance: the glandular trichome densities in the whitefly resistant ‘RTVC03’ are comparable or lower than in the whitefly susceptible ‘RTVC66’.
Acyl sugars, sesquiterpenes and methyl ketones, among others, are noteworthy among several allelochemicals found in wild tomato species (Neiva et al. 2013). Acyl sugars, such as acylglucoses and acylsucroses, have been reported in the leaf trichomes of the S. pennellii ‘LA 716’ (Dias et al. 2019) and the S. galapagense ‘LA 1401’ (Silva et al. 2019). Sesquiterpenes, mainly zingiberene, have been reported in the S. habrochaites var. hirsutum accession PI 127826 (Oliveira et al. 2018) and methyl ketone, 2-tridecanone, in the S. habrochaites var. glabratum accession PI 134417 (Neiva et al. 2013; Zeist et al. 2018a). These allelochemicals may lead to antibiosis and/or antixenosis plant responses against pests (Simmons and Gurr 2004; Zeist et al. 2018a).
Positive and significant correlations between non-glandular trichomes and the number of whitefly eggs and nymphs indicated that trichomes were not associated with whitefly resistance. Besides, a negative correlation between glandular and non-glandular trichomes was also found by Andrade et al. (2018), who suggested an ontogenetic relationship between these types of trichomes, where the presence of alleles responsible for allelochemical contents would be involved in the development of glandular structure at the tip of the trichomes.
It’s well known that non-glandular trichomes do not produce toxic exudates, so plant protection, if any, is only physical (Cho et al. 2017). ‘RVTC 66’, ‘AF 26970’, ‘Redenção’, F1(Redenção × RVTC 66) and F1(Redenção × AF 26970), which presented a lower number of glandular trichomes, displayed a higher incidence of whitefly eggs and nymphs (Table 1; Figs. 1, 2).
The potential of the RVTC 03 genotype to increase whitefly resistance in cultivated tomatoes is very promising. ‘RVTC’ 03 is part of the intraspecific S. lycopersicum variability (var. cerasiforme), producing fruits with a pattern similar to commercial varieties (Preczenhak et al. 2014). Because feral forms of var. cerasiforme are reported to be the more direct ancestors of the cultivated forms of S. lycopersicum (Nesbitt and Tanksley 2002; Bai and Lindhout 2007; Peralta et al. 2008), their genetic proximity to the latter makes accessions such as ‘RVTC 03’ a more convenient source of resistance for deployment in breeding programmers than other similarly resistant accessions found in S. pennellii, S. habrochaites and even in S. galapagense. Deployment of ‘RVTC 03’ as a source of resistance may represent the need for a smaller number of backcrosses towards the susceptible horticultural acceptable parent, thereby reducing time and resources needed to obtain an improved resistant tomato line (Rodríguez et al. 2010). The potential for using S. lycopersicum var. cerasiforme in the breeding of cultivated tomatoes has been commonly reported for several purposes (Ranc et al. 2008; Rodríguez et al. 2010; Leiva-Brondo et al. 2016). Thousands of genotypes are part of the cerasiforme variety, and their peculiarities should be further studied (Ranc et al. 2008; Islam et al. 2012; Preczenhak et al. 2014). This is evident when observing the contrasting differences between ‘RVTC 03’ and ‘RVTC 66’ relative to trichome types/densities and whitefly resistance, in which only the former demonstrated the potential for deployment in a resistance breeding.
References
Andrade MC, Silva AA, Carvalho RC, Santiago JA, Oliveira AMS, Francis DM, Maluf WR (2018) Quantitative trait loci associated with trichomes in the Solanum galapagense accession LA1401. Genet Resour Crop Evol 65:1671–1685. https://doi.org/10.1007/s10722-018-0644-3
Andrade MC, Silva AA, Neiva IP, Oliveira IRC, Castro EM, Francis DM, Maluf WR (2017) Inheritance of type IV glandular trichome density and its association with whitefly resistance from Solanum galapagense accession LA1401. Euphytica 213:52. https://doi.org/10.1007/s10681-016-1792-1
Baldin ELL, Souza DR, Souza ES, Beneduzzi RA (2007) Controle de mosca-branca com extratos vegetais em tomateiro cultivado em casa-de-vegetação. Hortic Bras 25:602–606. https://doi.org/10.1590/S0102-05362007000400022
Bai Y, Lindhout P (2007) Domestication and breeding of tomatoes: what have we gained and what can we gain in the future? Ann Bot 5:1085–1094. https://doi.org/10.1093/aob/mcm150
Cruz CD (2013) GENES - a software package for analysis in experimental statistics and quantitative genetics. Acta Sci Agron 35:271–276. https://doi.org/10.4025/actasciagron.v35i3.21251
Channarayappa SG, Muniyappa V, Frist RH (1992) Resistance of Lycopersicon species to Bemisiatabaci, a tomato leaf curl virus vector. Can J Bot 70:2184–2192. https://doi.org/10.1139/b92-270
Cho K, Kwon M, Cho J, Im J, Yong-Eun P, Hong S, Hwang I, Kang J (2017) Characterization of trichome morphology and aphid resistance in cultivated and wild species of potato. Hortic Environ Biotechnol 58:450–457. https://doi.org/10.1007/s13580-017-0078-4
Dias DM, Resende JTV, Zeist AR, Gabriel A, Santos MH, Vilela NC (2019) Resistance of processing tomato genotypes to leaf miner (Tutaabsoluta). Hortic Bras 37:40–46. https://doi.org/10.1590/s0102-053620190106
Firdaus S, van Heusden AW, Hidayati N, Supena EDJ, Visser RGF, Vosman B (2012) Resistance to Bemisiatabaci in tomato wild relatives. Euphytica 187:31–45. https://doi.org/10.1007/s10681-012-0704-2
Firdaus S, Van Heusden AW, Hidayati N, SupenaEDJ, Visser RGF, Vosman B (2013) Identification and QLT mapping of whitefly resistance components in Solanumgalapagense. Theor Appl Genet 126:1487–1501. https://doi.org/10.1007/s00122-013-2067-z
Glas JJ, Schimmel BCJ, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR (2012) Plant glandular trichomes as targets for breeding and engineering resistance to herbivores. Int J Mol Sci 13:17077–17103. https://doi.org/10.3390/ijms131217077
Gouveia BT, Oliveira AMS, Ribeiro GHMR, Maluf WR (2018) Resistance to whitefly (Bemisia argentifolii) and repellency to the two-spotted spider mite (Tetranychus urticae) in tomato plant hybrids with high leaf contents of acylsugar and the Mi gene. Euphytica 214:140. https://doi.org/10.1007/s10681-018-2224-1
Islam M, Mohanta H, Ismail M, Rafii MY, Malek MA (2012) Genetic variability and trait relationship in cherry tomato (Solanum lycopersicum l. Var. cerasiforme (Dunnal) A. Gray). Bangladesh J Bot 41:163–167. https://doi.org/10.3329/bjb.v41i2.13443
Jafarbeigi F, Samih MA, Alaei H, Shirani H (2020) Induced tomato resistance against Bemisia tabaci triggered by salicylic acid, β-aminobutyric acid, and trichoderma. Neotrop Entomol 49:456–467. https://doi.org/10.1007/s13744-020-00771-0
Lachhab N, Sanzani SM, Fallanaj F, Youssef K, Nigro F, Boselli M, Ippolito A (2015) Protein hydrolysates as resistance inducers for controlling green mould of citrus fruit. Acta Hortic 1065:1593–1598. https://doi.org/10.17660/ActaHortic.2015.1065.203
Leiva-Brondo M, Valcarcel M, Martí R, Roselló S, Cebolla-Cornejo J (2016) New opportunities for developing tomato varieties with enhanced carotenoid content. Sci Agric 6:512–519. https://doi.org/10.1590/0103-9016-2015-0427
Li N, Chen J, Shi Y-P (2015) Magnetic graphene solid-phase extraction for the determination of carbamate pesticides in tomatoes coupled with high performance liquid chromatography. Talanta 141:212–219. https://doi.org/10.1016/j.talanta.2015.04.018
Lucini T, Resende JTV, Oliveira JRF, Scabeni CJ, Zeist AR, Resende NCV (2016) Repellent effects of various cherry tomato accessions on the two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae). Genet Mol Res 15:1–6. https://doi.org/10.4238/gmr.15017736
Luckwill LC (1943) The genus Lycopersicon: a historical, biological and taxonomic survey of the wild and cultivated tomatoes. University of Aberdeen, UK
Mahmoud YA, Ebadah IMAEM, Attwa W, Moawad S, Omar N, El-Wahab TEA, Sadek H (2020) Susceptibility of different tomato, Solanum lycopersicum L., varieties to infestation with some insect pests in Egypt. Bull Natl Res Cent 44:46. https://doi.org/10.1186/s42269-020-00300-4
Maluf WR, Campos GA, Cardoso MG (2001) Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica 121:73–80. https://doi.org/10.1023/A:1012067505361
Moerkens R, Janssen D, Brenard N, Reybroeck E, Tellez MM, Rodríguez E, Bosmans L, Leirs H, SluydtsV (2020) Simplified modelling enhances biocontrol decision making in tomato greenhouses for three important pest species. J Pest Sci. https://doi.org/10.1007/s10340-020-01256-0
Momotaz A, Scott JW, Schuster DJ (2010) Identification of quantitative trait loci conferring resistance to Bemisia tabaci in an F2 population of Solanum lycopersicum × S. habrochaites accession LA1777. J Am Soc Hortic Sci 135(2):134–142. https://doi.org/10.21273/JASHS.135.2.134
Neiva IP, Andrade Júnior VC, Maluf WR, Oliveira CM, Maciel GM (2013) Role of allelochemicals and trichome density in the resistance of tomato to whitefly. Ciênc Agrotec 37:61–67. https://doi.org/10.1590/S1413-70542013000100007
Neiva IP, Silva AA, Resende JF, Carvalho RC, Oliveira AMS, Maluf WR (2019) Tomato genotype resistance to whitefly mediated by allelochemicals and Mi gene. Chil J Agric Res 79:124–130. https://doi.org/10.4067/S0718-58392019000100124
Nesbitt TC, Tanksley SD (2002) Comparative sequencing in the genus Lycopersicon: implication for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 162:365–379. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1462239
Oliveira JRF, Resende JTV, Maluf WR, Lucini T, Lima Filho RB, Lima IP, Nardi C (2018) Trichomes and allelochemicals in tomato genotypes have antagonistic effects upon behavior and biology of Tetranychus urticae. Front Plant Sci 9:1132. https://doi.org/10.3389/fpls.2018.01132
Peralta IE, Spooner DM, Knapp S (2008) Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides. sect. Juglandifolia. sect. Lycopersicon; Solanaceae). Syst Bot Monogr 8:1–186
Preczenhak AP, Resende JTV, Chagas RR, Silva PR, Schwarz K, Morales RGF (2014) Caracterização agronômica de genótipos de minitomate. Hortic Bras 32:348–356. https://doi.org/10.1590/S0102-053620140003000018
Rakha M, Hanson P, Ramasamy S (2017) Identification of resistance to Bemisia tabaci Genn. in closely related wild relatives of cultivated tomato based on trichome type analysis and choice and no-choice assays. Genet Resour Crop Evol 64:247–260. https://doi.org/10.1007/s10722-015-0347-y
Ranc N, Muños S, Santoni S, Causse M (2008) A clarified position for Solanum lycopersicum var. cera siformein the evolutionary history of tomatoes (Solanaceae). BMC Plant Biol 8:130. https://doi.org/10.1186/1471-2229-8-130
Resende JTV, Maluf WR, Cardoso MG, Nelson DL, Faria MV (2002) Inheritance of acylsugar contents in tomatoes derived from an interspecific cross with the wild tomato Lycopersicon pennellii and their effect on spider mite repellence. Genet Mol Res 1:106–116
Rodríguez GR, Pratta GR, Liberatti DR, Zorzoli R, Picardi LA (2010) Inheritance of shelf life and other quality traits of tomato fruit estimated from F1’s, F2’s and backcross generations derived from standard cultivar, nor homozygote and wild cherry tomato. Euphytica 176:137–147. https://doi.org/10.1007/s10681-010-0241-9
Silva AA, Carvalho RC, Andrade MC, Zeist AR, Resende JTV, Maluf WR (2019) Glandular trichomes that mediate resistance to green peach aphid in tomato genotypes from the cross between S. galapagense and S. lycopersicum. Acta SciAgron 41:e42704. https://doi.org/10.4025/actasciagron.v41i1.42704
Simmons AT, Gurr GM (2004) Trichome-based host plant resistance of Lycopersicon species and the biocontrol agent Malladasignata: are they compatible? Entomol Exp Appl 113:95–101. https://doi.org/10.1111/j.0013-8703.2004.00210.x
Simmons AT, Gurr GM (2005) Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric For Entomol 7:265–276. https://doi.org/10.1111/j.1461-9555.2005.00271.x
Youssef K, Roberto SR, de Oliveira AG (2019) Ultra-structural alterations in Botrytis cinerea—the causal agent of Gray Mold—treated with salt solutions. Biomolecules 9(10):582. https://doi.org/10.3390/biom9100582
Yule S, Chiemsombat P, Srinivasan R (2019) Detection of Tomato yellow leaf curl Thailand virus transmitted by Bemisia tabaci Asia I in tomato and pepper. Phytoparasitica 47:143–153. https://doi.org/10.1007/s12600-018-00712-z
Zanin DS, Resende JTV, Zeist AR, Oliveira JRF, Henschel JM, Lima Filho RB (2018) Selection of processing tomato genotypes resistant to two spotted spider mite. Hortic Bras 36:271–275. https://doi.org/10.1590/s0102-053620180221
Zeist AR, Faria MV, Resende JTV, Gabriel A, Nonato JJ, Santos MH (2020) Biomass association in specimens and interspecific hybrids of tomatoes. Acta Sci Agron 42:e42806. https://doi.org/10.4025/actasciagron.v42i1.42806
Zeist AR, Resende JTV, Faria MV, Gabriel A, Adriano E, Lima Filho RB (2018b) Photosynthetic characteristics in species and interspecific hybrids of tomato. Hortic Bras 36:352–360. https://doi.org/10.1590/s0102-053620180313
Zeist AR, Silva AA, Maluf WR, Resende JTV, Gabriel A, Guerra EP, Zanin DS (2018) Tomato breeding for insect-pest resistance. In: Nyaku ST, Danquah A (eds) Recent advances in tomato breeding and production. InTech Open Science, London, pp 79–88. https://doi.org/10.5772/intechopen.75978
Acknowledgements
To authors thank the National Council for Scientific and Technological Development (CNPq) for their support through a scholarship granted to the first author (Process 156025/2018-3).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeist, A.R., de Resende, J.T.V., Perrud, A.C. et al. Resistance to Bemisia tabaci in tomato species and hybrids and its association with leaf trichomes. Euphytica 217, 85 (2021). https://doi.org/10.1007/s10681-021-02815-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-021-02815-x