Abstract
Pest resistance in Solanum galapagense has been associated with the presence of type IV glandular trichomes and allelochemicals. Knowledge of the genetic factors involved in determining the presence and type of trichomes may assist in the process of gene introgression for development of pest resistant tomato cultivars. In this study, we sought to identify QTLs associated with the presence of type IV trichomes in an F2 population derived from the interspecific cross of Solanum lycopersicum TOM-684 × S. galapagense accession LA1401. Two QTLs contributing to type IV trichome occurrence and density were detected, one major QTL (gal.IV-2), responsible for 35.22% of phenotypic variation, was located on chromosome 2. The other QTL (gal.IV-3) was located on chromosome 3, and explained 23.35% of the phenotypic variation in the F2 population. These QTLs were responsible for 26.44 and 3.37% of the variation observed in a progeny derived from a backcross to TOM-684. The same QTLs were also associated with type II + III non-glandular trichomes, whose densities were negatively correlated to those of type IV trichomes, suggesting that the loci found may have pleiotropic effects for high type IV/low type II + III trichome densities. Our results indicate that the inheritance for type IV trichomes can be explained by a few genetic factors affecting the presence of a gland and the density of trichomes. These results further suggest a revision to trichome classification used for tomato may be appropriate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tomato plant may be attacked by a large number of insect pests that cause damage to foliage and fruit, thus reducing both yield and quality. The use of chemical products has been the focus of control for insects. However, their indiscriminate use, as well as the ability of pests to develop resistance, have reduced the efficiency of many products (Mutschler et al. 1996; Momotaz et al. 2010; Lucini et al. 2015).
A promising alternative for control is the use of pest resistant cultivars (Broekgaarden et al. 2011; Lucatti et al. 2014). To develop such resistant cultivars, it is first necessary to identify sources of resistance (Broekgaarden et al. 2011; Lucini et al. 2015). Knowledge of the cause and mechanism of resistance can also help to improve selection efficiency. Further, an understanding of the underlying genetic basis for pest resistance can lead to augmented selection strategies. Wild tomato species (Solanum pennellii Correll, Solanum habrochaites S. Knapp and D.M Spooner, Solanum pimpinellifolium L., Solanum galapagense S.C. Darwin et I. Peralta) are reported sources of resistance to pests such as whitefly Bemisia spp., South American tomato pinworm Tuta absoluta (Meyrick), and two spotted spider mites Tetranychus urticae Koch, among others (Maluf et al. 2001; Muigai et al. 2003; Maciel et al. 2011; Firdaus et al. 2012; Lucatti et al. 2013, 2014; Lucini et al. 2015; Andrade et al. 2017).
In wild species, the mechanism of resistance has often been related to the presence of glandular trichomes and to their association with production of allelochemicals (Goffreda et al. 1989; Maluf et al. 2001; Simmons and Gurr 2005; Maluf et al. 2007). Luckwill (1943) categorized cultivated and wild tomato trichomes into seven types (type I to type VII) based on their morphology. Luckwill’s classification considered the presence of a multicellular or unicellular base, the length of the supporting stalk, and the presence or absence of a unicellular or multicellular gland at the tip. He then classified the trichomes as either glandular (types I, IV, VI, and VII) or non-glandular (types II, III, and V). This classification accounts for observed variation but does not consider the ontogenetic relationship and the similarity among trichome types. As an example, the type I and IV glandular trichomes look physically similar to non-glandular trichomes, but they differ by the presence of glandular cells in the tip (McDowell et al. 2011). As we learn more about the genetic and developmental basis for trichomes, classification may need to be revised to reflect this knowledge.
In S. galapagense, S. pennellii, and S. pimpinellifolium, a high density of type IV trichomes are also associated with the secretion of acylsugars (Goffreda et al. 1989; Rodríguez-López et al. 2011; Firdaus et al. 2013; Lucatti et al. 2013; Lucini et al. 2015). The acylsugar and trichomes cause negative effects on arthropod pests, including increased mortality, reduction in oviposition and reduced adult survival (Simmons et al. 2004; Simmons and Gurr 2005). In a population derived from S. pennellii, accession LA0716, genotypes with high density of type IV trichomes and high levels of acylsugars showed resistance to two spotted spider mites Tetranychus urticae (Lucini et al. 2015). A study comparing whitefly resistance between the wild species S. galapagense and S. cheesmaniae (L. Riley) Fosberg found that all of the evaluated accessions of S. galapagense were resistant. In addition, they had a high density of type IV trichomes as well as elevated levels of acylsugars. All S. cheesmaniae accessions were susceptible, even the ones with acylsugar levels similar to those of S. galapagense. Based on the results, the authors suggest that to achieve an effective level of resistance it is necessary to have the presence of type IV glandular trichomes and a minimum level of acylsugars (Lucatti et al. 2013).
Knowledge of the genetic factors determining the presence and type of trichomes may facilitate and ensure the success of introgression of pest resistance into the cultivated tomato plant. Genomic regions which are associated with the presence of trichomes have been described as quantitative trait loci (QTL). QTLs associated with the density of type IV glandular trichomes have been identified for the species S. pennelli accession LA0716, S. galapagense accession PRI95004, and S. habrochaites accessions CGN1.1561 and LA1777 (Maliepaard et al. 1995; Blauth et al. 1998; Momotaz et al. 2010; Firdaus et al. 2013; Lucatti et al. 2014). In populations derived from the accession PRI95004 (S. galapagense), two QTLs associated with resistance to whitefly and the presence of type IV glandular trichomes were described (Firdaus et al. 2013). Evidence for one major QTL (Wf-1) on chromosome 2 and a minor QTL (Wf-2) on chromosome 9 were presented (Firdaus et al. 2013). In addition, it was suggested that inheritance of type IV trichomes is relatively simple, which favors their use in breeding programs (Firdaus et al. 2013).
The species S. galapagense has high resistance to whitefly, associated with the presence of type IV trichomes and acylsugars, and it constitutes an important source of resistance to be used in breeding programs (Lucatti et al. 2013). However, not all accessions of S. galapagense have the same performance in relation to pest resistance, especially to whitefly, as shown by Firdaus et al. (2012) and Lucatti et al. (2013), suggesting the need for more in-depth studies with different accessions. Among the accessions of S. galapagense evaluated by Lucatti et al. (2013), LA1401 has high levels of resistance to whitefly accompanied by high density of type IV trichomes as well as high acylsugar accumulation. Jouy et al. (1992) reported resistance of this accession to Liriomyza trifolii (Burgess) (leafminer), and they associated this resistance with production of acylsugars and glandular trichomes. The accession LA1401 has also been described as a source of resistance to salinity and high soluble solids content in fruits (Mahmoud et al. 1986; Shannon and Wrona 1992; Razdan and Mattoo 2006). In this study, we sought to identify QTLs associated with trichome type and density in a population derived from the interspecific cross between S. lycopersicum and S. galapagense accession LA1401.
Materials and methods
Populations and data for this study were developed through a partnership among the Universidade Federal de Lavras (UFLA) in Lavras, Hortiagro Sementes S.A. seed company in Ijaci, both in the State of Minas Gerais, Brazil, and The Ohio State University, Ohio Agricultural Research and Development Center (OSU-OARDC), in the United Sates. Phenotypic data were collected at UFLA/Hortiagro, while genotypic data collection and subsequent analyses were performed at the OSU-OARDC.
Plant material
Segregating populations were developed from S. galapagense (LA1401) and S. lycopersicum (TOM-684). LA1401 is a wild accession characterized by a high level of acylsugars, presence of type IV trichomes, and resistance to insects (Jouy et al. 1992; Lucatti et al. 2013). Previous studies at UFLA/Hortiagro demonstrated resistance of LA1401 to the whitefly Bemisia tabaci biotype B (Andrade et al. 2017). TOM-684 (susceptible to insects) is a proprietary fresh-market tomato inbred line from Hortiagro Sementes S.A. A cross was initially obtained between TOM-684 (female parent) and the accession LA1401 (male parent), thus obtaining the F1 generation. F1 plants were self-pollinated to obtain the F2 generation and also backcrossed with the parental accession TOM-684 to obtain the backcross BC1 [= (F1 × TOM-684)]. Segregating progeny and parental accessions were phenotyped for the density and type of trichomes according to the system of Luckwill (1943). The F2 population was used to identify QTLs associated with trichomes, and the F1BC1 backcross population was used to validate these QTLs. Tomato plants were grown in a greenhouse in a completely randomized design, with parents included as replicated checks. Experiments consisted of 20 plants from each parental line, 20 plants from the F1, and 140 individuals from the F2. For the backcross 120 individuals were included.
Identification and quantification of trichomes
When tomato plants were 3 months old, the fully-expanded fourth leaf from each plant was collected and stored in 70% ethanol in a beaker for 48 h. After storage, paradermal sections were prepared from the abaxial and adaxial surfaces of leaflets. The sections were cleared in 50% sodium hypochlorite for 20 s and washed three times in water to remove the excess hypochlorite. After that, sections were placed in 0.1% safranin dye for 20 s and then washed twice in water to remove excess dye. Six paradermal sections per surface were used per plant to make slides. Semi-permanent slides were prepared as mounts in glycerinated water (Freitas et al. 2002).
The slides were viewed under a 10× optical microscope (Carl Zeiss, model Axil l) with an attached camera (AxionCam ERc5s). Images were captured using the Axio Vision Rel. 4.8 program and analyzed using the Image Tool v. 3.00 software (Wilcox et al. 2002). Trichome densities were quantified (number of trichomes per mm2) on abaxial and adaxial surfaces of the leaflets for each of the trichome types based on Luckwill (1943).
Phenotypic analysis
Means, standard errors, and broad-sense heritabilities were calculated for each type of trichome. The total number of trichomes within each trichome type were obtained by the sum of abaxial and adaxial trichomes. Pearson correlation coefficients among densities of trichome types were calculated. All analyses were performed in R 3.1.1 (R Core Team 2014). Broad-sense heritabilities were estimated using the ratio of genotypic to phenotypic variances. Phenotypic (P), environmental (E), and genotypic (G) variances were estimated from generation variances as follows (Wright 1968):
Genotyping
The DNA, from the F2, BC1, and parent plants, was extracted from young leaves using the CTAB procedure (Ferreira and Grattapaglia 1998). The DNA was suspended in TE (1 mM Tris to 0.1 mM EDTA) and sent to the Horticulture and Crop Science Department at the OSU-OARDC/USA. DNA quality was checked using a Nano drop and by running samples on a 1% agarose gel and visually inspecting the ethidium bromide stained aliquots. The concentration was adjusted to 50 ng/µl, and 20 µl of each sample was used for genotyping. A total of 384 SNPs (Online Resource 1) previously optimized for fresh-market tomatoes, based on the SolCAP Infinium SNP Array (SIM et al. 2012), were used for genotyping. Genotyping was carried out using the Kompetitive Allele Specific (KASP) assay (LGC Genomics, United States).
Genetic map and QTL identification
A genetic map was developed using the polymorphic SNPs. Analyses were performed using r/QTL 1.36-6 (Broman et al. 2003). SNPs that showed distorted segregation patterns based on a Chi square test with the threshold at 5% and/or more than 10% missing data, were removed. The linkage groups were built based on maximum recombination frequency of 0.35 and minimum LOD score 4. Map quality was verified through reference to the physical map (Sim et al. 2012).
The association between chromosome intervals and trichome type or density was established using Simple Interval Mapping (SIM) and Composite Interval Mapping (CIM). Phenotypic distributions of trichome density were non-normal and, thus, several approaches were conducted to establish marker linkage to QTL. In SIM, both parametric and non-parametric models were considered. Significant thresholds for each trait were determined by permutation tests at the 5% level with 1000 replicates each.
Single marker-trait regression and Kruskal–Wallis tests were used to confirm SNP markers that were significantly associated in the interval mapping. In addition, these analyses provided an estimate of the proportion of phenotypic variation explained by each QTL, as well as the proportion of additive or dominance contribution to variances. Interaction analyses between significant SNPs were conducted to explore potential epistatic effects between QTLs. Analyses were performed using R 3.1.1(R Core Team 2014). The genetic linkage map, physical map, and QTL maps were drawn using Mapchart 2.3 (Voorrips 2002).
Validation of QTLs in backcross population
InDel Markers were developed for validation of QTLs in a backcross population [BC1 = (F1 × TOM-684)] with 120 individuals. Genome sequence data were used to find InDels which segregated in the crosses. Tomato now has extensive sequence data available (e.g. Aflitos et al. 2014; Lin et al. 2014). Among the 85 unique genomes available from the high-quality data generated through the 150 Tomato Genome Sequencing Consortium (Aflitos et al. 2014), there are three Galapagos Island accessions [LA1401 (S. galapagense), LA0483 and LA1044 (S. cheesmaniae)] and seven fresh-market accessions with descriptors similar to TOM-684 [EA00465, CGN20815, LYC 1410, TR00022, N020212, LYC 1365 (all S. lycopersicum)]. PI 406760 (S. lycopersicum var. cerasiforme) provided and outgroup for a cherry type tomato. The DNA sequences for these accessions were used to identify new markers near QTLs identified with SNPs. DNA sequences, of approximately 15 Kb inside the QTL regions identified, were obtained from the reference Genome Browser (SGN—Fernandez-Pozo et al. 2014). We then obtained matching sequences from the three Galapagos accessions and seven S. lycopersicum varieties using a custom script which uses BLAST to identify top hits in FASTA formatted sequences and extracts of the top match. Sequences were then aligned using MUSCLE (Edgar 2004). InDel Markers were identified by visual inspection of the resulting alignment. The criteria for identifying these markers were InDels which cluster S. galapagense separately from all the S. lycopersicum accessions and also were at least 10 bp in length. Flanking primers were designed using Primer3 (Untergasser et al. 2012).
Polymerase Chain Reaction (PCR) conditions were standardized for InDel markers with denaturation at 94 °C for 45 s, annealing at 56 °C for 45 s, and elongation at 72 °C for 45 s, repeated for 40 cycles. PCR products were separated on 4% agarose gel at 180 V run for 4 h. Confirmation of QTL was based on regression between InDel marker genotypes and phenotypic data on trichomes obtained as previously described.
Results
Type, density, and correlation among trichomes
The types and densities of trichomes found in the population derived from the cross between S. lycopersicum and S. galapagense are shown in Table 1. The parent LA1401 (S. galapagense) had a high density of the type IV glandular trichomes. The type I glandular trichomes, as well as the type II + III non-glandular trichomes, are present in LA1401, however, at low densities with count values near zero. The susceptible parent S. lycopersicum (TOM-684) has a high density of type II + III non-glandular trichomes and does not have either type I or IV glandular trichomes. Type V non-glandular trichomes are present at similar densities in both parents. Type I, VI, and VII glandular trichomes occurred at low density in the parents (when present) and in the F1 and F2 populations; therefore, they were not used for identification of QTLs (Table 1).
The density of glandular trichomes had high heritability. Type IV trichomes had a heritability of 0.85 and type I a heritability of 0.9. The F2 population had a mean of 7.0 trichomes per mm2 and range from 0 to 51.33 trichomes per mm2, with some individual plants at a density greater than the mean of the parent LA1401 (Table 1). High heritability was also found for the density of type II + III non-glandular trichomes (0.77).
There was a high negative correlation between the type IV glandular trichome and type II + III non-glandular trichomes. A significant positive correlation was found between the type I and IV glandular trichomes, and both are inherited from the wild parent LA1401. The type V non-glandular trichomes, present in both parents, did not show significant correlation with any of the other trichomes (Table 2).
Linkage map
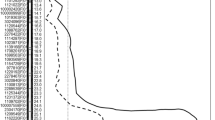
The genetic map was constructed based on 161 polymorphic SNPs, with a total length of 950.0 cM (Fig. 1). Thirteen linkage groups were formed with chromosome 1 represented by two linkage groups (1a and 1b). The physical position of each SNP was compared to the tomato genome assembly (SGN, solgenomics.net) and the physical and genetic maps showed consistent arrangement of markers (Fig. 1).
Genetic map based on an F2 population (S. lycopersicum × S. galapagense) and built using 161 SNP markers in the r/qtl-R program (left). Physical map based on physical map location of the SNPs in the tomato database at SGN (SL2.40) (Sim et al. 2012) (right)
Trichome QTLs
QTLs for the type IV glandular trichomes, and type V and type II + III non-glandular trichomes were identified through analyses of Composite Interval Mapping, Simple Interval Mapping, Single Marker-Trait Regression, and a Kruskal–Wallis test (Table 3). The QTLs detected using different techniques for analyses were in agreement. Despite the fact that phenotypic distributions of trichome density were non-normal, parametric and non-parametric models identified the same QTLs.
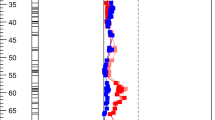
For the type IV trichomes, two QTLs were identified: one major QTL, called gal.IV-2, was located on chromosome 2 between markers “solcap_snp_sl_10796” and “solcap_snp_sl_36224”. The gal.IV-2 QTL explained 35.22% of the phenotypic variation. A second QTL, gal.IV-3, is located on chromosome 3 near SNP “CL017416.0406”, and explained 23.35% of the variation (Fig. 2).
Interval mapping of QTL affecting Type IV and Type II + III trichomes on chromosome 2 (upper) and 3 (lower). The schematic chromosome shows the name and the genetic distance in centiMorgan of the SNP markers and the position of the QTL. The Composite Interval Mapping graphs show the significant QTL for Type IV (black) and Type II + III (gray) trichomes on chromosome 2 (upper) and chromosome 3 (lower). Significant LOD score (y-axis) is 7.0 for trichome type IV and 3.0 for trichome type II + III
There was a significant difference between homozygous and heterozygous individuals for both QTLs (gal.IV-2 and gal.IV-3) (Fig. 3). Individuals that are homozygous for the alleles from S. galapagense have high density of type IV trichomes, whereas the homozygotes for the parent S. lycopersicum lack the type IV trichomes. Heterozygotes have type IV trichomes, but at a lower density than homozygotes for the S. galapagense alleles (Fig. 3). The additive effects for both QTLs were of greater magnitude than the effects of dominance (Table 3). The negative values and the magnitudes of the effects of dominance (Table 3) associated with the average density observed for the parents and populations for type IV trichome (Table 1) are indicative that the alleles for high density of type IV trichomes may be incompletely recessive to additive.
Type IV trichome density for homozygous and heterozygous genotypes for QTL gal.IV-2 on chromosome 2 (right) and QTL gal.IV-3 on chromosome 3 (left). The allele gal2 is from S. galapagense for QTL on chromosome 2; lyc2 is the S. lycopersicum allele for QTL at chromosome 2; gal3 is the S. galapagense allele for QTL on chromosome 3; lyc3 is the S. lycopersicum allele for QTL at chromosome 3. ***Significant interaction at the 0.00 level
A significant interaction was found between the QTL gal.IV-2 and QTL gal.IV-3 and the interaction explained 53.68% of the observed phenotypic variation (Table 3). The interaction occurred in the direction of increasing the density of type IV trichomes for the individuals that are (a) homozygotes for the allele of the S. galapagense parent for both QTLs (gal2/gal2 − gal3/gal3), (b) heterozygotes for QTL gal.IV-2 and homozygotes for parent S. galapagense QTL gal.IV-3 (gal2/lyc2 − gal3/gal3), (c) homozygotes for parent S. galapagense QTL gal.IV-2 and heterozygotes for QTL gal.IV-3 (gal2/gal2 − gal3/lyc3), and (d) heterozygotes for both the QTLs (gal2/lyc2 − gal3/lyc3) (Fig. 4).
Interaction effect between the QTL gal.IV-2 (chromosome 2) and QTL gal.IV-3 (chromosome 3) for type IV trichome density in the F2 population (S. lycopersicum × S. galapagense). The allele gal2 is the S. galapagense QTL on chromosome 2; lyc2 is the S. lycopersicum allele for QTL on chromosome 2; gal3 is the S. galapagense allele for QTL on chromosome 3; lyc3 is the S. lycopersicum allele for QTL on chromosome 3. ***Significant interaction at the 0.00 level. *Significant interaction at the 0.05 level
The QTLs gal.IV-2 and gal.IV-3 were also associated with type II + III non-glandular trichomes, but with lower values of the LOD score in Composite Interval Mapping (5.52 and 2.93, respectively). These QTLs were responsible for a lower phenotypic variation for this type of trichome representing 19.30 and 10.07% of the variation, respectively (Table 3). The coincidence of QTL gal.IV-2 and QTL gal.IV-3 for both type IV glandular trichome and type II + III non-glandular trichomes (Fig. 2) is not surprising given the strong negative correlations observed.
Two other minor QTLs were detected for type II + III trichomes, one located on chromosome 4 and the other on chromosome 5. These QTLs were responsible for 10.46 and 6% of phenotypic variation, respectively (Table 3). For the QTLs associated with type II + III trichomes, the S. galapagense alleles reduced the density. One QTL on chromosome 10 was detected for the type V non-glandular trichome.
Validation of QTL for type IV trichome in backcross population
As a validation strategy, InDel markers were developed for regions within the LOD confidence interval for the QTLs gal.IV-2 and gal.IV-3. Primers are described in Table 4. The presence of both QTLs was confirmed in the backcross population [(S. lycopersicum × S. galapagense) × S. lycopersicum] for type IV trichomes, but with a lesser effect than that found in the F2 population. The QTL gal.IV-2 explained 26.44% of phenotypic variation, whereas the QTL gal.IV-3 explained only 3.37% of the variation in BC1 progeny (Table 5).
Just as in the F2 population, there was significant interaction between the QTLs gal.IV-2 and gal.IV-3 in the backcross population (Fig. 5). The density of type IV trichomes is increased when the individual is heterozygous for both QTLs (gal2/lyc2 − gal3/lyc3). In homozygous individuals for the cultivated allele at QTL gal.IV-2 (lyc2/lyc2), the presence of the QTL gal3 allele from S. galapagense did not increase type IV trichome number above S. lycopersicum parent levels (Fig. 5).
Interaction effect between the QTL gal.IV-2 (chromosome 2) and QTL gal.IV-3 (chromosome 3) for type IV trichome density in a backcross population [(S. galapagense × S. lycopersicum) × S. lycopersicum]. gal2 is the S. galapagense allele for QTL at chromosome 2; lyc2 is the S. lycopersicum allele for QTL at chromosome 2; gal3 is the S. galapagense allele for QTL at chromosome 3; lyc3 is the S. lycopersicum allele for QTL at chromosome 3. ***Significant interaction at the 0.00 level
Discussion
Our study showed that the inheritance of type IV glandular trichomes from S. galapagense accession LA1401 is not complex and markers developed for QTLs gal.IV-2 and gal.IV-3 will be useful for indirect selection, assisting in the gene introgression process. The trichomes found in the greatest density in S. galapagense, accession LA1401, were the type IV glandular trichome (Table 1). LA1401 is resistant to whitefly, and this resistance is associated with the density of type IV trichomes and with the accumulation of high levels of acylsugars (Lucatti et al. 2013). Andrade et al. (2017) evaluated populations derived from the cross S. lycopersicum × S. galapagense ‘LA1401’ for whitefly resistance. LA1401 was resistant to B. tabaci biotype B and F2 progeny with a high density of type IV trichomes demonstrated levels of resistance similar to the S. galapagense parent. In a parallel study, Da Silva et al. (2016) cloned selected F2 plants based on extreme phenotypes for the highest and lowest density of type IV trichomes. These cloned genotypes permitted testing for resistance to Helicoverpa armigera (Lepidoptera: Noctuidae) with replication. Genotypes with a high density of type IV trichomes showed resistance to H. armigera, those with a low density did not. Several studies with different wild species of tomato have reported the importance of the presence of the type IV trichomes and an association with allelochemical content that confers pest resistance (Goffreda et al. 1989; Muigai et al. 2002; Simmons and Gurr 2005; Firdaus et al. 2012; Lucatti et al. 2013; Lucini et al. 2015). In our populations, the density of glandular trichomes, in general, and the type IV trichomes, specifically, showed high heritability suggesting that this trait may be easily selected and introgressed. High heritability for type IV trichomes has previously been reported for S. galapagense and other wild tomato species (Maliepaard et al. 1995; Freitas et al. 2002; Saeidi 2012; Firdaus et al. 2013; Andrade et al. 2017). We have observed no negative associations with trichome density and agronomic traits, though we have not completed introgression into the cultivated background and chromosome 2 contains several domestication loci including fw2.2 and exerted stigma (Frary et al. 2000; Chen and Tanksley 2004).
QTL on chromosome 2 (gal.IV-2) and on chromosome 3 (gal.IV-3) were associated with the type IV trichome densities. In the F2 population, these QTLs played an important role for the occurrence of these trichomes. Individuals homozygous for the alleles of the susceptible parent TOM-684 (S. lycopersicum) do not have type IV trichomes, whereas individuals homozygous for the alleles of the resistant parent LA1401 (S. galapagense) have trichome density similar to the parent LA1401 (Fig. 3). The gene action of these QTLs may be described as partially recessive to additive in the sense that a lower density of type IV glandular trichomes relative to the mid-parent was found for both loci. Both loci were validated in an independent BC1 population, confirming the ability to detect a genetic effect in heterozygotes.
The QTLs we identified add to an understanding of the genetic basis of trichomes from S. galapagense. In S. galapagense, accession PRI195004, a major QTL (Wf-1) on chromosome 2 and another minor QTL on chromosome 9 (Wf-2) were associated with density of type IV trichomes, with production of acylsugars, and with resistance to whitefly (Firdaus et al. 2013). The markers used to identify QTL Wf-1 lie approximately between 47.35 and 49.45 Mb, a region near the QTL gal.IV-2 found in our population (45.67–46.99 Mb) (Fernandez-Pozo et al. 2014). The identification of these QTLs (Wf-1 and gal.IV-2) emphasizes the importance of a region or regions on chromosome 2 for the presence of genes controlling type IV trichome presence in S. galapagense. The apparent lack of overlap of the physical regions identified for Wf-1 and gal.IV-2 may be due to marker choice or inexact mapping of QTL position. Further work will be required to determine whether these QTLs may be co-located and perhaps represent the same locus. Firdaus et al. (2013) also detected the presence of a QTL (Wf-2) on chromosome 9 associated with adult survival and the presence of type IV trichomes; however, the presence of this QTL was not confirmed in the F3 population. No QTL associated with trichomes was detected on chromosome 9 in our F2 population. Nevertheless, we identified the QTL gal.IV-3 on chromosome 3, a locus not detected in S. galapagense accession PRI195004 by Firdaus et al. (2013). These results may indicate the presence of different modifier genes in different accessions of S. galapagense, which in turn may explain the observed variation in S. galapagense accessions (Firdaus et al. 2012; Lucatti et al. 2013).
The QTLs found for type IV trichome density in S. galapagense appear to be different from those found in other wild species of tomato. In S. habrochaites accession CGN1.1561, Maliepaard et al. (1995) identified QTL on chromosome 5 (TriIV) and another on chromosome 9 (TriIV), located in a different region from the QTL Wf-2 identified by Firdaus et al. (2013). For the S. habrochaites accession LA1777, QTLs on chromosomes 9, 10, and 11 were associated with type IV trichome density and with oviposition rate of whitefly (Momotaz et al. 2010). In S. pennelli, seven QTLs, located on chromosomes 2, 4, 5, 6, 7, 10, and 11, were associated with the density of type IV trichomes and with production of acylsugars (Blauth et al. 1998). The S. pennelli QTL on chromosome 2 is located in a different region from QTL gal.IV-2.
The significant interaction between the QTLs gal.IV-2 and gal.IV-3 and the relative ranking of different allele combinations suggests that the action of the QTL gal.IV-3 is dependent on QTL gal.IV-2. This interaction suggests the possibility that QTL gal.IV-2 is responsible for the presence of type IV trichomes, while QTL gal.IV-3 acts by increasing the density of type IV trichomes. For QTL gal.IV-3 to express itself, it is necessary that there is at least one allele of the S. galapagense parent for the QTL gal.IV-2 (Fig. 4). Thus, individuals that have alleles of the parent S. galapagense only for the QTL gal.IV-3, even in the homozygous state do not have type IV trichomes. In contrast, individuals with alleles from S. galapagense only at QTL gal.IV-2 had type IV trichomes, even though at low densities. Thus, it may be inferred that there is epistasis in the sense of an increase in the density of type IV trichomes whenever there is simultaneous presence of at least one allele derived from S. galapagense (gal2 and gal3) at each one of the two loci in question.
The QTLs gal.IV-2 and gal.IV-3 were also associated with type II + III non-glandular trichomes, but with a negative correlation between the type IV and the type II + III trichomes. QTL gal.IV-2 was responsible for most of the variation for type II + III trichomes, just as it occurs for the type IV trichomes. The co-located QTL for type IV glandular trichome and type III non-glandular trichome was also described by Firdaus et al. (2013). The identification of the same QTLs for type IV glandular trichomes and type II + III non-glandular trichomes, and the negative correlation between these types of trichomes further suggest that the locus may be controlling the presence of the gland. In making selection for greater densities of type IV trichomes, one automatically selects for lower density of type II + III trichomes. The negative correlation between the density of type IV and type II + III trichomes, and the reported effect of the loci gal.IV-2 and gal.IV-3 in opposite directions for type IV and type II + III trichomes reinforce the potential for an ontogenetic relationship between these types of trichomes.
The type V non-glandular trichomes were found in accession LA1401, but at low density and did not show correlation with any other type of trichome. Simmons and Gurr (2004) also reported the presence of type V trichomes in S. galapagense. Different results were found by Firdaus et al. (2013), where type V trichomes were not identified in the accession S. galapagense PRI195004; in the F2 population, they observed a negative correlation for the presence of type IV and type V trichomes. The authors showed that the presence of type IV trichomes resulted in a low number of adults surviving and reduced the oviposition rate for whitefly. However, the presence of type IV trichomes combined with the presence of the type V trichomes caused an increase in the number of adults surviving and in the oviposition rate. For the type V non-glandular trichome, only one QTL was detected on chromosome 10 in our population, and no QTL were found to be co-located with the QTLs for type IV and type II + III trichomes.
Different classification systems are used for the trichomes of various plant families. For tomato the system of Luckwill (1943) has been widely adopted for taxonomic purposes. This system defines multiple types of trichomes and is reflective of systems used in the Solanaceae. However newer have attempt to organize the types into an ontology. For example, Inamdar and Patel (1973) recognize 24 types of trichomes in the Polemoniales (now Solanales). These are organized into an ontology that with the presence of glands a key bifurcation and also recognizes quantitative (gradation) differences in complexity. Qualitative grouping based on type may not reflect the quantitative genetic or morphogenetic processes that affect trichome density or development. More recent descriptions of trichome morphology focus on the distinction between glandular and non-glandular (eglandular) hairs, the number of cells in glands, and the number of cells in stalks (Edmonds 1982; Seithe 1979). These descriptions suggest that moving away from trichome types could simplify classification and better describe ontogenic relationships and the underlying genetic mechanisms affecting trichome morphology. In addition to the presence/absence of tip glands distinguishing type IV trichomes from type II and III non-glandular trichomes, type I and type IV glandular trichomes differ principally in their stalk length. In our work we found a positive correlation between type I and IV densities. However, type I trichome densities were low (near zero), and for this reason the QTL association analysis was not processed for this trichome type. In S. habrochaites, McDowell et al. (2011) suggested that type I and type IV trichomes may comprise the same type of trichome, differing only in stalk length, therefore the classification of either type I or IV would be artificial. The authors also report that these trichomes appear to be physically similar to non-glandular trichomes but terminate with a gland at the tip. A plausible hypothesis may be that both glandular and non-glandular trichomes originated from the same cell type, but during trichome development the alleles gal2 and gal3 would be involved in the development of glandular structure at the trichome tips. In Arabidopsis, trichome mutations that affect all the trichomes on the plant have been identified, which suggests that the development of multiple hair morphologies is controlled by the same genes (Marks 1997). This ontogenetic relationship hypothesis opens doors for new criteria of trichome classification based on gene action rather than on merely morphological description.
References
Aflitos S, Schijlen E, Jong H et al (2014) Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J 80:136–148. https://doi.org/10.1111/tpj.12616
Andrade MC, da Silva AA, Neiva IP, Oliveira IR, De Castro EM, Francis DM, Maluf WR (2017) Inheritance of type IV glandular trichome density and its association with whitefly resistance from Solanum galapagense accession LA1401. Euphytica 213:52. https://doi.org/10.1007/s10681-016-1792-1
Blauth SL, Churchill GA, Mutschler MA (1998) Identification of quantitative trait loci associated with acylsugar accumulation using intraspecific populations of the wild tomato, Lycopersicon pennellii. Theor Appl Genet 96:458–467. https://doi.org/10.1007/s001220050762
Broekgaarden C, Snoeren TA, Dicke M, Vosman B (2011) Exploiting natural variation to identify insect-resistance genes. Plant Biotech J 9:819–825. https://doi.org/10.1111/j.1467-7652.2011.00635.x
Broman KW, Wu H, Sen Ś, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890. https://doi.org/10.1093/bioinformatics/btg112
Chen KY, Tanksley SD (2004) High-resolution mapping and functional analysis of se2.1 A major stigma exsertion quantitative trait locus associated with the evolution from allogamy to autogamy in the genus lycopersicon. Plant Mol Biol Rep 168:1563–1573. https://doi.org/10.1007/s11105-018-1071-5
Core Team R (2014) R: a language and environment for statistical computing. R. Foundation for Statistical Computing, Austria
Da Silva AA, Andrade MC, de Castro CR, Neiva IP, Santos DC, Maluf WR (2016) Resistência à Helicoverpa armigera em genótipos de tomateiro obtidos do cruzamento de Solanum lycopersicum com Solanum galapagense. Pesq Agropec Bra 51:801–808. https://doi.org/10.1590/S0100-204X2016000700002
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Edmonds JM (1982) Epidermal hair morphology in Solanum L. section Solanum. Bot J Linn Soc 85:153–167
Fernandez-Pozo N, Menda N, Edwards JD et al (2014) The sol genomics network (SGN)-from genotype to phenotype to breeding. Nucleic Acids Res 43:D1036–D1041. https://doi.org/10.1093/nar/gku1195
Ferreira ME, Grattapaglia D (1998) Introdução ao uso de marcadores moleculares em análises genéticas. Embrapa-Cenargen, Brasília
Firdaus S, Heusden AWV, Hidayat N, Supena EDJ, Visser RGF, Vosman B (2012) Resistance to Bemisia tabaci in tomato wild relatives. Euphytica 187:31–45. https://doi.org/10.1007/s10681-012-0704-2
Firdaus S, Van Heusden AW, Hidayati N et al (2013) Identification and QLT mapping of whitefly resistance components in Solanum galapagense. Theor Appl Genet 126:1487–1501. https://doi.org/10.1007/s00122-013-2067-z
Frary A, Nesbitt TC, Grandillo S, van der Knaap E, Cong B, Liu J, Meller J, Elber R, Alpert KB, Tansley SD (2000) fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289:85–88
Freitas JA, Maluf WR, Das Graças Cardoso M, Gomes LA, Bearzotti E (2002) Inheritance of foliar zingiberene contents and their relationship to trichome densities and whitefly resistance in tomatoes. Euphytica 127:275–287. https://doi.org/10.1023/A:1020239512598
Goffreda JC, Mutschler MA, Avé DA, Tingey WM, Steffens JC (1989) Aphid deterrence by glucose esters in glandular trichome exudate of the wild tomato, Lycopersicon pennellii. J Chem Ecol 15:2135–2147. https://doi.org/10.1007/BF01207444
Inamdar JA, Patel RC (1973) Structure, ontogeny and classification of trichomes in some Polemoniales with 4 figures and 1 diagram. Feddes Repert 83:473–488. https://doi.org/10.1002/fedr.19730830702
Jouy N, Bordat D, Bessiere JM (1992) Identification of (2,3,4-tri-O-acyl)-a-D-glucopyranosyl-(3-O-acyl)-b-D-fructofuranoside, responsable of the righ level of leafminer resistance in Lycopersicon cheesmaniae. Rep Tomato Genet Cooperative 42:22
Lin T, Zhu G, Zhang J et al (2014) Genomic analyses provide insights into the history of tomato breeding. Nat Genet 46:1220–1226. https://doi.org/10.1038/ng.3117
Lucatti AF, Van Heusden AW, De Vos RCH, Visser RGF, Vosman B (2013) Differences in insect resistance between tomato species endemic to the Galapagos Islands. BMC Evol Biol 13:175. https://doi.org/10.1186/1471-2148-13-175
Lucatti AF, Meijer-Dekens FR, Mumm R, Visser RG, Vosman B, Van HS (2014) Normal adult survival but reduced Bemisia tabaci oviposition rate on tomato lines carrying an introgression from S. habrochaites. BMC Genet 15:142. https://doi.org/10.1186/s12863-014-0142-3
Lucini T, Faria MV, Rohde C, Resende JTV, De Oliveira JRF (2015) Acylsugar and the role of trichomes in tomato genotypes resistance to Tetranychus urticae. Arthropod Plant Interact 9:45–53. https://doi.org/10.1007/s11829-014-9347-7
Luckwill LC (1943) The genus Lycopersicon: an historical, biological and taxonomic survey of wild and cultivated tomatoes. Aberdeen University, Aberdeen
Maciel GM, Maluf WR, Silva VF, Gonçalves NA, Gomes LAA (2011) Híbridos pré-comerciais resistentes a Tuta absoluta obtidos de linhagem de tomateiro rica em acilaçúcares. Hort Brasil 29:151–156. https://doi.org/10.1590/S0102-05362011000200003
Mahmoud MH, Jones RA, El-Beltagy AS (1986) Comparative responses to high salinity between salt-sensitive and salt-tolerant genotypes of the tomato. Acta Hortic 190:533–544. https://doi.org/10.17660/ActaHortic.1986.190.62
Maliepaard C, Bas N, Van Heusden S et al (1995) Mapping of QTLs for glandular trichome densities and Trialeurodes vaporariorum (greenhouse whitefly) resistance in an F2 from Lycopersicon esculentum × Lycopersicon hirsutum f. glabratum. Heredity 75:425–433. https://doi.org/10.1038/hdy.1995.155
Maluf WR, Campos GA, Cardoso MG (2001) Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica 121:73–80. https://doi.org/10.1023/A:1012067505361
Maluf WR, Inoue IF, Ferreira RPD, Gomes LAA, Castro EM, Cardoso MG (2007) Higher glandular trichome density in tomato leaflets and repellence to spider mites. Pesq Agropec Bras 42:1227–1235. https://doi.org/10.1590/S0100-204X2007000900003
Marks MD (1997) Molecular genetic analysis of trichome development in Arabidopsis. Annu Rev Plant Biol 48:137–163. https://doi.org/10.1146/annurev.arplant.48.1.137
McDowell ET, Kapteyn J, Schmidt A, Li C, Kang JH, Descour A, Shi F, Larson M, Schilmiller A, An L, Jones AD (2011) Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol 155:524–539. https://doi.org/10.1104/pp.110.167114
Momotaz A, Scott JW, Schuster DJ (2010) Identification of quantitative trait loci conferring resistance to Bemisia tabaci in an F2 population of Solanum lycopersicum × Solanum habrochaites accession LA1777. J Am Soc Hortic Sci 135:134–142
Muigai SG, Schuster DJ, Snyder JC, Scott JW, Bassett MJ, Mcauslane HJ (2002) Mechanisms of resistance in Lycopersicon germplasm to the whitefly Bemisia argentifolii. Phytoparasitica 30:347–360. https://doi.org/10.1007/BF02979682
Muigai SG, Bassett MJ, Schuster DJ, Scott JW (2003) Greenhouse and field screening of wild Lycopersicon germplasm for resistance to the whitefly Bemisia argentifolii. Phytoparasitica 31:27–38. https://doi.org/10.1007/BF02979764
Mutschler MA, Doerge RW, Liu SC, Kuai JP, Liedl BE, Shapiro JA (1996) QTL analysis of pest resistance in the wild tomato Lycopersicon pennellii: QTLs controlling acylsugar level and composition. Theor Appl Genet 92:709–718
Razdan MK, Mattoo AK (2006) Genetic improvement of solanaceous crops: tomato. CRC, Boca Raton
Rodríguez-López MJ, Garzo E, Bonani JP, Fereres A, Fernández-Muñoz R, Moriones E (2011) Whitefly resistance traits derived from the wild tomato Solanum pimpinellifolium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of Tomato yellow leaf curl virus. Phytopathology 101:1191–1201. https://doi.org/10.1094/PHYTO-01-11-0028
Saeidi Z (2012) Inheritance of type IV glandular trichomes and two-spotted spider mite resistance in an interspecific cross of Lycopersicon esculentum × L. pennellii ‘LA2580’. J Res Agric Sci 8:157–163
Seithe A (1979) Hair types as taxonomic characters in Solanum. In: Hawkes JG, Lester RN, Skelding D (eds) The biology and taxonomy of the solanaceae. Academic Press, London, pp 307–319
Shannon MC, Wrona AF (1992) Salt tolerance of a selected line from the cross between a wild, tolerant tomato species (Lycopersicon cheesmaniae, ecotype LA1401) and a cultivated species (L. esculentum Mill.). HortScience 27:597
Sim SC, Durstewitz G, Plieske J et al (2012) Development of a large SNP genotyping array and generation of high-density genetic maps in tomato. PLoS ONE 7:e40563. https://doi.org/10.1371/journal.pone.0040563
Simmons AT, Gurr GM (2004) Trichome-based host plant resistance of Lycopersicon species and the biocontrol agent Mallada signata: Are they compatible? Entomol Exp Appl 113:95–101. https://doi.org/10.1111/j.0013-8703.2004.00210.x
Simmons AT, Gurr GM (2005) Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric For Entomol 7:265–276. https://doi.org/10.1111/j.1461-9555.2005.00271.x
Simmons AT, Gurr GM, McGrath D, Martin PM, Nicol HI (2004) Entrapment of Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) on glandular trichomes of Lycopersicon species. Aust J Entomol 43:196–200. https://doi.org/10.1111/j.1440-6055.2004.00414.x
Untergasser A, Cutcutache I, Koressaar T et al (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115. https://doi.org/10.1093/nar/gks596
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. https://doi.org/10.1093/jhered/93.1.77
Wilcox D, Dove B, McDavid D, Greer D (2002) Image tool for Windows, version 3.0. The University of Texas, Health Science Center in San Antonio, Texas
Wright S (1968) The genetics of quantitative variability. In: Wright S (ed) Evolution and genetics of populations, 2nd edn. Univeristy of Chicago, Chicago, pp 373–420
Acknowledgements
The authors thank all colleagues for their help with the laborious trichome phenotyping experiments. Acknowledgments to the Universities, agencies and company that supported this research project: Universidade Federal de Lavras (UFLA), The Ohio State University (OSU), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and HortiAgro Sementes S.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andrade, M.C., da Silva, A.A., Carvalho, R.d. et al. Quantitative trait loci associated with trichomes in the Solanum galapagense accession LA1401. Genet Resour Crop Evol 65, 1671–1685 (2018). https://doi.org/10.1007/s10722-018-0644-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-018-0644-3