Abstract
Aphis glycines Matsumura, the soybean aphid, first arrived in North America in 2000 and has since become the most important insect pest of domestic soybean, causing significant yield loss and increasing production costs annually in many parts of the USA soybean belt. Research to identify sources of resistance to the pest began shortly after it was found and several sources were quickly identified in the USDA soybean germplasm collection. Characterization of resistance expression and mapping of resistance genes in resistant germplasm accessions resulted in the identification of six named soybean aphid resistance genes: Rag1, rag1c, Rag2, Rag3, rag4, and Rag5 (proposed). Simple sequence repeat markers flanking the resistance genes were identified, facilitating efforts to use marker-assisted selection to develop resistant commercial cultivars. Saturation or fine-mapping with single nucleotide polymorphism markers narrowed the genomic regions containing Rag1 and Rag2 genes. Two potential NBS-LRR candidate genes for Rag1 and one NBS-LRR gene for Rag2 were found within the regions. Years before the release of the first resistant soybean cultivar with Rag1 in 2009, a soybean aphid biotype, named biotype 2, was found that could overcome the resistance gene. Later in 2010, biotype 3 was characterized for its ability to colonize plants with Rag2 and other resistance genes. At present, three biotypes have been reported that can be distinguished by their virulence on Rag1 and Rag2 resistance genes. Frequency and geographic distribution of soybean aphid biotypes are unknown. Research is in progress to determine the inheritance of virulence and develop DNA markers tagging virulence genes to facilitate monitoring of biotypes. With these research findings and the availability of host lines with different resistance genes and biotypes, the soybean aphid-soybean pest-host system has become an important model system for advanced research into the interaction of an aphid with its plant host, and also the tritrophic interaction that includes aphid endosymbionts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aphis glycines Matsumura (Hemiptera: Aphididae) is known as the soybean aphid and is a significant insect pest of soybean [Glycine max (L.) Merr.] in some Asian countries and the most important soybean insect pest in North America. It is the primary aphid species known to colonize soybean in North America (Hill et al. 2004a).
The soybean aphid was first described in Japan in 1917 (Matsumura 1917) and is native to China, Indonesia, Japan, Korea, Malaysia, the Philippines, Taiwan, and Thailand (Tilmon et al. 2011). One of the earliest reports on the significance of the soybean aphid was from China in 1962 (Wang et al. 1962). The first refereed publication on the soybean aphid occurring outside of Asia was from the United States in 2001 (Hartman et al. 2001). There were unconfirmed reports of earlier occurrences of the soybean aphid in the USA (Ragsdale et al. 2011). The soybean aphid became established across most of the main soybean-growing belt in both Canada and the USA within a few years (Venette and Ragsdale 2004). One of the key factors aiding its establishment in North America was the large pool of Rhamnus cathartica L. (common buckthorn) present on the continent, which serves as primary host for sexual reproduction and where the aphid overwinters as eggs.
The major concern for crop loss is in soybean, the secondary host, and not in the primary host that has no crop value (Ragsdale et al. 2004). Of the known secondary hosts, soybean is the most economically important. Soybean is the fourth-leading crop planted in the world with about 100 million ha in production (Hartman et al. 2011). The soybean aphid was highlighted as one of the top insect pest constraints to soybean production worldwide (Hartman et al. 2011). The global impact of the soybean aphid on this crop would even be greater if it occurred in Argentina and Brazil, because the combined soybean production of those two countries exceeds the production in the USA (Hartman et al. 2011). The economic impact of the soybean aphid on soybean production has been estimated to range from US $3.6 to $4.9 billion annually in North America (Kim et al. 2008a). Part of this loss in production is due directly to plant damage caused by the soybean aphid, such as plant stunting, leaf distortion, and reduced pod set and partly due to the increased crop input costs from insecticide applications used to control the pest (Ragsdale et al. 2004). In addition, aphid infestation can indirectly reduce crop production through soybean virus transmission and the buildup of black sooty mold on aphid honeydew that restricts photosynthesis (Hartman et al. 2001).
Although there are several management options, including modified cultural practices, application of insecticides, and release of natural enemies, this review focuses on soybean resistance and its interaction with the soybean aphid.
Biology of Aphis glycines
The lifecycle of the soybean aphid is heterocious as two hosts (primary and a secondary) are needed to complete its life cycle, and it is holocyclic since it undergoes sexual reproduction during part of its life cycle (Ragsdale et al. 2004; Wang et al. 1962). In North America, the common buckthorn serves as the main primary host (Ragsdale et al. 2004), while R. davurica Pall. (Dahurian buckthorn) and R. japonica Maxim. were identified as primary hosts in China and Japan (Takahashi et al. 1993). Native North American species R. alnifolia L’Hér. (alderleaf buckthorn), R. lanceolata Pursh (lanceleaf buckthorn) and glossy buckthorn, Frangula alnus Mill. (syn. Rhamnus frangula) are also known as winter hosts (Hill et al. 2010; Voegtlin et al. 2004b; Voegtlin et al. 2005). Soybean is the most common secondary host, but genotypes of other Glycine species such as wild soybean G. soja Sieb. and Zucc., and the perennial species G. latifolia (Benth.) Newell and Hymowitz also were reported to be colonized (Hill et al. 2004a). In addition, various other species of legumes, such as Medicago sativa L. (alfalfa), Trifolium pratense L. (red clover), T. subterraneum L. (subterranean clover), T. incarnatum L. (crimson clover), T. alexandrinum L. (berseem clover), and T. ambiguum M. Bieb. (kura clover) (Hill et al. 2004a) were reported to be colonized by soybean aphids in the greenhouse; however, to date, no reports of natural field colonization by soybean aphids on crops other than soybean are known.
The soybean aphid is a small (159 mm long) greenish-yellow aphid. Diagnostic morphological characters include pale antennae, dark siphunculi, and elongated oval cauda with 7–11 setae. These characters are often used to distinguish the soybean aphid from two closely related species, A. gosyppii (cotton-melon aphid) and A. nasturtii (buckthorn aphid), which also colonize the primary host, R. cathartica (Voegtlin et al. 2004a).
Maturation of the soybean aphid occurs through four instars before developing into either wingless (apteran) or winged (alate) adults (Wu et al. 2004). Alate development on the primary host is prompted by seasonal environmental cues, which facilitates the movement of mature aphids from the primary to the secondary host, while alate development on the secondary host soybean appears to be prompted by biotic factors such as overcrowding and poor plant quality that signals the aphids to seek new sources of food.
Insects are the most successful invertebrates known and their success is primarily attributed to extremely short developmental period combined with massive reproductive potential. For instance, with a development period of 5–7 days and fecundity potential of 20-75 aphids per female, the population doubling time of the soybean aphid in optimum conditions is less than 2 days, allowing the populations to explode at an exponential rate (McCornack et al. 2004; Michel et al. 2010). This propensity to rapidly multiply makes the soybean aphid the most important insect pest of soybean.
The life cycle of the soybean aphid starts with the hatching of first generation nymphs called fundatrices from surviving overwintered eggs on the primary host buckthorn. Egg hatching is tightly synchronized to the burst of buckthorn leaf buds during the months of March and April. Newly hatched fundatrices grow on developing buckthorn leaves into apterous (wingless) viviparous females that reproduce parthenogenetically (Ragsdale et al. 2004; Voegtlin et al. 2005). Development during this life stage is restricted to buckthorn, where the soybean aphid spends 2–3 generations, eventually producing viviparous alatae (winged aphids), also referred to as spring migrants. With the advent of warming temperatures and increasing photoperiod, spring migrants leave buckthorn seeking their summer host soybean.
Soybeans are receptive to soybean aphid colonization soon after emergence and colonies have been observed on developing unifoliolate leaves at the seedling stage (Heimpel et al. 2004; Ragsdale et al. 2004). Initial colonization on soybean has been reported as early as mid-June in North America (Hodgson et al. 2005). Upon successful landing on susceptible soybean plants, the aphids reproduce parthenogenetically giving birth to viviparous apterans, beginning the summer life stage. About 15–16 clonal generations occur on soybean during the crop season. The soybean aphid is multivoltine with overlapping generations and life stages occurring all through summer (Wu et al. 2004). Winged forms are produced in every generation, but a higher proportion of alates occur under crowded conditions (Lu and Chen 1993).
As the soybean crop matures, and with the onset of cooler autumn temperatures and decreasing photoperiod, the winged migrants, viviparous female gynoparae and sexual males, develop on senescing soybean plants and move to Rhamnus (Wu et al. 2004). The flight of gynoparae to Rhamnus is followed by winged males, which mate with mature oviparae produced by gynoparae. Mated oviparae move to lay fertilized eggs at the base of the dormant buckthorn shoot buds and twig crevices (Ragsdale et al. 2004). Egg hatch occurs in the spring, completing the life cycle of the soybean aphid.
Management of the soybean aphid
Soybean aphid outbreaks in North America are primarily managed by using three different techniques: chemical, biological, and more recently with host plant resistance. In the beginning of the soybean aphid invasion in the USA, the management focus was placed on identifying efficacious, easy-to-apply, economical, and readily available, registered insecticides. Organophospates, synthetic pyrethroids, and neonicotenoids are the most common chemicals shown to achieve effective control of soybean aphid as foliar sprays and the latter are also registered for seed treatment (Chandrasena et al. 2011). In addition, much work was done in determining the recommended economic threshold population size of about 250 aphids per plant prompting insecticide applications and giving a sufficient lead time before aphid populations cause economic damage (Ragsdale et al. 2007). While the timing of insecticide application is very critical for effective control, multiple sprayings and increasing use of insecticides pose a dire concern not only for potential environmental damage but also in promoting the development of insecticide resistance in soybean aphids, which has already been shown in a laboratory study (Chandrasena et al. 2011). Because of this, sex pheromones and insect growth regulators have been researched as potential alternate chemical strategies (Richardson and Lagos 2007; Zhu et al. 2006).
Although native to Asia, soybean aphid population outbreaks there have been sporadic and limited due to efficient control by natural enemies (Heimpel et al. 2004; Liu et al. 2004). In North America, endemic soybean aphid enemies include generalist aphidophagous predators, parasites, and entomopathogenic fungi (Fox et al. 2005; Kaiser et al. 2007; Nielsen and Hajek 2005; Rutledge et al. 2004). The predominant and most efficient predators include the multicolored Asian lady beetle, Harmonia axyridis Pallas (Coleopetra: Coccinellidae), minute pirate bug, Orius insidiosus (Say) (Hemiptera: Anthocoridae) (Costamagna et al. 2007; Fox et al. 2005; Mignault et al. 2006; Nielsen and Hajek 2005; Ragsdale et al. 2007; Rutledge et al. 2004; Schmidt et al. 2008), Aphidoletes aphidimyza Rondani and Allograpta obliqua Say predatory flies (Noma and Brewer 2008), and carabid beetles Elaphropus anceps (Le Conte), Clavina impressefrons Le Conte, Bembedion quadrimaculatum Say (Fox et al. 2005). In contrast, only six species of parasitoids were found associated with soybean aphid mummies in North America, of which two braconid wasps, Lysiphlebus testaceipes Cresson and Aphidius colemani Viereck were the most abundant (Kaiser et al. 2007; Noma and Brewer 2008). Even though there is a multitude of predators and parasites, suppression of soybean aphid outbreaks by natural enemies has not been effective in North America and is considered inefficient (Nielsen and Hajek 2005). This lack of efficiency of resident biological agents has encouraged entomologists to explore host-specific parasitoids of the soybean aphid in its native habitat of Asia. A parasitic wasp from China, Binodoxys communis (Gahan) (Chacón et al. 2008; Wyckhuys et al. 2009) was imported and released into the USA in 2007 and 2008, but has not been recovered to date, leading to speculation that the parasitoid was unable to overwinter (Hogg and Mahr 2007).
Discovery of soybean aphid resistance in soybean and allies
Plant insect resistance is the most cost effective and environmentally safe way to control insects such as the soybean aphid (Luginbill 1969). Resistance to insects is governed by genetic mechanisms similar to other plant traits (Auclair 1989). Dominant aphid resistance (R) genes have been identified in multiple crops, including cereals, forages, fruits, and vegetables, as reviewed in Smith (2005) and Smith and Boyko (2007). Two highly successful examples of the use of resistance to control aphids were the deployment of resistance to the Russian wheat aphid, Diuraphis noxia, on wheat (Triticum aestivum L.) (Basky 2003; Randolph et al. 2003) and on barley (Hordeum vulgare L.) (Bregitzer et al. 2003). Russian wheat aphid-resistant cultivars have been deployed widely throughout South Africa and the USA.
Although earlier studies conducted in China prior to the introduction of the soybean aphid into North America found resistance to aphid infestation in soybean genotypes (He et al. 1995; Hu et al. 1993; Yue et al. 1989), there were no known reports on the genetics of this resistance or the development of aphid-resistant breeding lines or cultivars. Multiple sources of resistance in soybean germplasm to the soybean aphid were first reported in North America in 2004 using a greenhouse choice-test procedure and a visual qualitative, non-parametric colonization rating scheme with a locally collected soybean aphid isolate (Hill et al. 2004b). First, commercial and pre-commercial soybean germplasm adapted to Illinois and surrounding states was screened and no resistance was found. Next, since the aphid migrated from Asia, a set of commercial Asian lines was screened without finding any sources of resistance. Then, a set of soybean ancestors and first progeny of ancestors representing 99 % of the genetic variability in North American public cultivars (Gizlice et al. 1994) was screened. A few of the ancestors expressed resistance in the choice tests (Hill et al. 2004b), including PI 548663 (cultivar Dowling) and PI 548657 (cultivar Jackson), important ancestors of current cultivars adapted to the southern USA Taking advantage of this finding, a set of current southern-adapted cultivars was screened for resistance; however, no resistance was found. Cultivars with Dowling or Jackson ancestry apparently lost aphid resistance during their development, possibly due to the lack of soybean aphids present to impose the selection pressure necessary for soybean breeders to maintain the resistance. Ancestors of Dowling and Jackson were screened to identify their aphid resistance donors. PI 548445 (cultivar CNS), a grandparent of Dowling, and PI 548657 (cultivar Palmetto), a parent of Jackson, were found to be resistant to the soybean aphid (Hill et al. 2004b).
With the knowledge that Mi root-knot nematode resistance in tomato also gave resistance to potato aphids (Rossi et al. 1998), sources of soybean root-knot nematode resistance were screened for resistance to the soybean aphid (Hill et al. 2004b). Strong antibiosis resistance was found in PI 200538 and PI 230977, both sources of resistance to Meloidogyne arenaria race 2 (Luzzi et al. 1995).
Resistance to the soybean aphid was reported in early maturing maturity group (MG) 0 to MG III germplasm accessions from northern China (Mensah et al. 2005). Using a semi-quantitative resistance rating scale, PI 567541B and PI 567598B were found to have antibiosis and PI 567543C and PI 567597C had antixenosis resistance expression. In addition, three aphid-resistant soybean germplasm accessions, PI 243540, PI 567301B, and PI 567324, were reported after screening a set of nearly 200 lines using a five point aphid score based on estimated numbers of aphids observed on individual plants (Mian et al. 2008a). Choice and no-choice testing revealed that PI 243540 expressed primarily antibiosis-type resistance while the other two accessions displayed mostly antixenosis.
A limited set of accessions of Glycine species was screened for soybean aphid resistance (Hill et al. 2004a). A few wild soybean, G. soja, lines expressed primarily antibiosis-type resistance. Resistance that was stronger or not significantly different from soybean Jackson was also found in germplasm accessions of Glycine species including G. argyrea, G. canescens, G. clandestine, G. curvata, G. cyrtoloba, G. falcata, G. microphylla, G. tabacina, and G. tomentella. Inheritance of resistance in these accessions is unknown so far and resistance from these sources has not been transferred to soybean.
Expression of soybean aphid resistance
Results of no-choice tests indicated that the resistance expression in Dowling, Jackson, and other G. max sources was primarily antibiosis (Hill et al. 2004b). This resistance was not transferred from resistant to susceptible stocks or scions in reciprocal grafts (unpublished results) and appeared to be expressed during all soybean growth stages (Hill et al. 2004b), in contrast with Mi-resistance in tomato, which was not expressed until plants were 6 weeks old (Kaloshian et al. 1995). Antixenosis-type resistance was primarily expressed in PI 71506 and PI 548445 (CNS). Further characterization of the expression of resistance indicated that the antibiosis in Dowling, Jackson, and PI 200538 caused a significant decrease in fecundity and longevity and increased mortality of soybean aphids compared to the susceptible soybean cultivar Pana (Li et al. 2004). Aphid longevity was 7 days longer on Pana than on Dowling and Jackson. First instar aphids placed on Dowling and PI 200538 leaves did not mature to adulthood. Starvation did not fully explain the effects of the antibiosis on the aphids. Aphids stayed on leaves of the three antibiotic genotypes for a significantly shorter time than on Pana, suggesting antixenosis-type resistance expression in addition to antibiosis.
Antixenosis-resistance expression was confirmed in Dowling, PI 71506, PI 230977, and in the breeding line G93-9223, as indicated by reduced aphid births in no-choice tests and reduced population development in choice tests with aphids from a local population collected in South Dakota (Hesler et al. 2007). Antibiotic resistance comparable to Jackson was found in the soybean cultivar Cobb (Hesler and Dashiell 2007) and resistance with moderate expression, compared to the resistance in Dowling, was also found in a few other soybean genotypes, which were sources of resistance to other insects (Hesler and Dashiell 2008).
Antibiosis-type resistance comparable to Dowling, Jackson, and Palmetto was found in soybean genotypes K1639 and Pioneer® 95B97, along with additional genotypes having moderate aphid resistance, after comparing nymphal population development during an initial screen followed by choice and no-choice tests to characterize resistance (Diaz-Montano et al. 2006). An electrical penetration graph technique used to evaluate aphid-feeding behavior on K1639, Pioneer® 95B97, Dowling, Jackson, and the susceptible genotype KS4202 indicated increased time to reach the first phloem sieve element and shorter time within the sieve element on the resistant genotypes compared to the susceptible genotype, suggesting that antibiosis-expression originated in the phloem (Diaz-Montano et al. 2007).
Genetics of soybean aphid resistance
Inheritance of soybean aphid resistance in Dowling (Hill et al. 2006a) and Jackson (Hill et al. 2006b) was found to be controlled by single, dominant genes. Both genes were mapped in the same genetic region on chromosome 7 or linkage group (LG) M in the soybean genome (Li et al. 2007), suggesting they were the same gene, allelic, or tightly linked. Because no common ancestors of Dowling and Jackson were known (Hill et al. 2004b), the Soybean Genetics Committee designated the gene in Dowling as Rag1 (resistance to Aphis glycines; Fig. 1)
(Hill et al. 2006a) and the gene in Jackson as Rag (Hill et al. 2006b). A subsequent genetic allelism test (unpublished results) found no susceptible plants among 1,000 Dowling × Jackson F2 plants, supporting the hypothesis that the genes were allelic. Simple sequence repeat (SSR) DNA markers were identified (Li et al. 2007) that flank the resistant allele at the Rag1 locus to facilitate incorporation of the gene into elite soybean genotypes through backcross breeding. Results of screening the flanking markers Satt435 and Satt463 in Dowling ancestor CNS and Jackson ancestor Palmetto indicated they carried the same resistance alleles as Dowling and Jackson, supporting the hypotheses that they were donors of soybean aphid resistance (Hill et al. 2004b) and that the genes in Dowling and Jackson were allelic (Somers et al. 2007).
Utilizing single nucleotide polymorphism (SNP) markers developed using near-isogenic lines, the Affymetrix Soybean GeneChip microarray (Kaczorowski et al. 2008), and additional SNP markers developed using advanced re-sequencing technique with a preliminary soybean draft sequence, Rag1 was located within a 115-kb genetic interval (Kim et al. 2010). Gene annotation predicted 13 putative genes within the interval; two genes appeared to be good candidates for Rag1 because they were nucleotide-binding, leucine-rich repeat (NBS-LRR) genes with high homology to disease resistance genes in Arabidopsis. NBS-LRR genes for aphid resistance have been found in tomato (Mi-1.2 gene), Lycopersicon peruvianum (L.) P. Mill. to Macrosiphum euphorbiae Thomas (Rossi et al. 1998) and melon (Vat gene), Cucumis melo L., to Aphis gossypii Glover (Klingler et al. 1998). The candidate Rag1 NBS-LRR sequences have been cloned. Work to confirm if one of them is Rag1 through transformation of susceptible soybean plants with the cloned genes is in progress.
Analyses of transcript expression profiles in Dowling and the susceptible genotype Williams 82 in response to soybean aphid infestation indicated that the gene expression response of Dowling plants was similar to the incompatible response induced by avirulent Pseudomonas syringae (Li et al. 2008). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) of three soybean defense genes indicated earlier and stronger response of Dowling toward aphid infestation than Williams 82. This result was consistent with gene-for-gene interactions found between plants and pathogens and other plant-aphid interactions (Smith and Boyko 2007).
Rag1 was found not to be associated with a yield drag, although it appeared to be associated with a slight delay in crop maturity (Kim and Diers 2009). With this encouraging result along with knowledge of flanking DNA markers (Li et al. 2007) enabling marker-assisted selection, soybean breeders proceeded to develop new cultivars with the gene, with the initial release in 2008 (Caspers-Simmet 2008).
A second resistance gene named Rag2 was identified in PI 200538 (Hill et al. 2009) and in PI 243540 (Kang et al. 2008; Mian et al. 2008b). Because the genes were mapped to the same location on soybean chromosome 13 in LG F and had similar response to different aphid isolates (Kim et al. 2008b), it is assumed that the genes are identical or allelic. Fine-mapping Rag2 in PI 200538 using SNP markers and re-sequencing narrowed the interval containing the resistance gene in the soybean genetic map to 54 kb (Kim et al. 2010). One candidate NBS-LRR-type resistance gene was identified within the region.
Additional soybean aphid resistance genes have been identified over the last few years. Two recessive resistance genes were identified in PI 567541B (Mensah et al. 2008). One of these genes mapped to the same region as Rag1 in LG M, and initially was named rag1_provisional, but is presently called rag1c; the other gene mapped to LG F in a different region from Rag2 and was named rag4 (Zhang et al. 2009). The potential allelic relationship between rag1c and Rag1 has not been determined. A dominant soybean aphid resistance gene found in PI 567543C mapped to soybean chromosome 16 in LG J and was named Rag3 (Zhang et al. 2010). A major gene controlling resistance to the soybean aphid in PI 567301B was mapped to chromosome 13 in LG F near Rag2; however, because resistance expressed by PI 567301B was antixenosis while Rag2-mediated resistance was antibiosis, this indicated that different genes were being expressed. Therefore, the gene in PI 567301B was considered different from Rag2 and was proposed to be called Rag5 (Jun et al. 2012). Antixenosis-type resistance in PI 71506 was primarily controlled by a single, dominant gene (Van Nurden et al. 2010). This gene apparently has not yet been mapped in the soybean genome.
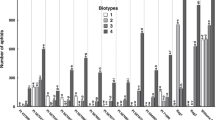
Known soybean aphid resistance genes in soybean are listed in Table 1, along with sources, map location, and resistance expression. Approximately a third of the 18,000-accession USDA soybean germplasm collection has been screened to date. It is possible that other resistance genes at new loci, or are allelic with presently known genes, will be found in the future with further screening.
Soybean aphid virulence
Most aphid species are specialized to feed on a particular plant family or even a few plant species within a family (Blackman and Eastop 2000; Powell et al. 2006). The soybean aphid is also highly specialized towards soybean and its closest relatives (Hill et al. 2004a). The bases for this specialization on Glycine species is not understood at present, however, it is likely the result of a long period of co-evolution between ancestors of the aphid and Glycine species in their center of origin in present day China (Blackman and Eastop 2000; Hymowitz and Bernard 1991). Biotypes of aphids, defined as aphid genotypes that can colonize specific host genotypes, have been reported in many host-aphid systems (Smith 2005; van Emden 2007).
Initial studies among soybean aphid populations indicated that virulence variability was lacking in North America. Results of early testing indicated that virulence among three soybean aphid isolates collected from a limited geographic area did not differ on a set of soybean genotypes and showed no host specialization (Hill et al. 2004b). In a recent study, based on molecular microsatellite marker screening, little genetic variation was found among aphid populations collected from a wide geographic range (Michel et al. 2009).
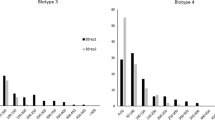
About the same time, while soybean breeding lines possessing Rag1 were being tested in the field in Ohio in 2006, dense aphid colonies were observed on the plants with Rag1, similar to levels of colonization observed on previously known susceptible lines. An isolate of aphids collected from these plants was tested in choice and non-choice experiments (Kim et al. 2008b). Results of the tests indicated that the Ohio isolate was a distinct biotype, now called biotype 2 (Hill et al. 2009, 2010) differing from the aphid isolate collected in Illinois, now called biotype 1, that was used to identify and map Rag1. The biotypes could be distinguished by their differential virulence on plants possessing Rag1. Biotype 2 developed dense colonies on plants with Rag1. In contrast, biotype 1 did not colonize plants with Rag1. The serendipitous discovery of biotype 2 was surprising because the resistance gene had not yet been deployed in soybean production. Another soybean aphid biotype was also recently identified (Hill et al. 2010), named biotype 3, that was distinguished by its virulence on plants with Rag2, but colonized plants with other resistance genes as well. A summary of the virulence of the three documented soybean aphid biotypes on plants with Rag1 and Rag2, or without the genes is presented in Table 2.
Discovery of soybean aphid biotypes before aphid resistant cultivars were deployed in production indicates a high potential for soybean aphid populations to rapidly adapt to resistance genes. This suggests that the effectiveness of deployed soybean aphid resistance genes, especially those expressing antibiosis-type resistance, could be short-lived in soybean production fields. Resistance gene adaptation has become a major concern to soybean breeders engaged in developing new soybean aphid resistant cultivars. It is probable that new soybean aphid biotypes will be identified as new soybean resistance genes are discovered and challenged with different aphid isolates. New sources of resistance may need to be continually sought and introduced into soybean to stay ahead of the ability of the aphid to adapt to host resistance genes.
However, the actual risk of soybean aphid resistance gene adaptation is uncertain at present because knowledge on the number and distribution of soybean aphid biotypes and their ability to survive and multiply is limited at present. Using a detached leaf bioassay, samples of aphids collected from different fields in Kansas, Ohio, and South Dakota were screened for virulence on soybean genotype Jackson (Michel et al. 2010). Moderate virulence on Jackson was found in Ohio, low virulence in Kansas, and only Jackson-avirulent aphids were found in South Dakota. More extensive sampling and testing is needed to assess the geographic virulence variability of soybean aphids in North America.
In contrast to the soybean aphid, it took several years after the Russian wheat aphid was found in the USA before biotypic variation was first discovered (Burd et al. 2006; Haley et al. 2004; Smith et al. 2004). Other secondary weedy grass hosts may have aided the evolution of Russian wheat aphid biotypes, as was found with greenbug (Anstead et al. 2003), Schizaphis graminum (Rondani), another important economic aphid pest of cereal crops. Several greenbug biotypes have been identified and their virulence characterized (Burd and Porter 2006). It is interesting to note that the most virulent isolates of greenbug were collected from non-cultivated hosts, which serve as a bridge host between winter wheat crops and are also important for aphid sexual reproduction. Although a few cultivated legume hosts, such as scarlet runner bean, Phaseolus coccineus L., and Trifolium species, were colonized by soybean aphids in greenhouse tests, with significantly lower populations than on susceptible G. max genotypes (Hill et al. 2004a), there are no reports of soybean aphid colonization in those crops in the field nor of additional secondary hosts. However, new biotypes in the soybean aphid may arise through sexual recombination on its primary host. Indeed, biotype 3 was found on Frangula alnus, the glossy buckthorn, which was previously not thought to be an important sexual host (Hill et al. 2010). Thus, the soybean aphid may not only be evolving new biotypes attacking specific soybean genotypes, but appears to also be adapting to new over-wintering hosts.
The basis for the interaction between the Russian wheat aphid and its host plants is thought to involve gene-for-gene interaction similar to that found between plants and plant pathogens (Flor 1971), involving an elicitor/effector from the aphid that is specifically recognized by a protein in the host plant, resulting in a cascade of biochemical defense responses (Botha et al. 2005). The gene-for-gene model has also been proposed for the tomato-potato aphid system (Kaloshian 2004). With the results of microarray analysis demonstrating the response of defense-related genes (Li et al. 2008), cloning of NBS-LRR resistance genes (Kim and Diers 2009; Kim et al. 2010), and the identification of biotypes (Hill et al. 2010; Kim et al. 2008b), supporting evidence for a gene-for-gene interaction between soybean and the soybean aphid appears to be accumulating.
On-going and future research
Research to identify and map virulence genes in the soybean aphid is one of our current projects and results will significantly increase our understanding of the interaction between soybean resistance genes and aphid virulence genes. Additionally, molecular markers flanking aphid virulence genes identified in this research could be used to diagnose virulence potential in soybean aphid samples and facilitate the intelligent deployment of soybean resistance genes in geographic regions where they would be most effective. This effort will be aided through sequencing the soybean aphid genome and transcriptomes of soybean aphid biotypes. On-going collaborations to complete the sequencing projects will facilitate the development of new molecular markers to complement markers developed by other research groups (Bai et al. 2010).
Potential roles of endosymbionts and gut bacteria in soybean aphid fitness and virulence need to be investigated. There is increasing evidence that these organisms may be involved in a tritrophic interaction with their aphid hosts and host plants (Francis et al. 2010; Tagu et al. 2008; Tsuchida et al. 2004; Walling 2000) and a nematode with a plant host (Cortada et al. 2011).
Research to improve the durability of genetic resistance in soybean through stacking of resistance genes is also underway. Identification and mapping of additional antixenosis-type resistance genes (Jun et al. 2012), may also facilitate development of increased resistance durability in soybean because that type of resistance may reduce selection pressure on soybean aphids that promotes adaptation on resistance genes by allowing limited colonization.
Currently, the interaction among different components of integrated management of the soybean aphid, such as use of host resistance, biological control, and chemical applications is unknown. Knowledge of potential negative or positive interactions among components will improve soybean aphid IPM and could increase the durability of resistance genes, insecticides, and biological control agents over time. Studies on the interaction between soybean aphid parasitoids with host resistance have begun (Ragsdale et al. 2011).
References
Anstead JA, Burd JD, Shufran KA (2003) Over-summering and biotypic diversity of Schizaphis graminum (Homoptera: Aphididae) populations on noncultivated grass hosts. Environ Entomol 32:662–667
Auclair JL (1989) Host plant resistance. In: Minks A, Harrewijn P (eds) Aphids: their viology, natural enemies, and control. Elsevier, New York, pp 225–265
Bai X, Zhang W, Orantes L, Jun TH, Mittapalli O, Mian MAR, Michel AP (2010) Combining next-generation sequencing strategies for rapid molecular resource development from an invasive aphid species, Aphis glycines. PLoS One 5:e11370
Basky Z (2003) Biotypic and pest status differences between Hungarian and South African populations of Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae). Pest Manage Sci 59:1152–1158
Blackman RL, Eastop VF (2000) Aphids on the World’s crops. Wiley, Chichester
Botha A, Li Y, Lapitan N (2005) Cereal host interaction with a homopteran insect, Russian wheat aphid: a review. J Plant Insect Interact 1:211–222
Bregitzer P, Mornhinweg DW, Jones BL (2003) Resistance to Russian wheat aphid damage derived from STARS 9301B protects agronomic performance and malting quality when transferred to adapted barley germplasm. Crop Sci 43:2050–2057
Burd JD, Porter DR (2006) Biotypic diversity in greenbug (Hemiptera: Aphididae): characterizing new virulence and host associations. J Econ Entomol 99:959–965
Burd JD, Porter DR, Puterka GJ, Haley SD, Peairs FB (2006) Biotypic variation among north American Russian wheat aphid (Homoptera: Aphididae) populations. J Econ Entomol 99:1862–1866
Caspers-Simmet J (2008) Soybean-aphid resistant varieties will come with a difference. In: Agri News. http://webstar.agrinews.com/agrinews/295310252644869.bsp
Chacón JM, Landis DA, Heimpel GE (2008) Potential for biotic interference of a classical biological control agent of the soybean aphid. Biol Control 46:216–225
Chandrasena D, DiFonzo C, Byrne A (2011) An aphid-dip bioassay to evaluate susceptibility of soybean aphid (Hemiptera: Aphididae) to pyrethroid, organophosphate, and neonicotinoid insecticides. J Econ Entomol 104:1357–1363
Cortada L, Sakai H, Verdejo-Lucas S, Mizukubo T (2011) Meloidogyne virulence locus molecular marker for characterization of selected Mi-virulent populations of Meloidogyne spp. is correlated with several genera of Betaproteobacteria. Phytopathology 101:410–415
Costamagna AC, Landis DA, Difonzo CD (2007) Suppression of soybean aphid by generalist predators results in a trophic cascade in soybeans. Ecol Appl 17:441–451
Diaz-Montano J, Reese JC, Schapaugh WT, Campbell LR (2006) Characterization of antibiosis and antixenosis to the soybean aphid (Hemiptera: Aphididae) in several soybean genotypes. J Econ Entomol 99:1884–1889
Diaz-Montano J, Reese JC, Louis J, Campbell LR, Schapaugh WT (2007) Feeding behavior by the soybean aphid (Hemiptera: Aphididae) on resistant and susceptible soybean genotypes. J Econ Entomol 100:984–989
Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopathol 9:275–296
Fox TB, Landis DA, Cardoso FF, Difonzo CD (2005) Impact of predation on establishment of the soybean aphid, Aphis glycines in soybean, Glycine max. BioControl 50:545–563
Francis F, Guillonneau F, Leprince P, De Pauw E, Haubruge E, Jia L, Goggin F (2010) Tritrophic interactions among Macrosiphum euphorbiae aphids, their host plants and endosymbionts: investigation by a proteomic approach. J Insect Physiol 56:575–585
Gizlice Z, Carter TE, Burton JW (1994) Genetic base for North American public soybean cultivars released between 1947 and 1988. Crop Sci 34:1143–1151
Haley SD, Peairs FB, Walker CB, Rudolph JB, Randolph TL (2004) Occurrence of a new Russian wheat aphid biotype in Colorado. Crop Sci 44:1589–1592
Hartman GL, Domier LL, Wax LM, Helm CG, Onstad DW, Shaw JT, Solter LF, Voegtlin DJ, D’Arcy CJ, Gray ME, Steffey KL, Isard SA, Orwick PL (2001) Occurrence and distrubution of Aphis glycines on soybeans in Illinois in 2000 and its potential control. Plant Health Progress. doi:10.1094/PHP-2001-0205-1001-HN
Hartman GL, West E, Herman T (2011) Crops that feed the world 2. Soybean-worldwide production, use, and constraints caused by pathogens and pests. Food Security 3:5–17
He F, Liu X, Yan F, Wang Y (1995) Soybean resistance to the soybean aphid. Liaoning Agric Sci 4:30–34
Heimpel GE, Ragsdale DW, Venette R, Hopper KR, O’Neil RJ, Rutledge CE, Wu Z (2004) Prospects for importation biological control of the soybean aphid: anticipating potential costs and benefits. Ann Entomol Soc Am 97:249–258
Hesler LS, Dashiell KE (2007) Resistance to Aphis glycines (Hemiptera: Aphididae) in various soybean lines under controlled laboratory conditions. J Econ Entomol 100:1464–1469
Hesler LS, Dashiell KE (2008) Identification and characterization of new sources of resistance to Aphis glycines Matsumura (Hemiptera: Aphididae) in soybean lines. Appl Entomol Zool 43:197–206
Hesler LS, Dashiell KE, Lundgren JG (2007) Characterization of resistance to Aphis glycines in soybean accessions. Euphytica 154:91–99
Hill CB, Li Y, Hartman GL (2004a) Resistance of Glycine species and various cultivated legumes to the soybean aphid (Homoptera: Aphididae). J Econ Entomol 97:1071–1077
Hill CB, Li Y, Hartman GL (2004b) Resistance to the soybean aphid in soybean germplasm. Crop Sci 44:98–106
Hill CB, Li Y, Hartman GL (2006a) A single dominant gene for resistance to the soybean aphid in the soybean cultivar Dowling. Crop Sci 46:1601–1605
Hill CB, Li Y, Hartman GL (2006b) Soybean aphid resistance in soybean Jackson is controlled by a single dominant gene. Crop Sci 46:1606–1608
Hill CB, Kim K-S, Crull L, Diers BW, Hartman GL (2009) Inheritance of resistance to the soybean aphid in soybean PI200538. Crop Sci 49:1193–1200
Hill CB, Crull L, Herman TK, Voegtlin DJ, Hartman GL (2010) A new soybean aphid (Hemiptera: Aphididae) biotype identified. J Econ Entomol 103:509–515
Hodgson EW, Venette RC, Abrahamson M, Ragsdale DW (2005) Alate production of soybean aphid (Homoptera: Aphididae) in Minnesota. Environ Entomol 34:1456–1463
Hogg DB, Mahr D (2007) Binodoxys communis field cage releases for control of the soybean aphid in Wisconsin. Wis Crop Manage 14:129–130
Hu Q, Zhao J, Cui D (1993) Relationship between content of secondary catabolite-lignin-in soybean and soybean resistance to the soybean aphid. Plant Prot 19:8–9
Hymowitz T, Bernard R (1991) Origin of the soybean and germplasm introduction and development in North America. In: Shands HL (ed) Use of plant introductions in cultivar development, part 1. vol 17. CSSA Special Publication, pp 149–167
Jun TH, Rouf Mian MA, Michel AP (2012) Genetic mapping revealed two loci for soybean aphid resistance in PI 567301B. Theor Appl Genet 124:13–22
Kaczorowski KAK, Diers KS, Hudson BW, Matthew E (2008) Microarray-based genetic mapping using soybean near-isogenic lines and generation of SNP markers in the Rag1 aphid-resistance interval. Plant Genome 1:89–98
Kaiser ME, Noma T, Brewer MJ, Pike KS, Vockeroth J, Gaimari SD (2007) Hymenopteran parasitoids and dipteran predators found using soybean aphid after its midwestern United States invasion. Ann Entomol Soc Am 100:196–205
Kaloshian I (2004) Gene-for-gene disease resistance: bridging insect pest and pathogen defense. J Chem Ecol 30:2419–2438
Kaloshian I, Lange WH, Williamson VM (1995) An aphid-resistance locus is tightly linked to the nematode-resistance gene, Mi, in tomato. Proc Natl Acad Sci USA 92:622–625
Kang S-T, Rouf Mian MA, Hammond RB (2008) Soybean aphid resistance in PI 243540 is controlled by a single dominant gene. Crop Sci 48:1744–1748
Kim K-S, Diers BW (2009) The associated effects of the soybean aphid resistance locus Rag1 on soybean yield and other agronomic traits. Crop Sci 49:1726–1732
Kim C, Schaible G, Garrett L, Lubowski R, Lee D (2008a) Economic impacts of the US soybean aphid infestation: a multi-regional competitive dynamic analysis. Agri Econ Rev 37:227–242
Kim K-S, Hill CB, Hartman GL, Mian MAR, Diers BW (2008b) Discovery of soybean aphid biotypes. Crop Sci 48:923–928
Kim KS, Bellendir S, Hudson KA, Hill CB, Hartman GL, Hyten DL, Hudson ME, Diers BW (2010) Fine mapping the soybean aphid resistance gene Rag1 in soybean. Theor Appl Genet 120:1063–1071
Klingler J, Powell G, Thompson GA, Isaacs R (1998) Phloem specific aphid resistance in Cucumis melo line AR 5: effects on feeding behaviour and performance of Aphis gossypii. Entomol Exp Appl 86:79–88
Li Y, Hill CB, Hartman GL (2004) Effect of three resistant soybean genotypes on the fecundity, mortality, and maturation of soybean aphid (Homoptera: Aphididae). J Econ Entomol 97:1106–1111
Li Y, Hill CB, Carlson S, Diers BW, Hartman GL (2007) Soybean aphid resistance genes in the soybean cultivars Dowling and Jackson map to linkage group. Mol Breed 19:25–34
Li Y, Zou J, Li M, Bilgin DD, Vodkin LO, Hartman GL, Clough SJ (2008) Soybean defense responses to the soybean aphid. New Phytol 179:185–195
Liu J, Wu K, Hopper KR, Zhao K (2004) Population dynamics of Aphis glycines (Homoptera: Aphididae) and its natural enemies in soybean in northern China. Ann Entomol Soc Am 97:235–239
Lu LH, Chen RL (1993) Production of the soybean aphid alatae Aphis glycines. Acta Entomol Sinica 36:143–149
Luginbill JP (1969) Developing resistant plants-the ideal method of controlling insects. US Government Print Office
Luzzi BM, Boerma HR, Hussey RS (1995) Inheritance of resistance to the peanut root-knot nematode in soybean. Crop Sci 35:50–53
Matsumura S (1917) A list of the Aphididae of Japan, with description of new species and genera. J College of Ag, Sapporo, Jap 7:387–388
McCornack BP, Ragsdale DW, Venette RC (2004) Demography of soybean aphid (Homoptera: Aphididae) at summer temperatures. J Econ Entomol 97:854–861
Mensah C, DiFonzo C, Nelson RL, Wang D (2005) Resistance to soybean aphid in early maturing soybean germplasm. Crop Sci 45:2228–2233
Mensah C, DiFonzo C, Wang D (2008) Inheritance of soybean aphid resistance in PI 567541B and PI 567598B. Crop Sci 48:1759–1763
Mian MAR, Hammond RB, St. Martin SK (2008a) New plant introductions with resistance to the soybean aphid. Crop Sci 48:1055–1061
Mian RM, Kang S-T, Beil S, Hammond R (2008b) Genetic linkage mapping of the soybean aphid resistance gene in PI 243540. Theor Appl Genet 117:955–962
Michel AP, Zhang W, Kyo Jung J, Kang ST, Mian M (2009) Population genetic structure of Aphis glycines. Environ Entomol 38:1301–1311
Michel AP, Mian MAR, Davila-Olivas NH, CaÒas LA (2010) Detached leaf and whole plant assays for soybean aphid resistance: differential responses among resistance sources and biotypes. J Econ Entomol 103:949–957
Mignault MP, Roy M, Brodeur J (2006) Soybean aphid predators in Quebec and the suitability of Aphis glycines as prey for three Coccinellidae. Biocontrol 51:89–106
Nielsen C, Hajek AE (2005) Control of invasive soybean aphid, Aphis glycines (Hemiptera: Aphididae), populations by existing natural enemies in New York State, with emphasis on entomopathogenic fungi. Environ Entomol 34:1036–1047
Noma T, Brewer MJ (2008) Seasonal abundance of resident parasitoids and predatory flies and corresponding soybean aphid densities, with comments on classical biological control of soybean aphid in the midwest. J Econ Entomol 101:278–287
Powell G, Tosh C, Hardie J (2006) Host plant selection by aphids: behavioral, evolutionary, and applied perspectives. Annu Rev Entomol 51:309–330
Ragsdale D, Voegtlin DJ, O’Neil RJ (2004) Soybean aphid biology in North America. Ann Entomol Soc Am 97:204–208
Ragsdale DW, McCornack BP, Venette RC, Potter BD, Macrae IV, Hodgson EW, O’Neal ME, Johnson KD, O’Neil RJ, DiFonzo CD (2007) Economic threshold for soybean aphid (Hemiptera: Aphididae). J Econ Entomol 100:1258–1267
Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N (2011) Ecology and management of the soybean aphid in North America. Annu Rev Entomol 56:375–399
Randolph T, Peairs F, Kroening M, Armstrong J, Hammon R, Walker C, Quick J (2003) Plant damage and yield response to the Russian wheat aphid (Homoptera: Aphididae) on susceptible and resistant winter wheats in Colorado. J Econ Entomol 96:352–360
Richardson ML, Lagos DM (2007) Effects of a juvenile hormone analogue, pyriproxyfen, on the apterous form of soybean aphid (Aphis glycines). J Appl Entomol 131:297–302
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci USA 95:9750–9754
Rutledge CE, O’Neil RJ, Fox TB, Landis DA (2004) Soybean aphid predators and their use in integrated pest management. Ann Entomol Soc Am 97:240–248
Schmidt NP, O’Neal ME, Dixon PM (2008) Aphidophagous predators in Iowa soybean: a community comparison across multiple years and sampling methods. Ann Entomol Soc Am 101:341–150
Smith CM (2005) Plant resistance to arthropods: molecular and conventional approaches. Kluwer Academic Publishers, Dordrecht
Smith CM, Boyko EV (2007) The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol Exp Appl 122:1–16
Smith CM, Belay T, Stauffer C, Stary P, Kubeckova I, Starkey S (2004) Identification of Russian wheat aphid (Homoptera: Aphididae) populations virulent to the Dn4 resistance gene. J Econ Entomol 97:1112–1117
Somers DS, Chen CY, Gu CG, Mensah CMC, Nelson RL, Wang D (2007) SSR marker diversity of soybean aphid resistance sources in North America. Genome 50:1104–1111
Tagu D, Klingler J, Moya A, Simon J (2008) Early progress in Aphid Genomics and consequences for plant-aphid interactions studies. Mol Plant Microbe Interact 21:701–708
Takahashi S, Inaizumi M, Kawakami K (1993) Life cycle of the soybean aphid Aphis glycines Matsumura, in Japan. Jan J Appl Entomol Zool 37:207–207
Tilmon K, Hodgson E, O’Neal M, Ragsdale D (2011) Biology of the soybean aphid, Aphis glycines (Hemiptera: Aphididae) in the United States. J Integr Pest Manage 2. doi:http://dx.doi.org/10.1603/IPM10016
Tsuchida T, Koga R, Fukatsu T (2004) Host plant specialization governed by facultative symbiont. Science 303:1989
van Emden HF (2007) Host-plant resistance. In: van Emden HF, Harrington R (eds) Aphids as crop pests. CAB International, Wallingford, UK
Van Nurden A, Scott R, Hesler L, Tilmon K, Glover K, Carter C (2010) Inheritance of soybean aphid resistance from PI 71506. J Crop Improv 24:400–416
Venette RC, Ragsdale DW (2004) Assessing the invasion by soybean aphid (Homoptera: Aphididae): where will it end? Ann Entomol Soc Am 97:219–226
Voegtlin DJ, Halbert SE, Qiao G (2004a) A guide to separating Aphis glycines Matsumura and morphologically similar species that share its hosts. Ann Entomol Soc Am 97:227–232
Voegtlin DJ, O’Neil RJ, Graves WR (2004b) Tests of suitability of overwintering hosts of Aphis glycines: identification of a new host association with Rhamnus alnifolia L’Héritier. Ann Entomol Soc Am 97:233–234
Voegtlin DJ, O’neil RJ, Graves WR, Lagos D, Yoo HJS (2005) Potential winter hosts of soybean aphid. Ann Entomol Soc Am 98:690–693
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Wang C, Siang N, Chang G, Chu H (1962) Studies on the soybean aphid, Aphis glycines Matsumura. Acta Entomol Sinica 11:31–44
Wu Z, Schenk-Hamlin D, Zhan W, Ragsdale DW, Heimpel GE (2004) The soybean aphid in China: a historical review. Ann Entomol Soc Am 97:209–218
Wyckhuys KAG, Koch RL, Kula RR, Heimpel GE (2009) Potential exposure of a classical biological control agent of the soybean aphid, Aphis glycines, on non-target aphids in North America. Biol Invasions 11:857–871
Yue D, Guo S, Shan Y (1989) Resistance of wild soybean Glycine soja to Aphis glycines. I. Screening for resistant varieties. Jilin Agric Sci 3:15–19
Zhang G, Gu C, Wang D (2009) Molecular mapping of soybean aphid resistance genes in PI 567541B. Theor Appl Genet 118:473–482
Zhang G, Gu C, Wang D (2010) A novel locus for soybean aphid resistance. Theor Appl Genet 120:1183–1191
Zhu J, Zhang A, Park K-C, Baker T, Lang B, Jyrenka R, Obrycki JJ, Graves WR, Pickett JA, Smiley D, Chauhan KR, Klun JA (2006) Sex pheromone of the soybean aphid, Aphis glycines Matsumura, and its potential use in semiochemical-based control. Environ Entomol 35:249–257
Acknowledgments
We thank the following funding agencies for partial support of our research program: Illinois Soybean Association, North Central Soybean Research Program, and the United Soybean Board. We also thank Dr. David Voegtlin for his editorial comments and Theresa Herman for her overall review. We also appreciate the cooperative efforts of Drs. Brian Diers and Rosanna Giordano on soybean aphid breeding and genomics research, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hill, C.B., Chirumamilla, A. & Hartman, G.L. Resistance and virulence in the soybean-Aphis glycines interaction. Euphytica 186, 635–646 (2012). https://doi.org/10.1007/s10681-012-0695-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0695-z