Abstract
Key message
Five soybean plant introductions expressed antibiosis resistance to multiple soybean aphid biotypes. Two introductions had resistance genes located in the Rag1, Rag2, and Rag3 regions; one introduction had resistance genes located in the Rag1, Rag2, and rag4 regions; one introduction had resistance genes located in the Rag1 and Rag2 regions; and one introduction had a resistance gene located in the Rag2 region.
Abstract
Soybean aphid (Aphis glycines Matsumura) is the most important soybean [Glycine max (L.) Merr.] insect pest in the USA. The objectives of this study were to characterize the resistance expressed in five plant introductions (PIs) to four soybean aphid biotypes, determine the mode of resistance inheritance, and identify markers associated with genes controlling resistance in these accessions. Five soybean PIs, from an initial set of 3000 PIs, were tested for resistance against soybean aphid biotypes 1, 2, 3, and 4 in choice and no-choice tests. Of these five PIs, PI 587663, PI 587677, and PI 587685 expressed antibiosis against all four biotypes, while PI 587972 and PI 594592 expressed antibiosis against biotypes 1, 2, and 3. F2 populations derived from PI 587663 and PI 587972 were evaluated for resistance against soybean aphid biotype 1, and populations derived from PIs 587677, 587685, and 594592 were tested against biotype 3. In addition, F2:3 plants were tested against biotypes 2 and 3. Genomic DNA from F2 plants was screened with markers linked to Rag1, Rag2, Rag3, and rag4 soybean aphid-resistance genes. Results showed that PI 587663 and PI 594592 each had three genes with variable gene action located in the Rag1, Rag2, and Rag3 regions. PI 587677 had three genes with variable gene action located in the Rag1, Rag2 and rag4 regions. PI 587685 had one dominant gene located in the Rag1 region and an additive gene in the Rag2 region. PI 587972 had one dominant gene located in the Rag2 region controlling antixenosis- or antibiosis-type resistance to soybean aphid biotypes 1, 2, or 3. PIs 587663, 587677, and 587685 also showed antibiosis-type resistance against biotype 4. Information on multi-biotype aphid resistance and resistance gene markers will be useful for improving soybean aphid resistance in commercial soybean cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean is the second most important field crop cultivated in the USA, with 36 million ha harvested in 2014, producing 1.09 MMT of grain valued at over $40.3 billion (NASS 2015). The soybean aphid, Aphis glycines Matsumura, now established since its introduction from Asia in 2000 (Hartman et al. 2001), has become one of the most economically important insect pests threatening USA soybean production (Kim et al. 2008a; Tilmon et al. 2011), prompting major changes in soybean pest management practices (Fernandez-Cornejo et al. 2014; Hill et al. 2012; Ragsdale et al. 2011). Heavy aphid colonization of soybean plants decreases plant productivity by reducing pod and seed set (Beckendorf et al. 2008), seed oil content (Riedell and Catangui 2006), and photosynthetic efficiency (Macedo et al. 2003). The soybean aphid is capable of transmitting soybean viruses (Hartman et al. 2001; Hill et al. 2001), although no major soybean virus epidemics have been reported to date.
Initially, the application of insecticides was the only commercially effective tool to control the soybean aphid (Ragsdale et al. 2011), but soybean cultivars with resistance have now been deployed in some USA soybean production areas (Hesler et al. 2013; Hill et al. 2012; McCarville et al. 2014). Even before soybean aphid-resistant cultivars became commercially available, at least four soybean aphid biotypes were discovered, three of which could overcome major resistance genes in the USA (Alt and Ryan-Mahmutagic 2013; Hill et al. 2010, 2012; Kim et al. 2008b; Pawlowski et al. 2014). Biotype 1 is avirulent on plants with Rag1 or Rag2. Biotype 2 is virulent on Rag1 and avirulent on Rag2. Biotype 3 is avirulent on Rag1 and virulent on Rag2. Biotype 4 is virulent on both Rag1 and Rag2, including pyramided lines. Biotypes were also subsequently reported from China (Liang et al. 2013; Zhong et al. 2014). The genetics of virulence of soybean aphid biotypes is unknown.
Evaluation of soybean germplasm resources for genetic resistance to the soybean aphid began soon after the pest arrived and continues today in the USA (Bansal et al. 2013; Bhusal et al. 2013, 2014; Fox et al. 2014; Hesler et al. 2012; Hill et al. 2012; Kim et al. 2014) and in China (Liu et al. 2014). Identification of sources of resistance effective against multiple soybean aphid biotypes has been discussed (Bansal et al. 2013; Hill et al. 2012; McCarville et al. 2014), and multi-biotype resistant germplasm accessions expressing resistance against three soybean aphid biotypes have been reported (Bansal et al. 2013).
An evaluation of over 3000 plant introductions (PIs) from the USDA Germplasm Collection, conducted at the University of Illinois, identified 50 PIs with resistance to both soybean aphid biotypes 1 and 2 (Kim et al. 2008b) and the genetics of resistance in 21 of the accessions was determined (Fox et al. 2014; Kim et al. 2014). We subsequently identified five additional PIs that expressed resistance to soybean aphid biotype 3 (Hill et al. 2010). With the discovery of soybean aphid biotype 4 in 2011 (Alt and Ryan-Mahmutagic 2013), the five PIs, 587663, 587677, 587685, 597972, and 594592 with resistance to soybean aphid biotypes 1, 2, and 3 were evaluated for resistance to biotype 4. The objectives of this study were to (1) characterize the resistance expressed in these five PIs to four soybean aphid biotypes through choice and no-choice testing to determines the type of resistance expression (antibiosis or antixenosis), (2) determine the mode of resistance inheritance, and (3) identify markers associated with resistance regions in these accessions.
Materials and methods
Aphid isolates and culturing
Soybean aphid isolates included soybean aphid biotypes 1, 2, 3 and 4. Biotype 1 was first collected on soybean in Illinois in 2000 (Hill et al. 2004), biotype 2 was found on soybean plants with the Rag1 resistance gene in Ohio in 2005 (Kim et al. 2008b), biotype 3 was collected from Frangula alnus in Indiana in 2007 (Hill et al. 2010), and biotype 4 was identified from soybean in Wisconsin in 2011 (Alt and Ryan-Mahmutagic 2013). Isolates of biotypes 1, 2, and 3 were maintained in our laboratory since their discovery. Researchers from the University of Wisconsin provided biotype 4 (Crossley and Hogg 2015). Methods to rear and maintain the aphids were previously described (Hill et al. 2004, 2010; Kim et al. 2008b). The soybean aphid biotypes 1, 2, 3, and 4 were maintained, respectively, on cultivar Williams 82, soybean breeding lines with Rag1, with Rag2, and on lines with both Rag1 and Rag2. Aphid isolates were cloned periodically from isolated nymphs and maintained in separate growth chambers for several viviparous, parthenogenetic generations prior to use.
Plant material
Known soybean aphid-resistant and -susceptible soybean genotypes were selected to use as checks and to compare resistance sources in resistance characterization tests (Table 1). Populations were generated from crosses made between the five aphid-resistant soybean PIs and aphid-susceptible soybean breeding lines. The crosses were LD02-5320 × PI 587663, LD03-6566 × PI 587677, LD03-10504 × PI 587685, LD03-10504 × PI 587972, and LD02-5320 × PI 594592, with soybean aphid-resistant PIs as male parents in all crosses. All five PIs originated from China. PIs 587663, 587677, 587685, and 594592 are classified as maturity group (MG) VII and PI 587972 as MG VI. LD02-5320, LD03-6566, and LD03-10504, (soybean breeding lines developed at the University of Illinois) were susceptible to biotypes 1, 2, and 3 in preliminary tests. They were not tested against biotype 4, which was not known when the crosses were made. The F1, F2, and F2:3 generations were developed as previously described (Fox et al. 2014; Hill et al. 2009).
Plant culture
Plants used in greenhouse experiments were planted in soil-less potting medium (Sunshine Mix, LC1, Sun Gro Horticulture Inc., Bellevue, WA, USA). For choice tests, plastic multi-pot inserts (Hummert Intl., Earth City, MO, USA) with each pot insert size 30 × 40 × 60 mm were placed inside plastic trays with holes (Hummert Intl.). The size of multi-pot inserts sometimes differed depending on the experimental design and number of test entries. The inserts were filled with the soil-less potting medium and then moistened to field capacity. Two seeds of each entry were placed in a shallow depression (approximately 0.5 cm deep) in the potting medium. Seedlings were thinned to one plant per pot after emergence. For no-choice tests, three seeds of each soybean genotype were planted into 13-cm-diameter plastic pots, filled with the soil-less medium and seedlings were thinned to one plant per pot after emergence. Fifteen ml of slow-release fertilizer pellets (Osmocote 19-6-12, NPK) were spread evenly on top of the soil-less growth medium to an approximate density of 2 to 3 pellets per cm2 following planting. Plants were manually irrigated as required to avoid drought stress.
Choice tests of resistance sources
The resistance sources were evaluated in choice tests conducted with each of the four soybean aphid biotypes over time to compare the overall soybean aphid resistance, which includes antixenosis and antibiosis-type resistance. The five PIs were tested along with eight soybean genotypes previously reported to have aphid resistance and the susceptible genotype Williams 82 (Table 1). The 14 genotypes were arranged in a randomized complete block design with four blocks. The soybean breeding lines introgressed with Rag1 (LD05-16611) and Rag2 (LD08-12422a) used in the biotype 1, 2, and 3 tests were different from the ones used in the biotype 4 test (LD11-4576a Rag1 and LD11-5431a Rag2) because seed was not available for the older (year after LD in the name) introgressed breeding lines. The experimental unit was one plant of each soybean genotype. There were two trials (repeat) of each biotype choice test and each trial had a different randomization of genotypes. Each test was infested with a single aphid biotype using methods previously described (Hill et al. 2010, 2009; Kim et al. 2008b; Pawlowski et al. 2014). Briefly, the tests were conducted inside an air-conditioned greenhouse set to maintain a constant 22 °C with a mixture of 1000 w high-pressure sodium vapor and metal halide lamps providing supplemental light 16 h day:8 h night. Soybean seedlings were infested with aphids during the VC to V1 growth stages (Fehr et al. 1971) by evenly distributing aphid-infested Williams 82 leaves and stems, containing aphids of all viviparous life stages, on top of the seedlings. Aphid colonization on each plant was evaluated when there was maximum aphid colonization on the susceptible check genotypes about 3 weeks after inoculation. A visual assessment of aphid colonization using a non-parametric, ordinal 1–4 scale of the degree of colonization and plant damage caused by aphid feeding was used (Fox et al. 2014; Hill et al. 2009, 2006a, b; Pawlowski et al. 2014). A rating of 1 = few solitary live aphids, often with dead aphids; 2 = several transient aphids present along with some viviparous aptera surrounded by a few nymphs, but without established colonies; 3 = dense aphid colonies; and 4 = dense colonies accompanied by plant damage such as leaf distortion and stunting. This scale describes four qualitative classes of phenotypes observed on the plants in response to infestation by soybean aphids, and has been consistently found to be appropriate for use in phenotyping qualitatively expressed soybean aphid resistance in our past research. The numbers 1–4 were used to rank the level of aphid colonization and not to quantify the number of aphids or be used for parametric analyses.
No-choice tests of resistance sources
No-choice tests were conducted over time to compare antibiosis-type resistance performance between multiple soybean genotypes. A randomized complete block design with three blocks was used with the five aphid-resistant PIs, eight genotypes reported to be aphid-resistant, and susceptible Williams 82 randomized within each block. The soybean breeding lines with Rag1 (LD05-16611) and Rag2 (LD08-12422a) used in the biotype 1, 2, and 3 tests were different from the ones used in the biotype 4 test (LD11-4576a Rag1 and LD11-5431a Rag2) due to insufficient amount of viable seed of the older breeding lines. The experimental unit was a pot with a single soybean genotype inoculated with a single soybean aphid biotype. The plants were inoculated at the Vc to V1 growth stage with aphids of a single biotype using 10 second or third instar nymphs as previously described (Chirumamilla et al. 2014; Hill et al. 2010; Pawlowski et al. 2014). Immediately following inoculation, each plant was isolated by covering it with a custom-made plastic (Cleartec® Packaging, Park Hills, MO) cylinder, 40 cm tall × 10.5 cm in diameter, closed on the top with a clear plastic cover and with the open bottom end pushed into the soil-less medium. Two rectangular windows (25 cm long × 6 cm wide) were cut out of each cylinder and covered with silk to enable ventilation and prevent aphid escape. The total number of aphids on each plant was counted 14 days after inoculation. The tests were conducted in a plant growth chamber (Conviron PGR15, Manitoba, Canada) illuminated with 500 µmol m2s−1 PAR fluorescent and incandescent lamps programmed for a 16- and 8-h light–dark cycle and a constant 22 °C. Each no-choice test for each biotype was repeated once and the two trials were conducted at different times.
Choice test evaluation of resistance in F2 populations and progeny testing
F2 populations derived from PI 587663 and PI 587972 were tested for resistance to soybean aphid biotype 1, and populations derived from PI 587677, PI 587685, and PI 594592 were tested against biotype 3. The parental breeding lines, 99–279 F2 plants from each population derived from the five aphid-resistant PI accessions (Table 2), and aphid-resistant and susceptible checks, were tested in the greenhouse using choice testing methods previously reported (Fox et al. 2014; Hill et al. 2006a, b, 2009). Each population was tested separately over time in a greenhouse with supplemental lighting set to provide a 13-h photoperiod and induce early blooming for subsequent F3 seed production. The greenhouse was air-conditioned and the temperature was set to 24 °C day and 18 °C night. The experimental design used to infest and phenotype the aphid resistance in the F2 populations was previously reported (Fox et al. 2014; Hill et al. 2006a, b, 2009). Four-pot rows of each F2 population, parental breeding lines, and resistant and susceptible checks were randomly planted in plastic multi-pot inserts containing 48 pots (Hummert Intl., Earth City, MO, USA). Each test included 25% aphid-susceptible genotypes to create aphid refuges and maintain high aphid pressure after the plants were infested. Infestation and evaluation of aphid colonization were conducted as described above for resistance characterization. The experimental unit was an individual plant of each soybean genotype or F2 plant.
F2 progeny testing was conducted using choice tests to determine the soybean aphid resistance genotypes of each F2 plant. After completion of F2 tests, plants were grown to maturity to produce F2:3 seed for progeny testing. F2:3 lines that had at least 12 seeds, regardless of F2 aphid resistance phenotype, were used in progeny tests. The tests of F2:3 progenies were conducted separately for each population at different times. Twelve to 16 F3 seeds were planted in multi-pot inserts in flats with drainage holes as described above. The progeny tests were set up as choice tests as previously described (Hill et al. 2006a, b, 2009). Biotype 3 was initially used in all F2 progeny tests. F2:3 plants, parents, and resistant and susceptible checks were planted and randomized in four-pot rows as described above, with the experimental unit each individual plant. Following the biotype 3 test, four seeds from each F2:3 family (of those families that still had four seeds remaining) were screened for resistance against soybean aphid biotype 2 using the same experimental design. Infestation and evaluation of aphid colonization using the 1–4 non-parametric ordinal scale, were as previously described.
Analysis of F2 plant and F2:3 family segregation
A rating of 1 or 2 was considered as a resistant response and a rating of 3 or 4 was considered susceptible (Fox et al. 2014; Hill et al. 2006a, b, 2009). For analysis of segregation in F2 populations, phenotypic ratios of resistant:susceptible plants were used to test Mendelian genetic models to determine the number of major genes controlling resistance in each PI resistance source. For analysis of the segregation of F2:3 families for resistance against biotypes 2 and 3, only families with at least 11 plants were used. Prior to Chi-square analyses, families were classified as all resistant if no susceptible plants were found, segregating if there were both resistant and susceptible plants, and all susceptible if no resistant plants were observed.
Genotyping and marker associations
Methods used to genotype F2 plants in the populations were previously described (Fox et al. 2014). Briefly, genomic DNA was extracted from young trifoliolate leaves of all F2 plants and the parents in each population using the CTAB extraction procedure (Keim and Shoemaker 1988). Single nucleotide polymorphisms (SNPs) and simple sequence repeat (SSR) genetic markers that map near the Rag1, Rag2, Rag3, and rag4 regions were used to identify markers that were polymorphic between the susceptible and resistant parents of the populations. One or two polymorphic markers mapping within each Rag region were selected (Fox et al. 2014) to genotype each F2 plant in each of the five populations. Polymerase chain reactions (PCR) for the SSR markers were performed following a previously reported protocol (Cregan and Quigley 1997) and the PCR products were analyzed using non-denatured polyacrylamide gel electrophoresis, using a previously reported protocol (Wang et al. 2003). SNP marker analyses were performed using TaqMan assays conducted with a Roche LightCycler 480 System (Roche Diagnostics, Indianapolis, IN) (Kaczorowski et al. 2008). Additionally, bulked segregant analysis (BSA) (Michelmore et al. 1991) was performed on DNA samples collected from the PI 587663, PI 587685, and PI 587972 parents and their F2-derived populations using the Illumina GoldenGate 1,536 Universal Soy Linkage Panel 1.0 (Hyten et al. 2010, 2008) after the initial marker screening. For each population, DNA from 10 F2 plants with resistant phenotypes and 10 plants with susceptible phenotypes were selected and bulked into two separate samples (Fox et al. 2014). The SNP genotypes of the resistant and susceptible bulks were compared with the SNP genotypes of the population parents to determine probable genetic regions associated with soybean aphid resistance. Marker genotypes present in F2:3 families were inferred from the parental F2 plant genotypes.
Not all F2 plants that were sampled for genotyping produced seed for subsequent testing. Also, the numbers of F2:3 plants derived from F2 plants that were genotyped were sometimes limited by the amount of seed F2 plants produced, which resulted in lower numbers of F2:3 families available for testing against different soybean aphid biotypes. Therefore, F2 plant seed productivity determined the population sizes that were evaluated for associations between marker genotypes and aphid colonization phenotypes.
Statistical analyses
Statistical analyses were performed with the aid of JMP 12 (SAS Institute, Cary, NC) software. Modes of aphid colonization ratings recorded from the resistance characterization choice tests were calculated using the JMP distribution procedure. Because there was an exponential range among no-choice aphid population numbers, the count data were transformed by adding 1 to the count and then taking the log10 of the total to correct for the heterogeneity of variance of aphid populations among the soybean genotypes. Using the JMP fit Y × X procedure and the unequal variance tests, with Y = transformed counts and X = trials, homogeneity of variance between trials of each no-choice test was analyzed to determine if the data from both trials could be combined for analysis of variance. The JMP Fit Model procedure was used to perform analysis of variance on the transformed no-choice count data using the model Y (log10 aphid count) = block + biotype + genotype + biotype × genotype + error. Soybean aphid biotype, soybean genotype, and block were fixed effects. Means were separated by least significant difference (LSD) at a significance level of α = 0.05, upon confirmation of significant differences among the sources of variation in the analysis of variance, and then were de-transformed by taking the antilog and subtracting 1 before presentation. χ 2 analyses were calculated using Microsoft Excel (v. 14) to analyze the segregation of resistant and susceptible plants in the F2 populations, and among the all resistant, segregating, and all susceptible F2:3 family classifications infested with biotype 3, based upon classes determined by the non-parametric, ordinal scale, as described above. Yates correction for continuity (0.5) was used in the calculations for the F2 analyses (Little and Hills 1978).
For statistical analyses of associations between F2 and F2:3 aphid colonization phenotypes and parent marker genotypes, F2 genotypes were inferred and assigned the value 0, 1, or 2, based on homozygosity for the marker alleles from the susceptible parent, or heterozygous or homozygous for the resistant parent marker alleles, respectively. F2:3 families were classified based on segregation of resistant plants, using the assigned value 0 for families with all susceptible plants (F2 homozygous susceptible), 1 for families segregating for resistance and susceptibility (F2 heterozygous), and 2 for families with all resistant plants (F2 homozygous resistant), and their F2 resistant PI parental genotypes were inferred based on the segregation classifications.
Because the 1–4 aphid colonization rating scale is a non-parametric ordinal scale, no parametric tests such as ANOVA were used to test for marker associations. Therefore, associations between F2 marker genotypes and F2 aphid colonization ratings (1, 2, 3, 4) against biotypes 1 or 3, and between F2:3 family resistance segregation classifications (0, 1, 2) against biotypes 2 or 3 were analyzed using the JMP multivariate procedure and Spearman’s correlation test. Spearman’s ρ is a correlation coefficient computed on the ranks of the data values instead of on the values themselves (Lehman et al. 2013). In addition, the non-parametric Wilcoxon/Kruskal–Wallis rank sums test was performed using the JMP Fit Y by X Non-Parametric procedure to test whether medians of the rank sums were located similarly across the three genotype groups (0, 1, 2). Paired comparisons between all three genotype groupings (0 and 1, 0 and 2, or 1 and 2) were then performed using the JMP Wilcoxon each pair test procedure. The Wilcoxon test for paired samples, the non-parametric equivalent of the paired samples t test, was used when the sample data were not normally distributed and log transformation did not normalize the data. The null hypothesis was that the median difference between pairs of observations is zero, or in other words, the group medians were in the same location in both groups. The level of significance for all statistical tests was P < 0.05.
Results
Choice tests of resistance sources
Table 2 shows the modes of the 1–4 non-parametric aphid colonization ratings for all soybean genotypes tested against the four soybean aphid biotypes. Four of the accessions under investigation in this study, PI 587663, PI 587677, PI 587685 and PI 587972, had rating modes of 2 for biotypes 1, 2, and 3, but each of them had a rating mode of 3 against biotype 4, showing that they expressed resistance to biotypes 1, 2, and 3, but not to biotype 4. The fifth accession, PI 594592, had relatively low colonization (rating mode = 2) by biotypes 1 and 2 and higher colonization by biotypes 3 and 4 (rating mode = 3). The soybean breeding line LD05-16611, containing the introgressed Rag1 gene, was tested against biotypes 1, 2, and 3, and had low aphid colonization levels (rating mode = 2) against biotype 1, indicating that it was resistant to biotype 1, however, it was susceptible to biotypes 2 and 3 (rating mode = 3). LD11-4576a, with the introgressed Rag1 gene, was tested against biotype 4 and had a rating mode = 3, indicating it was susceptible to that biotype. For soybean genotypes containing the introgressed Rag2 gene, LD08-12422a had lower colonization ratings (rating mode = 2) against biotypes 1 and 2 than against biotype 3 (rating mode = 3), and LD11-5431a, tested against biotype 4, had a relatively high level of biotype 4 colonization (rating mode = 3). Resistant checks PI 71506 (antixenosis resistance), PI 567541B, with rag1c and rag4, and PI 567543C, with Rag3, had low colonization by biotypes 1, 2, and 3 (rating mode = 2), but not biotype 4 (rating mode = 3). The mode for resistant check line PI 437696 was 2 across the four biotypes, indicating that accession had low levels of aphid colonization and expressed resistance against all of the biotypes. PI 567597C (antixenosis resistance) only had low levels of colonization against biotype 1 (rating mode = 2). Williams 82 had high levels of colonization by all four biotypes.
No-choice tests of resistance sources
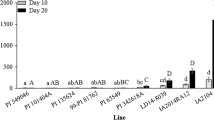
Results of four tests for homogeneity of variance between the two trials of each no-choice biotype test, including O’Brien’s, Brown–Forsythe, Levene’s, and Bartlett’s tests, were non-significant (P > 0.05), indicating homogeneous variances. Therefore, the data from both trials, where the second trial was a replication of the first with different genotype randomizations, were combined for analysis of variance. A highly significant (P < 0.0001) soybean aphid biotype x soybean genotype interaction (Fig. 1) was found for aphid population counts, indicating that aphid colonization was dependent on both biotype and soybean genotype. There were also highly significant differences found among the biotypes and among the genotypes. Differences among blocks with trials were non-significant (P > 0.05). Overall, resistant check genotypes PI 437696, PI 567543 C (Rag3), and PI 567597 C (antixenosis) had significantly lower populations of all four biotypes compared with Williams 82 (Fig. 1). Of the five PI accessions under investigation in this study, PI 587663, PI 587677, and PI 587685 also had significantly lower populations of all four biotypes compared with Williams 82. PI 587972 and PI 594592 had significantly lower populations of biotypes 1, 2, and 3, but not biotype 4, compared to Williams 82. The Rag1 introgressed breeding lines had significantly lower biotype 1 and biotype 3 populations, but non-significantly different populations of biotypes 2 and 4 compared to Williams 82. The Rag2 introgressed breeding lines had significantly lower biotype 1 and 2 populations, but non-significantly different biotype 3 and 4 populations than Williams 82. Resistant check genotypes PI 567541B (rag1c, rag4), PI 567598B (rag1b, rag3), and PI 71506 had significantly lower populations of biotypes 1, 2, and 3, but not biotype 4 compared to Williams 82. The biotype 4 isolate consistently had the highest levels of colonization on all of the soybean genotypes in this panel, compared to the other three soybean aphid isolates, with an overall mean of 461 aphids per plant, which was much higher than biotype 3 (117 aphids per plant), and an order of magnitude greater than colonization for biotype 1 (20 aphids per plant) and biotype 2 (14 aphids per plant).
Interactions between 14 soybean genotypes and four soybean aphid biotypes for aphid populations per plant 14 days after inoculation with 10 viviparous second or third instar aphids in a no-choice test. Letters above the bars indicate groupings of means not significantly different by least significant difference (LSD) at a significance level of α = 0.05. The Rag1 and Rag2 lines were Rag-region introgressed lines
Evaluation of resistance in F2 populations and progeny tests
Table 3 shows the number of F2 plants that were used in F2 resistance phenotyping, F2 marker genotyping, F2:3 progeny testing against biotype 3, and F2:3 progeny testing against biotype 2. The numbers of F2:3 families used were restricted by low seed production of some genotyped F2 plants.
No deviation from of the expected 3 resistant to 1 susceptible F2 plant segregation ratio was found in the χ 2 analyses for resistance to soybean aphid biotype 1 in PI 587663 and PI 587972, and resistance to biotype 3 in PI 587677 and PI 587685 (Table 4). This indicated that resistance to the biotypes tested was controlled by single dominant genes. The χ 2 test of a 3R:1S segregation for the PI 594592 F2 population was rejected, but a test of a 9R:7S ratio was not, which provided evidence two dominant genes with complementary epistatic interaction control resistance to biotype 3 exist in that accession. The χ 2 analyses of the segregation of F2:3 family classes failed to reject the expected 1 all resistant: 2 segregating:1 all susceptible ratio of family classifications, which supported evidence of single, dominant genes for resistance against soybean aphid biotypes 1, 2, or 3 for the five PIs tested.
Associations between F2 aphid colonization and F2:3 family resistance segregation classifications
Correlations of the aphid colonization ratings of biotypes 1 or 3 on F2 plants with the F2:3 family resistance segregation classifications when challenged against biotypes 2 or 3 are listed in Table 5. For the PI 587663-derived population, biotype 1 colonization ratings on F2 plants were negatively correlated with F2:3 family resistance segregation against biotype 2. This showed that resistance expressed against biotype 1 in parental F2 plants was also expressed against biotype 2 in their progeny plants because a higher number of F2:3 families segregating biotype 2-resistant plants tended to be associated with lower F2 biotype 1 colonization ratings. PI 587663 F2:3 family resistance segregation between biotypes 2 and 3 was also correlated but positive, indicating that parental F2 plants that were resistant to biotype 2 produced progeny that were susceptible to biotype 3. Similarly, the PI 587972 population biotype 1 colonization ratings on F2 plants were negatively correlated with F2:3 family resistance segregation against biotype 2, but also against biotype 3, while F2:3 family resistance segregation between biotypes 2 and 3 were positively correlated. F2:3 family segregation between biotypes 2 and 3 was also positively correlated for the PI 587677 population. There were significant negative correlations between aphid colonization ratings on F2 plants derived from PI 594592 against biotype 3 and F2:3 family resistance segregation classifications against biotype 2 and against biotype 3. For the population derived from PI 587685, the correlations between biotype 3 F2 plant aphid colonization ratings and biotype 3 F2:3 family resistance classifications, and between biotype 2 and biotype 3 F2:3 family resistance classifications, could not be calculated by JMP due to insufficient sample size of the biotype 3 F2:3 family resistance classifications, resulting from limited quantity of F2:3 seed from PI 587685 F2 plants available for the biotype 3 progeny testing (only F2 plants that produced a minimum of 12 seeds were used).
Genotyping and marker associations
Results of the BSA analysis with the Illumina GoldenGate assays of genomic DNA from PI 587663, PI 587685, PI 587972, and bulks of DNA from resistant and susceptible F2 plants indicated that all three PIs have a resistance gene mapping to the Rag2 region. Bulked segregant analysis was not done for the populations derived from PI 587677 and PI 594592.
Table 6 summarizes the non-parametric analyses of the effects of genetic regions associated with aphid-resistant PI marker alleles on aphid colonization on F2 plants and on F2:3 family resistance segregation classifications against biotypes 1, 2, or 3. There were significant correlations found for regions associated with aphid-resistant PI parent alleles for Rag1, Rag2, Rag3, and rag4.
PI 587663
There were significant positive correlations between Rag1 region markers Satt245 and Satt567 with F2:3 family resistance segregation when infested with biotype 3 (Table 6), indicating an association between the aphid-resistant PI Rag1 marker alleles with higher segregation of resistant plants against biotype 3. The effect of the region associated with the PI 587663 Satt245 allele on F2:3 family resistance segregation was partially dominant over the susceptible parent allele because the non-parametric comparisons 1 (heterozygous genotype) versus 0 (homozygous for susceptible parent alleles), and 2 (homozygous for resistant parent alleles) versus 1 were non-significant, while 2 versus 0 was significant. The allele effect was dominant for the region associated with the PI 587663 Satt567 allele because the 1 versus 0 and the 2 versus 0 comparisons were significant, while the 2 versus 1 was non-significant.
Significant negative correlations between PI 587663 Satt114, Satt335, and SNP2 marker alleles in the Rag2 region and F2 colonization rating against biotype 1 were found (Table 6), indicating that lower biotype 1 colonization was associated with PI 587663 alleles at those marker loci. Biotype 1 resistance in F2 plants associated with PI 587663 alleles at Satt114, Satt335, and SNP2 was partially dominant. Correlations between the PI 587663 alleles at Satt114, Satt335 and SNP2, with F2:3 family classification against biotypes 2 and 3, were positive and significant, indicating that PI 587663 alleles at those marker loci were associated with higher segregation of resistant plants when challenged against biotypes 2 and 3. Biotype 2 resistance associated with PI 587663 alleles at Satt114, Satt335, and SNP2 was additive against biotype 2, whereas for the biotype 3 resistance associated with the three markers, there was no effect, a recessive effect, or a partial dominance effect, respectively.
There was a significant correlation between the PI 587663 Satt285 marker allele in the Rag3 region and F2:3 family classification against biotype 3, indicating an association between the PI 587663 allele and biotype 3 resistance in F2:3 families. The effect of the allele was partially dominant.
PI 587677
A significant negative correlation between the PI 587677 Rag1 Satt540 allele and F2 plant biotype 3 colonization rating was found (Table 6). Biotype 3 resistance associated with that allele was dominant. There was a significant positive correlation between the PI 587677 Satt540 allele and biotype 3 resistance segregation in F2:3 families; however, the result of the analysis on the effect of the resistance associated with that allele was unclear.
Significant positive correlations were found between F2:3 family biotype 2 and biotype 3 resistance segregation classification and PI 587677 alleles at Satt335 in the Rag2 region and the Satt586 marker in the rag4 region. The effects of the biotype 2 and 3 resistance associated with the PI 587677 alleles varied from dominant to recessive, depending on the marker locus.
PI 587685
There were significant negative correlations found between F2 biotype 3 colonization ratings and two Rag1 region markers (Table 6) and the effects of the biotype 3 resistance associated with the PI 587685 alleles were dominant.
Additionally, there were significant positive correlations between F2:3 family biotype 2 resistance segregation classification and the PI 587685 alleles at markers Satt114, Satt335, and SNP1485 in the Rag2 region. The effects of the biotype 2 resistance associated with the PI 587685 Rag2 region Satt114 and Satt335 marker alleles were additive.
PI 587972
Significant negative correlations were found between F2 biotype 1 colonization ratings and PI 587972 alleles at three markers in the Rag2 region (Table 6). There were also significant positive correlations between the PI 587972 alleles at the same Rag2 region markers and F2:3 family biotype 2 and 3 resistance segregation classifications. The effects of the biotype 1, 2, and 3 resistance associated with the PI 587972 alleles at Satt114 in the Rag2 region were completely dominant, except for F2:3 biotype 3 resistance segregation classification associated with the Satt114 allele, which was additive. The effects of resistance against all three biotypes associated with the PI 587972 Satt510 allele in the Rag2 region were partially dominant.
PI 594592
F2 biotype 3 colonization ratings were significantly negatively correlated with the PI 594592 allele associated with marker Satt540 in the Rag1 region, with a dominant resistance effect, and with the allele at Satt654 in the Rag3 region, with a partially dominant resistance effect (Table 6). There was also a significant correlation between biotype 3 F2:3 resistance segregation classification and the PI 594592 Satt540 allele, with an additive effect of the resistance associated with the marker allele. A significant positive correlation between F2:3 family biotype 2 resistance segregation classification and the PI 594592 allele at Satt114 in the Rag2 region, with additive resistance expression, was found. The PI 594592 allele at Satt654 in the Rag3 region was negatively correlated with F2:3 family segregation for resistance to biotype 2.
Discussion
Resistance against soybean aphid biotypes 1, 2, 3 and 4 in three germplasm sources, PI 587663, PI 587677, and PI 587685, were reported for the first time. In addition, PI 437696, previously identified to have two aphid-resistance genes mapping in the Rag1 and Rag2 regions (Fox et al. 2014), was also found to be resistant to all four biotypes. A recent report (Bansal et al. 2013) that named two PI accessions, PI 606390A and PI 340034, as resistant to biotypes 1, 2, and 3, did not test for resistance to biotype 4.
Mendelian, correlation, and resistance allele effect analyses showed that PI 587663 had a single, dominant gene mapping in the Rag2 region, with a partially dominant effect that controlled resistance to biotype 1. That gene also controlled resistance to biotypes 2 and 3, with variable allele effects. The results of Golden Gate assay of bulked DNA samples of resistant or susceptible F2 plants confirmed there was a resistance gene in the Rag2 region. Correlation and resistance allele effects analyses also showed that there were resistance genes mapping in the Rag1 and in the Rag3 regions that contributed to resistance against biotype 3, with partially or completely dominant effects. Therefore, PI 587663 had three non-allelic, independently inherited soybean aphid resistance genes. Evidence showing an association between Rag1 region marker alleles and biotype 3 resistance in F2:3 plants, but no association between the marker alleles and biotype 1 in F2 plants, suggested that the gene in the Rag1 region controlled resistance to biotype 3 but not biotype 1. That result could mean that there was a different allele at that locus than the allele found in Dowling, the original source of Rag1 (Hill et al., 2006; Li et al. 2007) or that biotype 1 was virulent against the PI 587663 gene, while biotype 3 was not. The gene mapping in the Rag2 region gave resistance to all three biotypes, including biotype 3, suggesting it could be a new allele at the Rag2 locus providing resistance to biotype 3, whereas the original Rag2 allele found in PI 200538 (Hill et al. 2009) did not. The gene mapping in the Rag3 region that provided resistance to biotype 3 may be the same gene as reported in PI 567543C (Zhang et al. 2010) and may also provide resistance against biotype 4 because this PI was also found to be resistant to all four biotypes in this study.
As above, Mendelian, correlation and resistance allele effects analyses showed that a dominant gene in PI 587677, mapping in the Rag1 region, controlled resistance to biotype 3 and that genes mapping in the Rag2 and rag4 regions with variable allele effects controlled resistance to both biotypes 2 and 3. Either of these genes may be responsible for resistance to biotype 1. It is possible that the genes mapping in the Rag1, Rag2, and rag4 regions in PI587677 collectively controlled resistance to all four biotypes.
A gene in PI 587685 located in the Rag1 region, with dominant effects, controlled resistance to biotype 3. Also, a gene located in the Rag2 region, which was identified by the GoldenGate assay, controlled resistance to biotype 2 with additive effects. It is probable that either of these two genes controlled resistance to biotype 1 and it is also likely that there are either new alleles at the Rag1 or Rag2 loci, or closely linked genes, that control resistance to biotype 4. Testing the F2 population against biotype 1 could reveal the presence of duplicate dominant genes.
Resistance in PI 587972 to biotypes 1, 2 and 3 was clearly controlled by a gene located in the Rag2 region (also identified by the GoldenGate assay) that had dominant, partially dominant, or additive effects on resistance, depending on the marker and biotype tested. Because the gene controlled resistance to biotype 3, it is unlikely the same Rag2 allele found in PI 200538 (Hill et al. 2009) or PI 243540 (Mian et al. 2008), which were susceptible to biotype 3 (Hill et al. 2010; Pawlowski et al. 2014) and is more likely a new Rag2 allele or closely linked gene in the Rag2 region.
Genetic and marker association analyses showed that resistance to biotype 3 in PI 594592 was controlled by a gene in the Rag1 region, with dominant or additive effects. Additionally, a gene with partially dominant effects mapping in the Rag3 region contributed to resistance against biotype 3 and also was associated with resistance to biotype 2. Based on a higher coefficient of correlation, most of the resistance to biotype 2 appeared to be explained by a gene with additive effects mapping in the Rag2 region. The complementary, epistatic interaction (9R:7S ratio) for resistance to biotype 3 found in the Mendelian analysis of the resistance segregation in the F2 population indicated that two dominant alleles were interacting with each other resulting in complementary gene action. A dominant allele at the gene in the Rag2 region inherited from PI 594592 likely controlled resistance to biotype 2. Any of these resistance genes probably could control resistance to biotype 1 if they were challenged against it. Resistance phenotyping of the F2 population with biotype 1 could reveal more than one major gene segregating if biotype 1 was avirulent against the genes.
While seed production by F2 plants determined populations sizes used for evaluating marker genotype–aphid colonization phenotype associations, there was no obvious association between seed production and aphid colonization. The resistance genetics conclusions stated above are bolstered by the use of more than one soybean aphid biotype in the study. This demonstrates the utility of using multiple soybean aphid biotypes with variable virulence to determine soybean aphid resistance genetics in soybean cultivars previously identified (Kim et al. 2014). This is especially important if a completely avirulent biotype is not available for use.
Genetic allelism tests and fine mapping work will be required to discern between potential allelism at the Rag loci or if there are new, closely linked loci mapping within the Rag regions in these five aphid resistance sources. In any event, markers flanking these loci will be useful in marker-assisted selection procedures to transfer the resistance genes in the resistant PI accessions into elite, high-yielding soybean germplasm.
Results in this study showed that PIs 587663, 587677, 587685, and 594592 are natural multi-resistance gene pyramids. Other PIs with natural soybean aphid resistance gene pyramids have been reported (Bales et al. 2013; Chen et al. 2006; Fox et al. 2014; Jun et al. 2012; Kim et al. 2014). Recently, it was shown that a resistance gene pyramid, developed by our research group using marker-assisted selection to transfer and combine Rag1 and Rag2 from different sources, provided stronger field resistance and yield protection against soybean aphids than soybean lines with either resistance gene alone (McCarville et al. 2014; Wiarda et al. 2012). That result suggested the Rag1, Rag2 pyramid was resistant to multiple aphid biotypes.
In conclusion, the sources of resistance and the added information on the acting resistance genes reported in this study (Table 7) will be useful to soybean breeders developing new soybean aphid-resistant soybean cultivars. The strategy to develop and use resistance gene pyramids to manage soybean aphids in soybean has been discussed (McCarville et al. 2014), and results of the multi-biotype resistance expressed in natural resistance gene pyramids found in this study would complement this effort. Although it is challenging for soybean breeders to develop multiple resistance gene pyramids in elite, high-yielding genetic backgrounds, there is much potential therein to increase resistance gene durability similar to resistance gene pyramids used to combat plant diseases (Crute and Pink 1996; Djian-Caporalino et al. 2014; Mundt 1991). There is also the possibility that pyramids would be more attractive to soybean producers (McCarville et al. 2014), which validates current and future efforts to combine soybean aphid resistance genes into pyramids.
Author contribution statement
CBH and GLH conceived the project; CBH, DS, and CF conducted the research; CBH completed the data analyses and drafted the manuscript; and all authors contributed to the final submission.
Abbreviations
- PCR:
-
Polymerase chain reaction
- SNP:
-
Single nucleotide polymorphisms
- SSR:
-
Simple sequence repeat
References
Ajayi-Oyetunde OO, Diers BW, Lagos-Kutz DM, Hill CB, Hartman GL, Reuter-Carlson U, Bradley CA (2016) Differential reactions of soybean isolines with combinations of aphid resistance genes Rag1, Rag2, and Rag3 to four soybean aphid biotypes. J Econ Entomol 109:1431–1437
Alt J, Ryan-Mahmutagic M (2013) Soybean aphid biotype 4 identified. Crop Sci 53:1491–1495
Bales C, Zhang G, Liu M, Mensah C, Gu C, Song Q, Hyten D, Cregan P, Wang D (2013) Mapping soybean aphid resistance genes in PI 567598B. Theor Appl Genet 126:2081–2091
Bansal R, Mian M, Michel AP (2013) Identification of novel sources of host plant resistance to known soybean aphid biotypes. J Econ Entomol 106:1479–1485
Beckendorf EA, Catangui MA, Riedell WE (2008) Soybean aphid feeding injury and soybean yield, yield components, and seed composition. Agron J 100:237–246
Bhusal SJ, Jiang G-L, Tilmon KJ, Hesler LS (2013) Identification of soybean aphid resistance in early maturing genotypes of soybean. Crop Sci 53:491–499
Bhusal SJ, Jiang G-L, Hesler LS, Orf JH (2014) Soybean aphid resistance in soybean germplasm accessions of maturity group I. Crop Sci 54:2093–2098
Chen Y, Mensah C, DiFonzo C, Wang D (2006) Identification of QTLs underlying soybean aphid resistance in PI 567541B. The ASA-CSSA-SSSA International Annual Meetings
Chirumamilla A, Hill CB, Hartman GL (2014) Stability of soybean aphid resistance in soybean across different temperatures. Crop Sci 54:2557–2563
Cregan P, Quigley C (1997) Simple sequence repeat DNA marker analysis. In: Caetano-Anolles G, Gresshoff P (eds) DNA markers: protocols, applications and overview. Wiley, New York, pp 173–185
Crossley MS, Hogg DB (2015) Rag virulence among soybean aphids (Hemiptera: Aphididae) in Wisconsin. J Econ Entomol 108:326–338
Crute IR, Pink D (1996) Genetics and utilization of pathogen resistance in plants. The Plant Cell 8:1747
Djian-Caporalino C, Palloix A, Fazari A, Marteu N, Barbary A, Abad P, Sage-Palloix A-M, Mateille T, Risso S, Lanza R (2014) Pyramiding, alternating or mixing: comparative performances of deployment strategies of nematode resistance genes to promote plant resistance efficiency and durability. BMC Plant Biol 14:53. http://www.biomedcentral.com/1471-2229/14/53
Fehr WR, Caviness CE, Burmood DT, Pennington JS (1971) Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci 11:929–931
Fernandez-Cornejo J, Nehring R, Osteen C, Wechsler S, Martin A, Vialou A (2014) Pesticide Use in US Agriculture: 21 Selected Crops, 1960–2008. United States Department of Agriculture, Economic Research Service
Fox CM, Kim K-S, Cregan PB, Hill CB, Hartman GL, Diers BW (2014) Inheritance of soybean aphid resistance in 21 soybean plant introductions. Theor Appl Genet 127:43–50
Hartman GL, Domier LL, Wax LM, Helm CG, Onstad DW, Shaw JT, Solter LF, Voegtlin DJ, D’Arcy CJ, Gray ME, Steffey KL, Isard SA, Orwick PL (2001) Occurrence and distribution of Aphis glycines on soybeans in Illinois in 2000 and its potential control. Online Plant Health Progress doi:101094/PHP-2001-0205-01-HN. Plant Health Progress
Hesler LS, Prischmann DA, Dashiell KE (2012) Field and laboratory evaluations of soybean lines against soybean aphid (Hemiptera: Aphididae). J Econ Entomol 105:608–615
Hesler LS, Chiozza MV, O’Neal ME, MacIntosh GC, Tilmon KJ, Chandrasena DI, Tinsley NA, Cianzio SR, Costamagna AC, Cullen EM (2013) Performance and prospects of Rag genes for management of soybean aphid. Entomol Exp Appl 147:201–216
Hill JH, Alleman R, Hogg DB, Grau CR (2001) First report of transmission of Soybean mosaic virus and Alfalfa mosaic virus by Aphis glycines in the New World. Plant Dis 85:561
Hill CB, Li Y, Hartman GL (2004) Resistance to the soybean aphid in soybean germplasm. Crop Sci 44:98–106
Hill CB, Li Y, Hartman GL (2006a) A single dominant gene for resistance to the soybean aphid in the soybean cultivar Dowling. Crop Sci 46:1601–1605
Hill CB, Li Y, Hartman GL (2006b) Soybean aphid resistance in soybean Jackson is controlled by a single dominant gene. Crop Sci 46:1606–1608
Hill CB, Kim K-S, Crull L, Diers BW, Hartman GL (2009) Inheritance of resistance to the soybean aphid in soybean PI 200538. Crop Sci 49:1193–1200
Hill CB, Crull L, Herman TK, Voegtlin DJ, Hartman GL (2010) A new soybean aphid (Hemiptera: Aphididae) biotype identified. J Econ Entomol 103:509–515
Hill C, Chirumamilla A, Hartman G (2012) Resistance and virulence in the soybean-Aphis glycines interaction. Euphytica 186:635–646
Hyten DL, Song Q, Choi I-Y, Yoon M-S, Specht JE, Matukumalli LK, Nelson RL, Shoemaker RC, Young ND, Cregan PB (2008) High-throughput genotyping with the GoldenGate assay in the complex genome of soybean. Theor Appl Genet 116:945–952
Hyten DL, Choi I-Y, Song Q, Specht JE, Carter TE, Shoemaker RC, Hwang E-Y, Matukumalli LK, Cregan PB (2010) A high density integrated genetic linkage map of soybean and the development of a 1536 universal soy linkage panel for quantitative trait locus mapping. Crop Sci 50:960–968
Jun T-H, Mian MR, Michel AP (2012) Genetic mapping revealed two loci for soybean aphid resistance in PI 567301B. Theor Appl Genet 124:13–22
Kaczorowski KA, Kim K-S, Diers BW, Hudson ME (2008) Microarray-based genetic mapping using soybean near-isogenic lines and generation of SNP markers in the Rag1 aphid-resistance interval. Plant Gen 1:89–98
Keim P, Shoemaker RC (1988) Construction of a random recombinant DNA library that is primarily single copy sequence. Soybean Genet Newsl 15:147–148
Kim C, Schaible G, Garrett L, Lubowski R, Lee D (2008a) Economic impacts of the US soybean aphid infestation: a multi-regional competitive dynamic analysis. Agr Resource Econ Rev 37:227–242
Kim K, Hill CB, Hartman GL, Diers BW (2008b) Discovery of soybean aphid biotypes. Crop Sci 48:923–928
Kim K-S, Chirumamilla A, Hill CB, Hartman GL, Diers BW (2014) Identification and molecular mapping of two soybean aphid resistance genes in soybean PI 587732. Theor Appl Genet 127:1251–1259
Lehman A, O’Rourke N, Hatcher L, Stepanski E (2013) JMP for basic univariate and multivariate statistics: methods for researchers and social scientists. SAS Institute
Li Y, Hill CB, Carlson SR, Diers BW, Hartman GL (2007) Soybean aphid resistance in the soybean cultivars Dowling and Jackson map to linkage group M. Mol Breed 19:25–34
Liang X, Biao W, Tianlong W (2013) Identification of Two Soybean Aphid Biotypes. Crops 4:033
Little TM, Hills FJ (1978) Agricultural Experimentation: Design and Analysis. John Wiley and Sons, New York
Liu G, Xing H, Diao Y, Yang X, Sun D, Wang Q, Qi N, Lin H (2014) Identification of resistance to soybean aphids in early germplasm. Crop Sci 54:2707–2712
Macedo TB, Bastos CS, Higley LG, Ostlie KR, Madhavan S (2003) Photosynthetic responses of soybean to soybean aphid (Homoptera: Aphididae) injury. J Econ Entomol 96:188–193
McCarville MT, O’Neal ME, Potter B, Tilmon KJ, Cullen EM, McCornack BP, Tooker JF, Prischmann-Voldseth D (2014) One gene versus two: a regional study on the efficacy of single gene versus pyramided resistance for soybean aphid management. J Econ Entomol 107:1680–1687
Mensah C, DiFonzo C, Nelson RL, Wang D (2005) Resistance to soybean aphid in early maturing soybean germplasm. Crop Sci 45:2228–2233
Mian MAR, Kang ST, Beil SE, Hammond RB (2008) Genetic linkage mapping of the soybean aphid resistance gene in PI 243540. Theor Appl Genet 117:955–962
Michelmore RW, Paran I, Kesseli R (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci 88:9828–9832
Mundt C (1991) Probability of mutation of multiple virulence and durability of resistance gene pyramids: Further comments. Phytopathology 81:240–242
NASS (2015) National Agricultural Statistics Service. United States
Pawlowski M, Hill CB, Voegtlin DJ, Hartman GL (2014) Soybean aphid intrabiotype variability based on colonization of specific soybean genotypes. Insect Sci. doi:10.1111/1744-7917.12169
Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N (2011) Ecology and management of the soybean aphid in North America. Annnu Rev Entomol 56:375–399
Riedell W, Catangui M (2006) Greenhouse studies of soybean aphid (Hemiptera: Aphididae) effects on plant growth, seed yield and composition. J Agric Urban Entomol 23:225–235
Tilmon K, Hodgson E, O’Neal M, Ragsdale D (2011) Biology of the soybean aphid, Aphis glycines (Hemiptera: Aphididae) in the United States. JIPM 2:A1–A7
Wang D, Shi J, Carlson SR, Cregan PB, Ward RW, Diers BW (2003) A low-cost, high-throughput polyacrylamide gel electrophoresis system for genotyping with microsatellite DNA markers. Crop Sci 43:1828–1832
Wiarda SL, Fehr WR, O’Neal ME (2012) Soybean aphid (Hemiptera: Aphididae) development on soybean with Rag1 alone, Rag2 alone, and both genes combined. J Econ Entomol 105:252–258
Zhang G, Gu C, Wang D (2009) Molecular mapping of soybean aphid resistance genes in PI 567541B. Theor Appl Genet 118:473–482
Zhang G, Gu C, Wang D (2010) A novel locus for soybean aphid resistance. Theor Appl Genet 120:1183–1191
Zhong YP, Xiao L, Wang B, Jiang YN, Yan JH, Cheng LJ, Wu TL (2014) Biotypic variation among soybean aphid isolates from four provinces in China. Crop Sci 54:2023–2029
Acknowledgements
We thank the United Soybean Board for partial funding for this research. We also thank Brian Diers for critically reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors state that the experiments comply with the current laws of the country in which they were performed (USA).
Additional information
Communicated by Henry T. Nguyen.
Rights and permissions
About this article
Cite this article
Hill, C.B., Shiao, D., Fox, C.M. et al. Characterization and genetics of multiple soybean aphid biotype resistance in five soybean plant introductions. Theor Appl Genet 130, 1335–1348 (2017). https://doi.org/10.1007/s00122-017-2891-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-017-2891-7