Abstract
The Chinese wheat landrace Huangfangzhu (HFZ) has a high level of resistance to Fusarium head blight (FHB). To identify chromosomal regions that are responsible for FHB resistance in HFZ, F8 recombinant inbred lines (RIL) were developed from a cross between HFZ and Wheaton, a U.S. hard spring wheat. FHB was evaluated by single floret inoculation in both greenhouse and field environments. Two quantitative trait loci (QTL) with major effects were identified. One QTL was located on the short arm of chromosome 3B, and explained 35.4% of the phenotypic variation; the other QTL was assigned to 7AL and explained 18.0% of the phenotypic variation for FHB response. In addition, three minor QTL were detected on chromosomes 1AS, 1B and 5AS by single marker regression. HFZ contributed all favorable alleles. The RIL with HFZ alleles at the QTL on 3BS and 7AL displayed significantly lower percentages of infected spikelets than RIL without these alleles in both greenhouse and field environments. HFZ combined several alleles from germplasm reported previously and is a promising alternative source for improving wheat FHB resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight (FHB) caused by Fusarium graminearum is a destructive disease in wheat (Triticum aestivum L.) worldwide (Bai and Shaner 2004). It not only reduces grain yield and quality but also contaminates wheat grain with mycotoxins such as deoxynivalenol (DON), rendering the grain unsuitable for human or animal consumption (Trail 2009). FHB resistance in common wheat is a quantitative trait and controlled by a few major genes and some modifier genes (Liu et al. 2009). More than 200 quantitative trait loci (QTL) have been reported on all 21 chromosomes of hexaploid wheat after 46 wheat accessions were studied worldwide, and 19 loci have been identified in multiple mapping populations (Buerstmayr et al. 2009).
Growing resistant cultivars within an integrated cultural system is the most economic, effective and environmentally safe approach to reducing losses caused by this disease. Because environments significantly affect FHB response, large-scale phenotypic selection for resistance is difficult and requires costly and laborious field evaluations with poor repeatability among testing seasons and locations. Marker-assisted selection (MAS) may greatly facilitate selection efficiency. To date, progress has been made in breeding for resistance to FHB; some resistant varieties have been released for commercial production. Sumai 3 and its derivatives have been the major sources of resistance used in breeding programs worldwide (Bai and Shaner 2004). However, only a limited number of resistant sources have been genetically dissected to date, and these provide wheat breeders with only limited choices to enhance FHB resistance. Further sources of resistance therefore need to be genetically analyzed to identify major-effect QTL for gene pyramiding in wheat breeding programs by MAS.
Several Chinese landraces show high levels of resistance (Yu et al. 2006, 2008a). The QTL for FHB resistance in these landraces have not been investigated. Huangfangzhu (HFZ) is a Chinese spring wheat landrace with superior resistance to FHB (Yu et al. 2006, 2008a). The objectives of this study were to investigate QTL for type II resistance in HFZ and to quantify their effects using recombinant inbred lines (RIL) of HFZ/Wheaton.
Materials and methods
Plant materials and FHB evaluation
An SSD population of 106 F8 RIL was developed from a cross between Wheaton, a susceptible U.S. wheat variety, and HFZ, a resistant wheat landrace from Jiangsu Province. The RIL were grown in 1.0 L Dura pots (Hummert International, St. Louis, MO, USA) filled with Metro-mix 360® growing medium (Hummert International) on a greenhouse bench at 17 ± 2°C (night) and 22 ± 5°C (day) with supplemental light for 12 h and evaluated for FHB response in three consecutive greenhouse (GH) experiments from 2007 to 2008 at Kansas State University (KSU), and one field experiment (2009) at KSU Rocky Ford FHB Nursery, Manhattan, KS. A F. graminearum conidia suspension was prepared following Bai et al. (1999). Wheat spikes were inoculated by delivering 10 μL of conidial suspension (100 conidia/μL) into the floral cavity between the lemma and palea of one floret of a middle spikelet per spike using a syringe. Five spikes per RIL in each pot were inoculated. Following exposure to 100% relative humidity for 48 h in a mist chamber, the pots were returned to a greenhouse bench for further FHB development. Experiments were arranged in a randomized complete block design with two replicates (pots) of five plants per pot. In the field experiment, the RIL population and both parents were arranged in a randomized complete block design with two replications (blocks), with about 50 seeds per entry sown in a one-row plot in each replication. At anthesis, five spikes per row were inoculated by single floret injection as described for the greenhouse experiments. Between heading and the late dough stage, plants in the FHB nursery were misted for 10 min every hour using sprinklers. In both field and greenhouse experiments, the total number of spikelets and the number of infected spikelets were counted for each inoculated spike at 21 day after inoculation. The percentage of infected spikelets (PIS) per spike was calculated.
DNA extraction and marker analysis
Genomic DNA was isolated from 2-week-old wheat leaves of each RIL using a modified CTAB method (Maguire et al. 1994). The harvested wheat leaves were dried in a freeze dryer (ThermoSavant, Holbrook, NY) for 48 h and ground using a Mixer Mill (MM 300, Retsch, Germany) before DNA extraction.
A total of 1,125 SSR primer pairs including primer sets with BARC, WMC, GWM, KSM, CFA, CFD and DUP (http://wheat.pw.usda.gov) designations were used to screen the parents. Primer pairs that detected polymorphism between the parents were used to screen two bulks with contrasting FHB responses. The resistant bulk was constructed by mixing equal amounts of DNA from 10 highly resistant RIL and the susceptible bulk was constructed by mixing equal amounts of DNA from 10 highly susceptible RIL. Primer pairs that detected polymorphism between the contrasting bulks were used to genotype the entire RIL population. For SSR analysis, each 10 μL PCR mixture contained 40 ng template DNA, 1 mM each of reverse and M13-tailed forward primers, 0.2 mM of each dNTP, 1× PCR buffer, 2.5 mM MgCl2, and 0.6 U Taq polymerase. For PCR detection, 1 pmol of fluorescence-labeled M13 primer was added to each PCR. A touchdown PCR program was used for PCR amplification, in which the reaction mixture was incubated at 95°C for 5 min, followed by five cycles of 45 s of denaturing at 95°C, 5 min of annealing at 68°C with a decrease of 2°C in each sequential cycle, and 1 min of extension at 72°C. For another five cycles, the annealing temperature started at 58°C for 2 min with a decrease of 2°C for each sequential cycle. PCR continued through an additional 25 cycles of 45 s at 94°C, 2 min at 50°C, and 1 min at 72°C with a final extension at 72°C for 5 min. The amplified PCR fragments were separated in an ABI 3730 DNA Analyzer (Applied Biosystems, Foster City, CA). All marker data were scored using GeneMarker 1.6 (Softgenetics Inc. LLC), and visually checked twice to remove ambiguous data.
Genetic map construction and QTL analysis
Genetic linkage maps were constructed with SSR markers using JoinMap version 3.0 (Van Ooijen and Voorrips 2001) and the Kosambi function (Kosambi 1944). The threshold for logarithm of odds (LOD) value was set at 3.0 to claim linkage between markers with a maximum fraction of recombination at 0.4.
For QTL analysis, composite interval mapping (CIM) was performed using WINQTL Cartographer version 2.5 (Wang et al. 2007) model 6. Five markers were used as cofactors with a window size of 10 cM. QTL were analyzed using line means from individual experiments and from combined line means across all experiments. The LOD threshold for declaring a significant QTL was determined by 1,000 permutations. Single marker regression (SMR) was used to reveal marker-phenotype associations when a QTL was not significant either using CIM or simple interval mapping (SIM).
Statistical analysis
Broad sense heritability (H 2) was calculated for trait PIS based on ANOVA results using the formula \( H^{2} = \sigma_{\text{G}}^{2} /\sigma_{\text{G}}^{2} + \left( {\sigma_{{{\text{GE}}/{\text{e}}}}^{2} } \right) + \left( {\sigma_{re}^{2} } \right) \), where σ 2G = genotypic variance, σ 2e = residual error variance, σ 2GE = genotype × environment variance, r = number of replicates (pots) and e = number of experiments (seasons) following Jayatilake et al. (2011). Multiple comparisons of PIS among groups of RIL harboring different numbers of QTL were conducted using the least significant difference (LSD) method at α = 0.05. Statistical analyses were performed using Matlab software (MathWorks Inc., Natick, MA, USA, 2007).
Results
FHB variation in RIL
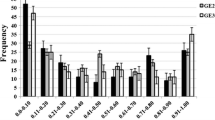
In the greenhouse experiments, PIS for the resistant parent (HFZ) averaged 15.3%, ranging from 11.5 to 22.1%, and 100% for the susceptible parent (Wheaton). The frequency distributions of PIS among RIL were continuous with an average PIS of 64.7%, ranging from 9.6 to 100% (Fig. 1). The most resistant RIL showed PIS similar to that of the resistant parent (HFZ), but most RIL means were distributed toward the susceptible parent, with about 75% of RIL having an average PIS higher than 50%.
In the field experiment, PIS ranged from 5.8 to 14.6% for HFZ with an average of 9.3%, and from 92.7 to 100% for Wheaton with an average of 97.0%. The frequency distributions of PIS among RIL were continuous with an average PIS of 49.1%, ranging from 6.5 to 100% in the field experiment (Fig. 1). The disease levels on RIL were less severe than in the greenhouse experiments and half of them had PIS less than 50%. The χ2 test of homogeneity demonstrated that the data from individual greenhouse and field experiments were not significantly different (χ2 = 3.36, P Χ df = 0.34), and thus could be combined. The PIS differences among RIL, environment, and genotype × environment interaction were highly significant (Table 1). Significant correlations were observed among the three greenhouse experiments (r > 0.42, P > 0.0001) and between greenhouse mean FHB data and field FHB data (r = 0.43, P < 0.0001). The mean heritability of PIS for RIL was 0.90 over three-greenhouse experiments and was 0.80 over the combined greenhouse and field experiments.
QTL for type II resistance
Among 1,125 primer pairs screened, 318 markers were polymorphic between the parents. Among them, 27 from five chromosomes were polymorphic between the contrasting bulks. Polymorphic markers from all five chromosomes were genotyped for all RIL and five linkage groups were constructed, covering 85.0 cM in genetic distance. CIM detected two QTL with major effects on type II resistance in HFZ. One QTL on chromosome 3BS was detected in all individual greenhouse experiments and the combined field-greenhouse data. SSR marker Xbarc147 and STS marker Xumn10 flanked this QTL which coincided with Fhb1 and explained 23.0–28.0% of the phenotypic variation in individual greenhouse experiments, 35.6% for mean greenhouse data and 35.4% for combined greenhouse-field data (Table 2; Fig. 2). It was not detected in the field experiment alone when CIM was conducted; however, single marker analysis showed that Xbarc147 on 3BS accounted for 12.9% of PIS variation (Table 3; Fig. 2).
A second major effect QTL on 7AL was flanked by SSR markers Xgwm276 and Xbarc121. This QTL was detected in the two 2007 greenhouse experiments, mean greenhouse data and combined greenhouse-field data (Table 2; Fig. 2), but not in the 2008 greenhouse and 2009 field experiments when CIM was used, although SSR markers Xgwm276 and Xbarc121 were significantly associated with the PIS in SMR analyses (Table 3; Fig. 2).
Single marker regression detected five additional markers on 1AS, 1B and 5AS associated with FHB resistance (Table 3; Fig. 2), each with R 2 values smaller than 0.12. Markers Xwmc120.2 on 1AS, Xbarc207 on 1B and Xbarc186/Xbarc117 on 5AS were significantly associated with mean greenhouse data and combined greenhouse-field data, whereas marker Xwmc24 on 1AS associated with FHB resistance only in the field experiment.
Effects of QTL on type II resistance
The segregations of contrasting alleles at each SSR locus closely linked to QTL exhibited 1:1 ratios. In the greenhouse experiments, the average PIS for RIL carrying HFZ alleles at Xumn10 on 3BS and Xgwm276 on 7AL were 50.0 and 56.0%, respectively, while the average PIS of RIL carrying the Wheaton alleles were 78.0 and 74.0%, respectively. In the field experiment, the PIS of RIL with HFZ alleles at Xumn10 and Xgwm276 were 38.0 and 43.0%, respectively, and those with Wheaton alleles were 58.0 and 55.0%, respectively. For the other three markers on 1AS, 1B and 5AS, the average PIS of RIL with HFZ alleles in greenhouse experiments ranged from 58.0 to 59.0%, compared with 71.0 to 74.0% for those with the corresponding Wheaton alleles. In the field experiment, the average PIS of the RIL with HFZ alleles ranged from 44.0 to 47.0% compared with 51.0 to 54.0% for those with the Wheaton alleles. The lower average PIS of RIL with HFZ alleles and the negative effects of all five Wheaton alleles confirmed that all favorable alleles for FHB resistance were contributed by HFZ. The 3BS QTL contributed the largest effect on FHB resistance and the 7AL QTL was next.
To elucidate the effect of single and combined QTL on FHB response, the RIL were divided into five groups: group 1 contained the HFZ alleles at QTL on 3BS and 7AL ignoring the effects of the minor QTL; group 2 carried the HFZ allele on 3BS but not the HFZ allele on 7AL; group 3 carried only the HFZ allele on 7AL; group 4 contained only HFZ minor alleles (1–3); and group 5 carried only Wheaton alleles at all five loci. Frequencies of lines within the five groups ranged from 8.8 to 26.5%. In the greenhouse experiments, the mean PIS of groups 1 and 2 were significantly lower (LSD, α = 0.05) than those of groups 3, 4 and 5 (Fig. 3). Group 3 had significantly lower PIS than groups 4 and 5. In the field experiment, group 1 showed lower PIS than the other four groups, and groups 2, 3 and 4 had almost the same PIS but all were lower than group 5. However, differences were significant only between groups 1 and 5 (LSD, α = 0.05).
Comparisons of percentage infected spikelets (PIS) among genotypes with different QTL combinations based on FHB data in greenhouse experiments. G1 = Qfhb.uhgl-3BS + Qfhb.uhgl-7AL + 0–3 minor QTL; G2 = Qfhb.uhgl-3BS + 0–3 minor QTL; G3 = Qfhb.uhgl-7AL + 0–3 minor QTL; G4 = 1–3 minor QTL; G5 = no identified QTL. The solid circle on the vertical line is the mean PIS of each group and the length of the line represents the confidence interval. Two groups not sharing a horizontal dashed line are significantly different at LSD.05. Numbers in parentheses on the horizontal axis are frequencies of RIL in each group
Discussion
Five putative QTL for type II resistance to FHB were identified on chromosomes 3BS, 7AL, 5AS, 1AS and 1B of Chinese landrace HFZ. The QTL on 3BS was first reported in Sumai 3, designated as Qfhs.ndsu-3BS (Waldron et al. 1999) and in Ning 7840 (Bai et al. 1999). This QTL has been detected in at least 26 different studies and shows a stable major effect on type II resistance (resistance to fungal spread within spikes) (Buerstmayr et al. 2009; Liu et al. 2009). In addition to Sumai 3 and its derivatives, including Ning7840 (Bai et al. 1999; Zhou et al. 2002), Ning 894037 (Shen et al. 2003), CM-82036 (Buerstmayr et al. 2002), W14 (Chen et al. 2006), CJ 9306 (Jiang et al. 2007a, b) and Huapei 57-2 (Bourdoncle and Ohm 2003; Shen et al. 2003), this QTL was also reported in materials not related to Sumai 3, such as Wangshuibai (Lin et al. 2004; Zhang et al. 2004; Zhou et al. 2004; Mardi et al. 2005; Yu et al. 2008b) and Nyu Bai (McCartney et al. 2007). Because of its large effect on FHB response, this QTL was fine mapped as a single Mendelian gene within a 1.2 cM interval, and renamed as Fhb1 (Cuthbert et al. 2006; Liu et al. 2006). Xumn10 was proposed as the best marker for prediction of Fhb1 (Liu et al. 2008). Xumn10 was also the closest marker in the present study indicating the QTL is most likely Fhb1. The non-significance of the QTL in CIM analysis of the field experiment may be due to confounding effects of further infections. In the field experiment, plants were infected by both single floret injection and naturally. Thus disease rating reflects not only disease spread from the artificially inoculated site but also from natural infections at other positions in the spike. Single marker analysis showed that flanking markers Xumn10 and Xbarc147 were significantly associated with PIS in the field experiment. Another problem could be the large differences in flowering time across the RIL population leading to non-uniform conditions for FHB development between early and late flowering lines.
A QTL flanked by Xgwm276 and Xbarc121 was identified on 7AL of HFZ. Like the 3BS QTL, this QTL was also non-significant in CIM analysis of the field experiment, but was significant in SMR of Xbarc121. A QTL on 7AL was also reported in Wangshuibai (Zhou et al. 2004; Jia et al. 2005), NK93604 (Semagn et al. 2007) and Ritmo (Klahr et al. 2007). Xgwm276 was the most closely linked marker to the QTL in Wangshuibai (Jia et al. 2005) and NK93604. In another study, a QTL on T. dicoccoides 7AL (Kumar et al. 2007), was tightly associated with Xbarc121. This result suggests that the 7AL QTL may be the same QTL as previously reported in these various lines.
Three QTL on 5AS, 1AS and 1B showed only minor effects on type II resistance and were detected only by SMR. QTL from several sources were reported on chromosome 5AS. These were associated with either type I or type II resistance and explained 4–26% of the phenotypic variation in different experiments (Buerstmayr et al. 2002, 2003; Steiner et al. 2004; Yang et al. 2005; Chen et al. 2006; Jiang et al. 2007a, b; Liu et al. 2007; McCartney et al. 2007). In our study, markers Xbarc117 and Xbarc186 on 5AS were associated with mean PIS in the three greenhouse experiments, but not the field experiment, suggesting that a QTL with a minor effect on type II resistance might be present in HFZ. According to the linked common marker location, it may be the same QTL as described by Chen et al. (2006).
CJ 9306 carried a QTL for FHB resistance on 1AS (QFhs.nau-1AS), which reduced PIS by 11.7–21.2%. The QTL detected on 1AS in our study also enhanced type II resistance. Marker Xwmc120.2 was the closest marker for the QTL in HFZ.
The QTL on chromosome 1B was significantly associated with SSRs Xbarc207 and Xbarc181. In previous reports, a QTL from Arina was detected on 1BL (Semagn et al. 2007). Twelve QTL for type II resistance reported on 1BL fell into three different regions when subjected to a meta-analysis (Liu et al. 2009). Because common markers were not found between this study and others, the relationship of the present QTL on 1B to others remains unknown.
In summary, FHB resistance in HFZ investigated in this study was contributed by a combination of five QTL that were probably reported previously in different germplasms. The QTL on chromosomes 3BS and 7AL contributing major effects on type II resistance and consistently detected in multiple experiments in this and other studies should be used together to improve FHB resistance in breeding. Three other QTL showing minor effects and detected in only some experiments need further validation before they are used in breeding. Thus with a unique combination of QTL compared to other resistance sources, HFZ can be used as a valuable alternative source for improvement of FHB resistance.
References
Bai G, Shaner G (2004) Management and resistance in wheat and barley to fusarium head blight. Annu Rev Phytopathol 42:135–161
Bai G, Kolb FL, Shaner G, Domier LL (1999) Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling scab resistance in wheat. Phytopathology 89:343–348
Bourdoncle W, Ohm HW (2003) Quantitative trait loci for resistance to Fusarium head blight in recombinant inbred wheat lines from the cross Huapei 57–2/Patterson. Euphytica 131:131–136
Buerstmayr H, Lemmens M, Hartl L, Doldi L, Steiner B, Stierschneider M, Ruckenbauer P (2002) Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (Type II resistance). Theor Appl Genet 104:84–91
Buerstmayr H, Steiner B, Hartl L, Griesser M, Angerer N, Lengauer D, Miedaner T, Schneider B, Lemmens M (2003) Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. II. Resistance to fungal penetration and spread. Theor Appl Genet 107:503–508
Buerstmayr H, Ban T, Anderson J (2009) QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed 128:1–26
Chen J, Griffey CA, Maroof MAS, Stromberg EL, Biyashev RM, Zhao W, Chappell MR, Pridgen TH, Dong Y, Zeng Z (2006) Validation of two major quantitative trait loci for Fusarium head blight resistance in Chinese wheat line W14. Plant Breed 125:99–101
Cuthbert PA, Somers DJ, Thomas J, Cloutier S, Brule-Babel A (2006) Fine mapping Fhb1, a major gene controlling Fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor Appl Genet 112:1465–1472
Jayatilake DV, Bai GH, Dong YH (2011) A novel quantitative trait locus for Fusarium head blight resistance in chromosome 7A of wheat. Theor Appl Genet 122:1189–1198
Jia G, Chen PD, Qin GJ, Bai GH, Wang X, Wang SL, Zhou B, Zhang SH, Liu DJ (2005) QTLs for Fusarium head blight response in a wheat DH population of Wangshuibai/Alondra’s. Euphytica 146:183–191
Jiang GL, Dong Y, Shi J, Ward RW (2007a) QTL analysis of resistance to Fusarium head blight in the novel wheat germplasm CJ 9306. II. Resistance to deoxynivalenol accumulation and grain yield loss. Theor Appl Genet 115:1043–1052
Jiang GL, Shi JR, Ward RW (2007b) QTL analysis of resistance to Fusarium head blight in the novel wheat germplasm CJ 9306. I. Resistance to fungal spread. Theor Appl Genet 116:3–13
Klahr A, Zimmermann G, Wenzel G, Mohler V (2007) Effects of environment, disease progress, plant height and heading date on the detection of QTLs for resistance to Fusarium head blight in an European winter wheat cross. Euphytica 154:17–28
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Kumar S, Stack RW, Friesen TL, Faris JD (2007) Identification of a novel Fusarium head blight resistance quantitative trait locus on chromosome 7A in tetraploid wheat. Phytopathology 97:592–597
Lin F, Kong ZX, Zhu HL, Xue SL, Wu JZ, Tian DG, Wei JB, Zhang CQ, Ma ZQ (2004) Mapping QTL associated with resistance to Fusarium head blight in the Nanda2419 × Wangshuibai population. I. Type II resistance. Theor Appl Genet 109:1504–1511
Liu S, Zhang X, Pumphrey MO, Stack RW, Gill BS, Anderson JA (2006) Complex microcolinearity among wheat, rice, and barley revealed by fine mapping of the genomic region harboring a major QTL for resistance to Fusarium head blight in wheat. Funct Integr Genomics 6:83–89
Liu S, Abate ZA, Lu H, Musket T, Davis GL, McKendry AL (2007) QTL associated with Fusarium head blight resistance in the soft red winter wheat Ernie. Theor Appl Genet 115:417–427
Liu SX, Pumphrey MO, Gill BS, Trick HN, Zhang JX, Dolezel J, Chalhoub B, Anderson JA (2008) Toward positional cloning of Fhb1, a major QTL for Fusarium head blight resistance in wheat. In: 3rd International FHB Symposium, Szeged, Hungary. Cereal Res Commun 36:195–201
Liu S, Hall MD, Griffey CA, McKendry AL (2009) Meta-analysis of QTL associated with Fusarium head blight resistance in wheat. Crop Sci 49:1955–1968
Maguire TL, Collins GG, Sedgley M (1994) A modified CTAB DNA extraction procedure for plants belonging to the family Proteaceae. Plant Mol Biol Rep 12:106–109
Mardi M, Buerstmayr H, Ghareyazie B, Lemmens M, Mohammadi SA, Nolz R, Ruckenbauer P (2005) QTL analysis of resistance to Fusarium head blight in wheat using a ‘Wangshuibai’-derived population. Plant Breed 124:329–333
McCartney CA, Somers DJ, Fedak G, DePauw RM, Thomas J, Fox SL, Humphreys DG, Lukow O, Savard ME, McCallum BD, Gilbert J, Cao W (2007) The evaluation of FHB resistance QTLs introgressed into elite Canadian spring wheat germplasm. Mol Breed 20:209–221
Semagn K, Skinnes H, Bjornstad A, Maroy AG, Tarkegne Y (2007) Quantitative trait loci controlling Fusarium head blight resistance and low deoxynivalenol content in hexaploid wheat population from ‘Arina’ and NK93604. Crop Sci 47:294–303
Shen X, Zhou M, Lu W, Ohm H (2003) Detection of Fusarium head blight resistance QTL in a wheat population using bulked segregant analysis. Theor Appl Genet 106:1041–1047
Steiner B, Lemmens M, Griesser M, Scholz U, Schondelmaier J, Buerstmayr H (2004) Molecular mapping of resistance to Fusarium head blight in the spring wheat cultivar Frontana. Theor Appl Genet 109:215–224
Trail F (2009) For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol 149:103–110
Van Ooijen J, Voorrips R (2001) JoinMap® 3.0, software for the calculation of genetic linkage maps. Plant Research International, Wageningen
Waldron BL, Moreno-Sevilla B, Anderson JA, Stack RW, Frohberg RC (1999) RFLP mapping of QTL for fusarium head blight resistance in wheat. Crop Sci 39:805–811
Wang S, Basten C, Zeng Z-B (2007) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Yang Z, Gilbert J, Fedak G, Somers DJ (2005) Genetic characterization of QTL associated with resistance to Fusarium head blight in a doubled-haploid spring wheat population. Genome 48:187–196
Yu JB, Bai GH, Cai SB, Ban T (2006) Marker-assisted characterization of Asian wheat lines for resistance to Fusarium head blight. Theor Appl Genet 113:308–320
Yu JB, Bai GH, Cai SB, Dong YH, Ban T (2008a) New Fusarium head blight-resistant sources from Asian wheat germplasm. Crop Sci 48:1090–1097
Yu JB, Bai GH, Zhou WC, Dong YH, Kolb FL (2008b) Quantitative trait loci for Fusarium head blight resistance in a recombinant inbred population of Wangshuibai/Wheaton. Phytopathology 98:87–94
Zhang X, Zhou MP, Ren LJ, Bai GH, Ma HX, Scholten OE, Guo PG, Lu WZ (2004) Molecular characterization of Fusarium head blight resistance from wheat variety Wangshuibai. Euphytica 139:59–64
Zhou WC, Kolb FL, Bai GH, Shaner G, Domier LL (2002) Genetic analysis of scab resistance QTL in wheat with microsatellite and AFLP markers. Genome 45:719–727
Zhou WC, Kolb FL, Yu JB, Bai GH, Boze LK, Domier LL (2004) Molecular characterization of Fusarium head blight resistance in Wangshuibai with simple sequence repeat and amplified fragment length polymorphism markers. Genome 47:1137–1143
Acknowledgments
This project is partly funded by NSFC (Grant no. 31171537), the Priority Academic Program Development of Jiangsu Higher Education Institution, Jiangsu Provincial Natural Science Foundation of China (Grant no. BK2010312), and National Research Initiative Competitive Grants CAP project 2011-68002-30029 from the USDA National Institute of Food and Agriculture. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. This is contribution no. 11-207-J from the Kansas Agricultural Experiment Station, Manhattan, Kansas, USA.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, T., Bai, G., Wu, S. et al. Quantitative trait loci for resistance to Fusarium head blight in the Chinese wheat landrace Huangfangzhu. Euphytica 185, 93–102 (2012). https://doi.org/10.1007/s10681-012-0631-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0631-2