Abstract
In order to characterise quantitative trait loci (QTLs) for Type I and Type II resistance against Fusarium head blight (FHB) in wheat, a population of recombinant inbred lines derived from the cross Cansas (moderately resistant)/Ritmo (susceptible) was evaluated in spray-inoculated field trials over three seasons. Map-based QTL analysis across environments revealed seven QTLs on chromosomes 1BS, 1DS, 3B, 3DL, 5BL, 7BS and 7AL (QFhs.whs-1B, QFhs.whs-1D, QFhs.whs-3B, QFhs.whs-3D, QFhs.whs-5B, QFhs.whs-7A, QFhs.whs-7B) associated with FHB resistance. They accounted for 56% of the phenotypic variance. QFhs.whs-1D primarily appeared to be involved in resistance to fungal penetration, whereas the other QTLs mainly contributed to resistance to fungal spread. FHB resistance was significantly correlated with plant height (PH) and heading date (HD). Including all single environments, corresponding overlaps of QTLs for FHB resistance and QTLs for PH/HD occurred at six loci, among them two consistently detected QTLs, QFhs.whs-5B and QFhs.whs-7A. When significant effects of PH and HD on FHB resistance were eliminated by covariance analysis, a second QTL analysis revealed possible escape mechanisms for the majority of the coincidental loci.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FHB is one of the major diseases in wheat (Triticum aestivum L.) and has reached epidemic dimensions in many parts of the world. Besides yield loss and reduced seed quality resulting in lower test weights and market grades the most serious concern is accumulation of mycotoxins, that make the grain unfit for both human and livestock consumption. FHB response shows oligogenic as well as polygenic inheritance and is significantly affected by environment (Buerstmayr et al. 2002). It is therefore imperative that inheritance studies be conducted under different environmental conditions in order to separate QTLs expressed in different situations.
Five types of FHB resistance in wheat have been described (Mesterházy 1995). Most research has focused on Type II resistance (resistance to fungal spread in infected tissue), because it can be easily studied under controlled conditions by injection of spore suspensions into a single floret. Although Type I resistance (resistance to initial infection) has been documented by phenotypic evaluation (Rudd et al. 2001), genetic factors specifically involved in resistance to fungal penetration have not been adequately described. Differences in the temporal expression patterns of general defence response genes between the resistant cultivar Sumai3 and the susceptible cultivar Wheaton were observed, such as higher and earlier transcript accumulation of pathogenesis-related proteins PR-4 (chitinase types I, II) and PR-5 (thaumatin-like protein) in Sumai3 (Pritsch et al. 2000). Moreover, little is known about the dynamics of FHB QTLs during disease development. Marker-aided tagging of QTLs involved in the resistance of initial infection and different phases of disease development is the first step towards pyramiding QTLs operating at distinct stages of host defence.
Observations of taller and early heading genotypes showing fewer FHB symptoms (Miedaner 1997; Steiner et al. 2004) have led to contradictory results. Whereas significant phenotypic associations of FHB resistance, HD and PH were supported by coincidences of QTLs for these traits in the studies of Gervais et al. (2003) and Steiner et al. (2004), Somers et al. (2003) detected no overlaps of such QTLs. Thus, passive resistance mechanisms in the wheat–Fusarium pathosystem allowing a plant to escape from infection (passive resistance) due to morphological or developmental features may be an obstacle when separating undesired morphological traits from QTLs for FHB resistance.
In the present study we analysed (a) the stability of QTLs conferring Type I and II resistance under diversified environmental conditions, (b) the functional pattern of QTLs during disease development and (c) the influence of PH and HD on the detection of FHB QTLs. In comparing the present results with those reported elsewhere we were particularly interested in determining if chromosomal segments characterised in QTL analysis of FHB response coincided with the positions of genes involved in developmental/morphological traits.
Material and methods
Plant material and field evaluation
A population of F4 recombinant inbred lines (RILs) was developed from a cross between the winter wheats Ritmo (highly susceptible, Hobbit//Line-1320/Wizard/3/Marksman/4/Virtue) and Cansas (moderately resistant, Kristall/Marksman) by single-seed descent to the F4. F5 bulks were sown as F6 lines in the first year of field evaluation (1998). From each F6 line 25 random spikes were bulked and sown as F7 in field trials in 1999. The same procedure was applied to obtaining the F8 generation used for the field trials in 2000. The RILs were evaluated at Pettenbrunn, Germany, in 1998, 1999, and 2000 and at Pulling in 2000. Phenotypic and QTL analyses were thus performed with data from four environments, designated Pe98, Pe99, Pe00 and Pu00. Plots consisted of double rows 1.20 m in length and arranged in a randomised block design. The trials included the RILs with two replications and, only for Pe99, Pe00 and Pu00, five replicated entries of the parents. Non-inoculated control plots were included in the different year/location experiments, again with two replications. The inoculum consisted of a spore suspension (1 × 106 spores per ml) representing a Bavarian regional spectrum of field isolates of Fusarium culmorum. Cultures were grown according to Miedaner et al. (1996). When plots reached 50% anthesis, a global inoculation was performed using a motor-driven sprinkler to spray the spore suspension on to the spikes at a rate of 800 l/ha. The inoculation was repeated 4 days later. These artificial inoculations were carried out in the evening in order to benefit from the higher relative humidity required for infection. Disease assessment began with the appearance of the first symptoms 7–14 days after inoculation (1st score = S1), followed by three consecutive scores (S2, S3 and S4) at intervals of 4–5 days. Each score estimated the percentage (0–100%) of visually infected spikelets for each plot. All four scores were used to calculate the area under disease progress curve (AUDPC) (Buerstmayr et al. 2000) for each plot in individual environments. HD and PH were recorded from the non-inoculated control plots, measured as the number of days from May 1 until 50% spike emergence and the height to the top of the spikes. In each trial each replicate was scored to obtain the means for the phenotypic traits FHB AUDPC, HD and PH.
Statistical analysis of the phenotypic assessments
All statistical analyses were based on means and were conducted with SPSS version 10.0 (SPSS, Chicago) and PLABSTAT version 2N (Utz 1995). The Kolmogorov–Smirnov test was performed to confirm normal distributions for the phenotypic traits FHB AUDPC, PH and HD. The phenotypic data were analysed by two-factorial ANOVA with environments and replications considered as random. Broad sense heritability (h 2) was estimated from mean squares as described by Hallauer and Miranda (1981):
where σ 2g = genotypic variance, σ 2ge = genotype × environment interaction variance, σ2 = error variance, r = number of replications and e = number of environments. Multiple mean comparisons were made by one-way ANOVA, followed by an a posteriori test. The significances of transgressive segregations for all traits were tested by t-test (LSD). Correlations between traits and FHB severity among environments were estimated by the Pearson correlation coefficient.
Where a significant correlation between FHB severity and PH or HD existed, visual scores of FHB AUDPC were adjusted for differences in HD/PH by covariance analysis, where PH and HD were individually used as covariables in separate analyses. The adjusted data were used in a second scan for FHB QTLs. This analysis allowed the evaluation of FHB QTLs unaffected by PH and HD. Therefore, true FHB resistance could be differentiated from passive resistance, i.e., escape mechanisms based on favourable plant architecture and/or developmental characters.
Molecular marker scoring and map construction
Using the 94 F4 RILs of Cansas/Ritmo, a genetic linkage map was constructed as described by Klahr et al. (2004). The final map used for QTL scanning consisted of 211 AFLPs, 37 SSRs and the resistance gene analogue (RGA) marker locus Xwhs2001-1D. These markers mapped to 34 linkage groups spanning 1,219 cM. Quantitative scoring of dominant, i.e., null-allelic molecular markers (114 AFLPs, six SSRs and RGA locus Xwhs2001-1D), enabled codominant assessment and resulted in 59% codominantly analysed markers. The SSRs permitted the assignment of linkage groups to chromosomes according to published genetic maps (Röder et al. 1998; Somers et al. 2004). All chromosomes, except 4B, 4A, 4D and 6D, were covered with at least partial maps. The linkage group harbouring QFhs.whs-5B (see results) was erroneously assigned to chromosome 7BL in Klahr et al. (2004) and, therefore, relocated to chromosome 5BL according to the consensus microsatellite map of Somers et al. (2004).
QTL analysis
Simple and composite interval mapping (SIM, CIM) using PLABQTL software (Utz and Melchinger 1996) was applied. The chosen model included all additive and two-loci epistatic additive effects. For CIM, cofactor selection was done automatically with an F-to-enter threshold of 7. The LOD values were calculated from the sum of squares of the regression in a model with a putative QTL versus the sum of squares of the regression in a model without QTL. LODs of 3.4 and 4.1 were computed as thresholds for significance of QTLs at P = 0.05 and P = 0.01, respectively. Estimates of QTL positions were obtained at the maximum LOD score in the region under consideration. QTL positions were given in cM from the end of the short arms of chromosomes or from the starting point of partially mapped chromosomes corresponding to maximum peaks of the LOD plot curve. Support intervals referred to a LOD fall-off of 1.0. QTLs with non-overlapping support intervals were regarded as different. Phenotypic variances explained by individual QTL effects were calculated as the square of the partial correlation coefficient R 2a for significant additive gene effects. The percentage of phenotypic variance explained by all QTLs (R 2P ), the genotypic variance explained by the detected QTLs (σQTL) across all environments, and the significance of QTL × E interactions were calculated in the ‘‘final simultaneous fit’’. Only QTLs found to be significant in the analysis across all environments (stable or consistent QTLs) were designated according to the guidelines for nomenclature of loci and alleles controlling quantitative characters (McIntosh et al. 1998).
QTL calculation for FHB resistance in single environments and across all environments was performed on AUDPC means based on the four scores in each environment. For phase-specific analysis characterising the development of consistent QTLs for FHB resistance throughout the progress of the disease, AUDPC from the inoculation day to S1 (S-1), from S1 to S2 (S-2), from S2 to S3 (S-3) and from S3 to S4 (S-4) were used separately for joint analysis across all environments. QTL analysis for PH and HD was performed on means of the ratings taken for each environment.
Results
Phenotypic variation of FHB resistance
FHB severity followed normal distributions in single environments and for the means across all environments. The segregation of FHB severities in the population differed between environments. The parents displayed significantly different FHB severities in each experiment. The moderate resistance of Cansas varied among environments. In Pe99 and Pe00 there were no significant differences between parent and RIL means, whereas in Pu00 and over averaged environments, the population means were significantly lower than the parent means (Table 1). Correspondingly, transgression towards resistance was always stronger than towards susceptibility, although transgressive segregants with AUDPC values less than Ritmo occurred in some environments (data not shown). Averaged over all environments, six lines were more resistant than Cansas, but no line was significantly more susceptible than Ritmo (LSD5% = 29). Correlations of FHB phenotypes for RILs among environments were always significant (P = 0.01) and ranged between moderate and high levels. Averaged over all environments the correlation coefficient was r = 0.55 (Table 2).
Phenotypic variation of FHB resistance during disease development
ANOVA calculated for FHB AUDPC at S-1, S-2, S-3 and S-4 across all environments detected significant differences for genotypes and environments as sources of variation (Table 3). Despite the importance of environmental factors genotypic effects were significant for all ratings, and increased considerably at the later stages. Heritability reached the highest value (h 2 = 82.3) in S-4.
QTL analysis of FHB AUDPC resistance in single and across all environments
The results of the QTL analysis across the four tested environments based on CIM are given in Table 4. Seven QTLs on chromosomes 1BS, 1DS, 3B, 3DL, 5BL, 7AL and 7BS (QFhs.whs-1B, QFhs.whs-1D, QFhs.whs-3B, QFhs.whs-3D, QFhs.whs-5B, QFhs.whs-7A, QFhs.whs-7B) were identified in the joint analysis across all environments, and accounted for 16.5, 8.2, 11.2, 11.1, 20.0, 9.9 and 11.0%, respectively, of the phenotypic variance. Each QTL was detected in one to three individual environments. A simultaneous fit for all QTLs in the joint analysis explained 56.0% of the phenotypic variance. The locations on chromosomes and the LOD profiles of the seven consistent QTLs are shown in Fig. 2. SIM reinforced the significance of QFhs.whs-1B, QFhs.whs-3D, QFhs.whs-5B and QFhs.whs-7A found by CIM across environments. However, QFhs.whs-1D, QFhs.whs-3B and QFhs.whs-7B did not reach the critical LOD thresholds in the SIM analysis. The largest effects on FHB resistance were associated with QFhs.whs-5B and QFhs.whs-1B. The resistance allele of QFhs.whs-1B was located on the T1BL·1RS wheat-rye translocated chromosome carried by the resistant parent Cansas. Three of the seven QTLs increasing FHB resistance were inherited from the susceptible parent, Ritmo, possibly explaining the occurence of the resistant transgressives. No epistatic effects were found. Eleven additional loci were found to be associated with FHB resistance considering all single environments (data not shown).
Effects of plant height and heading date on FHB resistance
Across all environments the average PH of Cansas and Ritmo were 87.7 cm and 81.0 cm, respectively. The parents showed only a 2 days difference in heading, with Cansas heading earlier (Table 1). For HD, means of the RIL population across environments showed a continuous distribution. In contrast, the distribution for PH was bimodal and characterised by main and minor peaks with the majority of RILs exceeding the height of Cansas (data not shown). ANOVA for both traits revealed significant effects of σ2 G, σ2 E and σ2 G × E (data not shown). The heritabilities of PH and HD were 96.1 and 89.6, respectively.
Correlations between FHB AUDPC and either PH or HD were significant for two and three, respectively, of the four environments and varied from weak to moderate levels (Table 5). PH and FHB AUDPC were negatively correlated in each environment, whereas there were both negative and positive correlations for HD and FHB AUDPC. QTLs for PH and HD across all environments are shown in Table 6. Five loci of the 18 FHB AUDPC QTLs (including all single environments) overlapped with QTLs for HD or PH, among them two stable FHB QTLs (QFhs.whs-5B, QFhs.whs-7A). In order to evaluate the actual genetic variance of FHB response not confounded by effects of PH and HD, adjusted FHB AUDPC data were obtained by covariance analysis using HD or PH as covariables. In a second QTL analysis using the adjusted AUDPC data, only one (QFhs.whs-5B) of the two stable FHB QTLs, which overlapped with QTLs for PH or HD, remained significant (Fig. 1). Further loci affected by overlapping were found on chromosomes 5A and 5B in single environments (Tables 7, 8). None of these QTLs associated with FHB resistance was detected by the second analysis.
Chromosome positions of consistent QTLs for FHB AUDPC Type I and II resistance as well as for PH and HD on chromosomes, where stable FHB QTLs were found. Cumulative genetic distances are given after starting point of mapping. QTLs for FHB resistance are shown for the calculation of the original disease scores and those adjusted for PH and HD. The threshold for QTL significance (P = 0.05) is indicated by a dotted line. Centromeres are indicated by black bars
Effects of FHB QTLs throughout the disease progress
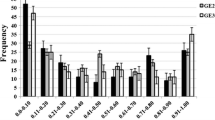
The R 2 values of the seven consistently detected QTLs measured by AUDPC were recorded over the consecutive disease observation phases S-1, S-2, S-3, S-4. The R 2 values showed marked differences during the course of the epidemic. Four QTLs (QFhs.whs-3B, QFhs.whs-3D, QFhs.whs-5B and QFhs.whs-7B) reached maximum R 2 values at S-2, and one (QFhs.whs-7A) was maximum at S-3. QFhs.whs-1B increased from S-1 to S-2 and remained almost stable until the end of the disease. In contrast, the R 2 value of QFhs.whs-1D was highest at S-1 and ceased to be significant after S-2 (Fig. 2).
Discussion
Influence of environmental factors on FHB resistance
A set of different environments (years and locations) was used to evaluate FHB response, which is confounded by factors such as humidity, inoculum level, temperature and wind (Miedaner 1997). The moderate inter-environmental correlation coefficient confirmed the complexity of FHB resistance. This was corroborated by the high proportion of environment-specific QTLs, found to be involved. Despite this, G × E effects were not significant throughout disease development, and QTL analysis revealed seven stable QTLs.
Effects of PH and HD on FHB resistance
Despite spray-inoculation, providing the same amount of inoculum to each ear independent of PH, the significant negative correlations between FHB resistance and PH support the hypothesis that semi-dwarf genotypes are more subject to infection by Fusarium due to higher moisture and humidity promoting the spread of the fungus. These observations agree with other spray-inoculation experiments (Gervais et al. 2003), even when mist irrigation for humidity control was used (Buerstmayr et al. 2000). In agreement with the results of our study, the correlation between FHB severity and HD seems not to be as congruent as for FHB severity and PH, as both positive and negative correlations between FHB resistance and HD were reported (Gervais et al. 2003; Somers et al. 2003; Steiner et al. 2004). Coincident QTLs for FHB resistance, PH and HD may contribute to the significant phenotypic correlations in some individual environments as well as in the overall analysis. QTL analysis based on AUDPC data adjusted for effects of PH and HD by covariance analysis allowed differences between QTLs conferring resistance per se and those reflecting possible escape to be distinguished. Upon second-pass QTL scanning, four of five FHB QTLs, previously colocalised with QTLs for PH and HD, were not identified implying that resistance effects conferred by these loci were not based on true resistance mechanisms. Only the stable QTL QFhs.whs-5B remained significant after the second QTL scan and, thus appeared not to be related to disease escape. The haplotypes of coinciding QTLs supported the results of covariance analysis; in cases of FHB QTLs that were no longer detected, the alleles for either resistance and tallness, or resistance and early heading, were contributed by one parent. In contrast, at QFhs.whs-5B and QEet.whs-5B.2 the alleles for resistance and early heading were not derived from one parent, but were from Cansas and Ritmo, respectively. Paillard et al. (2004) also detected a coincidence of QTLs for FHB resistance and HD calculated from AUDPC data already corrected for HD/PH at approximately the same genomic position. Using different resistance sources Gervais et al. (2003) and Steiner et al. (2004) mapped overlapping QTLs for FHB resistance and PH to the marker interval Xgwm639–Xgwm617 on chromosome 5AL. On this map segment we also detected coinciding QTLs for FHB resistance and HD. This genomic region was found to harbour a QTL for PH by Cadalen et al. (1998) and a gene for vernalisation requirement (Vrn-A1) by Kato et al. (1999).
QTL expression in different phases of disease progress
Epidemic-specific expression of QTLs provided evidence of different genes involved in resistance at different phases of disease development. This points to a complex relationship between pathogen development and host response, and prevents a reliable evaluation of QTLs based on a single disease assessment. As the first score covered the initial disease symptoms there was a higher probability of recognising Type I resistance at S-1. Thus, the significantly different infection levels for S-1 indicated that the segregating lines varied in Type I resistance. The results of QTL analyses at consecutive stages of the disease progress indicated a unique role of QFhs.whs-1D in the early stages of disease development. This QTL almost coincided with the RGA locus Xwhs2001-1D, which shows close sequence homology to RGH 2a/b, a RGA located in the Mla region of barley (Wei et al. 2002). We suggest that Type I resistance is expressed as delay in initial infection. For S-1 we found the lowest heritability (h 2 = 58.9) and the highest effect of environment. This agrees with previous indications pointing to greater environmental variation associated with the phenotypic evaluation of resistance types other than Type II (Kolb et al. 2001). QFhs.whs-5B and QFhs.whs-3D were effective at all stages of disease development, whereas QFhs.whs-1B was not identified in the early phase suggesting its major role in Type II resistance. These findings are in accordance with the early speculations of Schroeder and Christensen (1963), who hypothesised that certain physiological factors may condition both resistance to initial penetration and fungal spread whereas other factors are solely responsible for one or other aspect. Our results showed that the knowledge of specific QTL activity enables the selection of genotypes with genes operating at different phases during the course of the disease. Thus, a resistance response throughout the epidemic will be ensured.
Sources of FHB resistance in different genetic backgrounds
QFhs.whs-1D was not found in other FHB resistance studies. QFhs.whs-1B, located on the T1BL·1RS wheat-rye translocated chromosome appears to agree with loci detected in other crosses between European wheat cultivars (Shen et al. 2003; Schmolke et al. 2005). In this study, no QTL was associated with the genomic region of chromosome 3BS, which harbours the most prominent QTL for FHB resistance derived from the Chinese resistance source Sumai 3 (Bai et al. 1999; Waldron et al. 1999; Anderson et al. 2001; Buerstmayr et al. 2002). This QTL does not seem to be present in European sources of FHB resistance.
References
Anderson JA, Stack RW, Liu S, Waldron BL, Fjeld AD, Coyne C, Moreno-Sevilla B, Mitchell Fetch J, Song QJ, Cregan PB, Frohberg RC (2001) DNA markers for Fusarium head blight resistance QTLs in two wheat populations. Theor Appl Genet 102:1164–1168
Bai G, Kolb FL, Shaner G, Domier LL (1999) Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling scab resistance in wheat. Phytopathology 89:343–348
Buerstmayr H, Steiner B, Lemmens M, Ruckenbauer P (2000) Resistance to Fusarium head blight in winter wheat: heritability and trait associations. Crop Sci 40:1012–1018
Buerstmayr H, Lemmens M, Hartl L, Doldi L, Steiner B, Stierschneider M, Ruckenbauer P (2002) Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (Type II resistance). Theor Appl Genet 104:84–91
Cadalen T, Sourdille P, Charmet G, Tixier MH, Gay G, Boeuf C, Bernard S, Leroy P, Bernard M (1998) Molecular markers linked to genes affecting plant height in wheat using a doubled-haploid population. Theor Appl Genet 96:933–940
Gervais L, Dedryver F, Morlais JY, Bodusseau V, Negre S, Bilous M, Groos C, Trottet M (2003) Mapping of quantitative trait loci for field resistance to Fusarium head blight in an European winter wheat. Theor Appl Genet 106:961–970
Hallauer AR, Miranda JB (1981) Quantitative genetics in maize breeding. Iowa State University Press
Kato K, Miura H, Sawada S (1999) QTL mapping of genes controlling ear emergence time and plant height on chromosome 5A of wheat. Theor Appl Genet 98:472–477
Klahr A, Mohler V, Herz M, Wenzel G, Schwarz G (2004) Enhanced power of QTL detection for Fusarium head blight resistance in wheat (T. aestivum) by means of codominant scoring of hemizygous markers. Mol Breed 13:289–300
Kolb FL, Bai GH, Muehlbauer GJ, Anderson JA, Smith KP, Fedak G (2001) Symposium on genetic solutions to Fusarium head blight in wheat and barley: challenges, opportunities, and imperatives. Crop Sci 41:611–619
McIntosh RA, Hart GE, Devos KM, Gale MD, Rogers WJ (1998) Catalogue of gene symbols for wheat: 1998 edition. http://wheat.pw.usda.gov/ggpages/wgc/98
Mesterházy Á (1995) Types and components of resistance to Fusarium head blight of wheat. Plant Breed 114:377–386
Miedaner T (1997) Breeding wheat and rye for resistance to Fusarium diseases. Plant Breed 116:201–220
Miedaner T, Gang G, Geiger HH (1996) Quantitative-genetic basis of aggressiveness of 42 isolates of Fusarium culmorum for winter rye head blight. Plant Dis 80:500–504
Paillard S, Schnurbusch T, Tiwari R, Messmer M, Winzeler M, Keller B, Schachermayr G (2004) QTL analysis of resistance to Fusarium head blight in Swiss winter wheat (Triticum aestivum L.). Theor Appl Genet 109:323–332
Pritsch C, Muehlbauer GJ, Bushnell WR, Somers DA, Vance CP (2000) Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol Plant Microbe In 13:159–169
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier M-H, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Rudd JC, Horsley RD, McKendry AL, Elias EM (2001) Host plant resistance genes for Fusarium head blight: sources, mechanisms, and utility in conventional breeding systems. Crop Sci 41:620–627
Schmolke M, Zimmermann G, Buerstmayr H, Schweizer G, Miedaner T, Korzun V, Ebmeyer E, Hartl L (2005) Molecular mapping of Fusarium head blight resistance in the winter wheat population Dream/Lynx. Theor Appl Genet 111:747–756
Schroeder HW, Christensen JJ (1963) Factors affecting the resistance of wheat to scab caused by Gibberella zeae. Phytopathology 53:831–838
Shen X, Ittu M, Ohm HW (2003) Quantitative trait loci conditioning resistance to Fusarium head blight in wheat line F201R. Crop Sci 43:850–857
Somers DJ, Fedak G, Savard M (2003) Molecular mapping of novel genes controlling head blight resistance and deoxynivalenol accumulation in spring wheat. Genome 46:555–564
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Steiner B, Lemmens M, Griesser M, Scholz U, Schondelmaier J, Buerstmayr H (2004) Molecular mapping of resistance to Fusarium head blight in the spring wheat cultivar Frontana. Theor Appl Genet 109:215–224
Utz HF (1995) PLABSTAT: a computer program for statistical analysis of plant breeding experiments. Version 2N. Institute of Plant Breeding, Seed Science and Population Genetics, University of Hohenheim, Germany. http://www.uni-hohenheim.de/∼ipspwww/soft.html
Utz HF, Melchinger AE (1996) PLABQTL: a computer program to map QTL. Release Version 1.1. Institute of Plant Breeding, Seed Science and Population Genetics, University of Hohenheim, Germany. http://www.uni-hohenheim.de/∼ipspwww/soft.html
Waldron BL, Moreno-Sevilla B, Anderson JA, Stack RW, Frohberg RC (1999) RFLP mapping of QTL for Fusarium head blight resistance in wheat. Crop Sci 39:805–811
Wei F, Wing RA, Wise RP (2002) Genome dynamics and evolution of the Mla (powdery mildew) resistance locus in barley. Plant Cell 14:1903–1917
Acknowledgements
The work was funded by the Bavarian State Ministry of Agriculture. We thank Dr. L. Hartl, Dr. G. Backes and Dr. M. Herrmann for support in the statistical analyses as well as Sven Jahnsen for critically reading and Bob McIntosh for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to the memory of Gerhard Schwarz who passed away during completion of this manuscript.
Rights and permissions
About this article
Cite this article
Klahr, A., Zimmermann, G., Wenzel, G. et al. Effects of environment, disease progress, plant height and heading date on the detection of QTLs for resistance to Fusarium head blight in an European winter wheat cross. Euphytica 154, 17–28 (2007). https://doi.org/10.1007/s10681-006-9264-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-006-9264-7