Abstract
Coastal aquifer is a fragile environment due to the interaction of groundwater with seawater, especially in arid environments. Groundwater along Kuwait’s Bay is polluted due to discharge of waste from desalination plants, power plants, and other anthropogenic activities. Earlier studies on submarine groundwater discharge in Kuwait’s Bay region have reported the transfer of nutrient flux from the groundwater to Kuwait's Bay. The current study focused on nitrate sources and processes governing their distribution in groundwater samples collected from the southern part of Kuwait’s Bay. The concentration of nitrate in the samples ranged from 22.7 to 803.9 mg/L. Higher values were noted in the samples collected inland and a few samples adjacent to the Bay. Spearman’s correlation analysis of the data indicated that NO3− has a strong positive correlation with SO42− and moderate positive correlation with Na + , TDS/EC. The PCA analysis and factor scores revealed the different sources for groundwater nitrate contamination as follows: leakage of sewer lines in the urban region has led to the infiltration of contaminated sewage, high saline environment due to seawater intrusion, chemical weathering, and influence of denitrifying bacteria. The health risk has resulted due to the NO3− concentration being above the standard limit for adults. Furthermore, the nitrate concentration was higher in the region adjoining the landfills. In addition, the discharge of groundwater with higher nitrate to the adjacent open water in the Bay may lead to eutrophication. Hence, proper management strategies are to be adopted to control the nitrate pollution in groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an essential source of drinking water in many countries globally (Li & Qian, 2018; Li et al., 2018). However, groundwater quality is degrading due to anthropogenic activities and natural pollution from many sources (Bain et al., 2014; Li et al., 2016). One of the common pollutants that affect groundwater quality is nitrate (NO3−). Nitrate contamination in groundwater is chiefly influenced by the presence of pollutant sources and regional environmental conditions, where it infiltrates to groundwater either naturally or due to anthropogenic activities (He et al., 2022a, b). According to WHO (2017), the maximum permissible limit of nitrate in drinking water is 50 mg/L. People are mostly exposed to polluted groundwater via two routes: ingestion of drinking water or oral exposure and cutaneous interaction (Li et al., 2019). In general, a high concentration of nitrate in water and food leads to human health risks like stomach cancer, gastrointestinal malignancies, and hypertension (Ward et al., 2018). Reduction of nitrate to nitrite and on reaction with amines and amide it forms nitrosoamine and nitrosoamide, which leads to carcinogenicity (Jain et al., 2020). In addition, the risk of methemoglobinemia, a condition in which the blood lacks the capacity to deliver enough oxygen to individual body cells, increases (Shah & Joshi, 2017).
Nitrate has a nitrogen molecule present in small levels in various environments, derived predominantly from the atmosphere and soil. Anthropogenic activities like industrial emissions also play a significant role in increasing the concentration of NO3− in groundwater through infiltration process. Apart from the atmospheric source, sources of pollution can be classified as point or non-point source. Point source regions of nitrate contamination are intense livestock confinement, leaking septic or sewage systems, and chemical or manure storage (Haller et al., 2013). Point sources can result in exceptionally high nitrate concentrations in localized areas (Zhou et al., 2015). Agricultural operations are well-known non-point sources of nitrate contamination in groundwater via fertilizers applications (Pisciotta et al., 2015). Nitrates are the most common material added to and/or produced by agricultural soils because they are highly soluble and easily leached in the form of fertilizers (Forster et al., 1982).
In addition, NO3− is the principal source of inorganic nitrogen in the soil and required for growth and development of crops (Pisciotta et al., 2015). Nitrate is an extremely soluble compound; as a result, it is rapidly absorbed by soils and leaches into groundwater (Alex et al., 2021). Generally, in many types of soil, the N2 increases with rainfall events, as the oxygen levels decrease due to the respiration process of microorganisms (Zaady et al., 1996; Kieft et al., 1987). This phenomenon in arid regions helps generate the denitrification process (Peterjohn & Schlesinger, 1990). Apart from the infiltration process, the presence of NO3− in groundwater may be due to natural minerals that contain nitrogen, especially in arid regions like soda niter or (NaNO3), niter or (KNO3), and nitrocalcite [Ca(NO3)2.4H2O], their deposition is associated with halite, gypsum, and other soluble nitrates and sulfates (Hamilton et al., 1983).
However, the deposition of environment plays a significant role in the variation of nitrate concentration. In a desert ecosystem, the primary nitrogen source comes from fixing N2 from the atmosphere by organisms, eolian transport, and deposits of NO3− salts, NH4+, and NO3− during precipitation periods (Boring et al., 1988). The atmosphere has 78% of natural nitrogen gas apart from those derived from anthropogenic sources like burning fossil fuels and other pollution sources (Uhlman et al., 2011). The deposition of atmospheric nitrogen in the soil is mainly governed by the formation of biological soil crust and canopy covers (Zaady, 2005). The deposition of nitric acid or ammonia and sea spray is considered a chief source of nitrogen inputs from the atmosphere (Michalski et al., 2004). Recent studies on nitrate pollution in groundwater have also focused on a Graphical Interface System database Normalized Difference Vegetation Index and time-series (Rodriguez-Galiano et al., 2018). The effect of hydrogeological conditions on groundwater nitrate pollution was studied by DRASTIC and HHRA model and evaluated the human health risk assessment (Zhang et al., 2021). A review on global groundwater nitrate pollution indicated that the sources of pollution were due to agriculture, industry, sewage, septic tanks, and landfills (Abascal et al., 2021). Studies have also identified that water level fluctuation and land use pattern influenced NO3− level in groundwater along with sewage, septic sewage, and manure contributed to NO3− pollution in groundwater (Panda et al., 2022).
The availability of freshwater resources is scanty, especially in arid regions like Kuwait. In Kuwait, groundwater is mainly used for domestic purposes, irrigation, and livestock rearing. The earlier studies on groundwater from different regions of Kuwait have reported higher nitrate concentrations (> 10 mg/L) in the central and southern regions of Kuwait (Mukhopadhyay et al., 2008). Since then, there has been a drastic change in living conditions, industrialization, and urbanization. An increase in population and modernization may lead to an increase in NO3− concentrations over the years. The southern part of Kuwait’s Bay has experienced rapid urbanization and industrialization. Therefore, the current study has attempted to quantify nitrate concentration in groundwater, determine the significant sources south of Kuwait’s Bay region, and perform scenarios of health risk assessment considering oral consumption for female and male adults. Furthermore, the study also aims to determine the spatial variation of the nitrate concentration in groundwater and the process governing its distribution.
Study area
The study area is located inland south of Kuwait’s Bay with a ground surface elevation varying from 4 m along the seashore to about 45 or 50 m above mean sea level in the city towards the southern suburbs (Al-Rashed & Sherif, 2001). Samples were collected chiefly in areas around agriculture, landfills, and urban areas. Kuwait City, its environs, and industrial zones cover roughly 600 2 km. The region also includes sites for oil wells, pipelines, and processing facilities (Omar & Shahid, 2013). The population for 2021 is approximately 813,618 (Worldpopulationreview, 2021), with a higher population density with various plants and trees distributed in private houses, public shopping areas, public parks, and along the streets. The most common plants and trees found around the study area are Conocarpus, Palms, Nerium Copa, and Albizia Lebbeck. Kuwait, in general, is known for having a very hot climate, especially in summer seasons, with an annual average temperature of 26 °C with an average rainfall of 110–120 mm (Dhanu Radha et al., 2022).
Geology and hydrogeology
Kuwait has two main aquifers with brackish water, mostly utilized for irrigation and animal husbandry. The clastic Kuwait Group Aquifer (KGA) that overlies the dolomitic limestone is referred to as the Dammam formation (DF). A clayey aquitard zone separates both formations (Omar et al., 1981). The thickness of KGA and DF varies from 0 to 300 m and 120 to 300 m, respectively. Both aquifers show an increase in thickness in northeastern parts of Kuwait (Mukhopadhyay et al., 2008). The groundwater in Kuwait generally flows from the southwest to the northeast part of the country, where it flows towards the sea. A study by Al-Senafy and Fadlelmawla (2014) illustrated that the submarine groundwater discharge (SGD) is the result of groundwater flowing directly into the sea as it descends a gradient whenever an inland aquifer is linked to the sea. Furthermore, the SGD was estimated by calculating the nutrient inputs and radioactive tracers along coastal areas (Al-Senafy & Fadlemawla, 2014). Al-Sulaimi (1988) reported that KGA is composed of a thick succession of unconsolidated, coarse, pebbly to gravelly sand of terrestrial fluviatile origin, potentially corresponding to the Oligocene Ghar formation and the Lower Fars formation representing the lower Miocene. The calcareous sandstone, fine-grained limestone, and muddy sand with modest amounts of granules and dispersed pebbles are the most common rock types found in KGA. In addition, the study identified the topsoil layer of eolian dust, which was transported by the northwesterly wind from Iraq to Kuwait (Al-Sulaimi & Al-Ruwaih, 2005). Kuwait City underlines Ghar and Fars formations, both belonging to KGA, with minor regional variations (Al-Sulaimi & Al-Ruwaih, 2005). Calcrete is abundant in the undifferentiated Ghar and Fars formations in the south, which is found in a thick sand succession. Gypcrete is predominantly associated with the Dibdibah formation’s top part in the north (Al-Sulaimi & Pitty, 1995). The widespread presence of calcrete, dolocrete, and gypcrete deposits, several meters thick and with limited permeability, has been documented in shallow excavations and boreholes in Kuwait City (Al-Rashed & Sherif, 2001).
Methodology
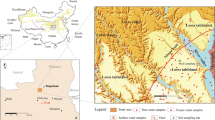
Nineteen groundwater samples were collected from monitoring wells distributed in different regions of the study area (Fig. 1) during September 2021 by adopting standard procedures (SMEWW, 2017). The location of the samples is based upon the availability of sampling wells and accessibility to the locations. The well depth of the collected samples ranges from 9.8 to 49.8 m. The samples were filtered using 0.45 µm Millipore filter paper and transferred to glass bottles for further analysis in the Water Research Center (WRC) laboratories of the Kuwait Institute for Scientific Research (KISR). Nitrate and other physical, chemical, and microbiological parameters like pH, electrical conductivity, total dissolved solids, alkalinity, major cations and anions, silica, and sulfide were analyzed using the standard methods (SMEWW, 2017; ASTM, 2009). The variables were chosen for analysis based on their respective relevance in terms of groundwater contamination potential/availability. The QC/QA procedures were adapted to the analytes as per the standard methods such as duplication, standard checks, QC sample, and ion balance error equation were used. The Statistical Package for Social Sciences (SPSS v26) software was used to analyze the data.
All groundwater sample parameters (pH, EC, TDS, SiO2, nitrate, and major ion concentrations) were subjected to descriptive statistics (minimum, maximum, and average values). The results of applying statistical analysis to the raw dataset showed that not all variables were normally distributed, and hence all data for each parameter was used in the analysis without removing any outliers. Spearmen’s correlation method is preferred because the total number of variables is less than 30, and the data is not following a normal distribution. PCA is an effective approach for reducing the dimensionality of a data set with many connected variables while preserving as much variability as possible (Ren et al., 2021; Wu et al., 2020). Factor loadings are categorized as (strong, moderate, or weak), with absolute loading values of > 0.75, 0.75–0.50, and 0.50–0.30, respectively (Liu et al., 2003). Four factors were extracted as primary principal components using PCA with Varimax rotation and Kaiser normalization, identifying the variables impacting each principal component for the physico-chemical parameters. MapInfo Software was used to generate the spatial distribution of nitrate in the study area.
The Hazard Index (HQ) is the ratio of a single drug exposure level (dose or concentration) during a specified time period to the Oral toxicity Reference (RfD) calculated for the same substance over the same time period. When the ratio is higher than one, the chemical concentration is high enough to have long-term non-carcinogenic effects.
This value is according to the USEPA’s Integrated Risk Information System (USEPA, 2014). The NO3− exposure dose is calculated using the estimated daily intake (EDI) of NO3− from groundwater, by calculating various variables (Table 1). Equation 1 from the US Environmental Protection Agency was used to compute the EDI values. EDI can be determined using the following equation (Ji et al., 2020; Wang & Li, 2022; Wei et al., 2022):
Results and discussion
Statistical analysis
The pH values varied between 7.33 and 8.26, showing neutral to basic water type. Also, it indicates the type of sediments that are alkaline and carbonate in nature. Limestone and carbonate minerals are two substances that may help to buffer pH variations in water (Jeong et al., 2020). Calcium carbonate (CaCO3) and other bicarbonates may neutralize pH by combining hydrogen or hydroxyl ions (McNally & Metha, 2004). The DO (Dissolved Oxygen) values varied from 4.9 to 7.9 mg/L. The analytical results of samples reflect nitrate concentrations ranging from 22.7 to 803.7 mg/L with an average of 143.8 mg/L (Table 2). The highest concentration of nitrate was found in the groundwater sample collected from Abdullah Al Mubarak (AB.M-2). ABM is a residential area adjoining the south side of a non-active landfill area called Jleeb Al Shuyoukh (Fig. 2).
Distribution of active and non-active landfill locations in Kuwait. (Modified after EPA, 2010)
At an approximate distance of 9 km from the south of AB.M-2, two large landfills were reported by EPA (2010), located in the north and south parts of the seventh ring road, whereas the north landfill is non-active and the one in the south is active. The deepest sample (49.8 m) was collected from well SUL-7, with a nitrate concentration of 535.9 mg/L, which considers the second-highest value of NO3−. SUL-7 is located along the Sulabiya road, and on the west and northwest side of the well sample are livestock farms, and agricultural areas were observed, respectively. Another old landfill in the Kabd area is found almost south of sampled well at an approximate distance of 12 km. This landfill is non-active and characterized by animal waste dumping sites (EPA, 2010). The lowest NO3− concentration recorded was observed in sample BNQ-1, with a value of 22.7 mg/L. BNQ-1 sample located in Bnied Algar area along the gulf road near the Arabian gulf sea considered an urbanized area with tall medical buildings and apartments.
Spearman’s correlation
The correlation coefficient measures the relationship between variables. The correlation coefficient of 0.25 to 0.5 and > 0.5 are considered moderate correlation and good positive correlation, respectively. The correlation coefficient was observed only for sixteen parameters (Table 3). The ion distribution in a groundwater sample is non-parametric. Therefore, Spearman’s correlation was attempted to understand the relationship between nitrate and other parameters. EC and TDS exhibited an excellent positive correlation between them, with the highest value (r = 0.98) and had a moderate correlation with pH, which reflects that electrical conductivity (EC) is dependent on total dissolved solids (TDS). Na+ and Cl− resulted in a good positive correlation (r = 0.79), indicating the saline nature, especially in arid and semi-arid regions (Ganyaglo et al., 2010), due to the availability of salt deposits in that area due to chemical weathering or saline water infiltration.
The molar NO3−/Cl− a ratio of the groundwater is generally assessed to the surface water and the rainwater to assess the conservative nature of the coupled ions and to unravel the vertical migration history during recharges (Gates et al., 2008). On comparing Cl− and NO3− ratios to depth profiles, few samples showed that Cl− increases with depth and NO3 decreases (Fig. 3). It is due to the process of progressive evaporation (Phillips, 1994). Moreover, the decrease of NO3 with depth results from the plant uptake and biological activity in the shallow zone (Jobbagy & Jackson, 2001). Especially Cl− ion is excluded from soil vapor, and the plant membranes avoid uptake during transpiration. As water returns to the atmosphere, subsequent rain enhances the Cl− infiltration to the deeper layers. The second trend shows that nitrogen increases with depth which might be governed by EOR that is adopted in the adjoining oil fields, where the nitrogen is facilitated by structural and geological controls (Carman, 1996).
The correlation between Ca2+ and SO42− is moderate, indicating no significant mineral dissolution, especially with gypsum and anhydrite in the study area. NO3− has a strong positive correlation with SO42− with a value of r = 0.54, which probably indicates the presence of nitrogen-type fertilizers that infiltrated to groundwater (Babiker, 2004). Sulfate is a common anion in water that has been overlooked as a source of groundwater contamination in the past (Wang and Zhang, 2019). Through geothermal processes, seawater intrusion, and atmospheric deposition, SO42− is released into the water as part of residential wastewaters, industrial wastes, and wastewaters through mining, smelting, steel production, kraft pulp, paper mills, and flue gas desulphurization circuits. Sulfate minerals (e.g., gypsum), oxidation of sulfide minerals (e.g., pyrite), rainfall, and volcanic activity are responsible for greater amounts of SO42− in groundwater (Fernando et al., 2018). However, the study area is dominated by sedimentary rocks. And the surrounding area, mainly southwest of the state of Kuwait, has many oil fields. Enhance Oil Recovery (EOR) preferred in the oil field could be another reason for increasing nitrate concentration. The generation of H2S is observed during the secondary recovery of oil due to the development of SRB (Sulfate Reducing Bacteria) during seawater injection to maintain high pressure during oil extraction (Vigneron et al., 2017). This development of H2S is mainly due to the processes of high sulfatic in reducing environment in seawater.
Moreover, this sulfate is anaerobically reduced by bacteria and organic acids, hydrocarbon, and H2, facilitating an ideal environment for SRB. The mechanism involved in this process is sulfate anaerobically reduced by bacteria SRB converted into H2S. Hence to prevent the formation of H2S, nitrate is injected into a system where the growth of NRB is enhanced as it is a high-energy electron accepter, then sulfate, thereby reducing the formation of H2S (Dolfing & Hubert, 2017). Further, the formation of sulfur or sulfate from sulfides could result from oxidation process due to chemo lithospheric bacteria.
The dissolved sulfides also react abiotically with NRB (Nitrogen Reducing Bacteria) (Davidova et al., 2001)
A moderate positive correlation was observed between NO3− with Na + (r = 0.40), Li + (r = 0.32), TDS (r = 0.35), and EC (r = 0.31). Lithium in groundwater may come from various sources, including waste disposal leachate. Kjolholt et al. (2003) determined a concentration of 330 g/L in leachate from a waste disposal site. Lithium excreted from medicinal users into wastewater has been identified as a possible source in septic systems and sewage treatment facilities (Bexfield et al., 2019). Additionally, soils’ lithium concentrations have been linked to synthetic fertilizers made from rock phosphates and naturally occurring brines (Ebensperger et al., 2005). Weak correlations were observed between NO3− and F−, similarly with K+ and Ca2+. NO3− also shows a weak negative correlation with HCO3 (r = − 0.09), indicating that denitrification took place in groundwater wherein acts as nitrate removal (Laftouhi et al., 2003). The presence of dissolved oxygen in the arid environment deters the process of biotic denitrification and favors the persistence of NO3− in the saturated zone for a very long period (Edmunds, 2003). Interrelationships between NO3− and other variables were also considered from previous studies to characterize the sources and processes leading to nitrate occurrence.
Principal component analysis (PCA)
Four PCA components of eigenvalue greater than 1 are of variance up to 80% (Table 4). The first component (PCA-1) is the most significant in PCA (37% variance) with loadings of EC, TDS, Cl−, NO3−, SO42−, Li+, and Na+ while an intermediate representation of pH, K+, and Mg2+ was observed. As a result, the substantial positive loadings of the principal ions and EC and TDS in PCA-1 imply that the groundwater in the region is brackish/saline, indicated by the association of Na+, Cl−, and TDS. This saline water reflects anthropogenic contamination by the loadings of NO3−, SO42−, and Li+ in the study area, such as industrial, agricultural, and domestic sewage (Yang et al., 2013). Hence, the PCA-1 reflects sewage contamination, either septic tanks discharge or leakage of sewage pipelines (Mukhopadhyay et al., 2007). The second component (PCA-2) represents a 25% variance with the association between Cl−, NH4+, and Ca2+, which is mainly due to the utilization of chemical fertilizers such as NH4HCO3, CO(NH2)2, NH4NO3, (NH4+)2SO4, Ca(H2PO4)2, and KCl (Zhang et al., 2014). The third component (PCA-3) with 11% variance attributes K+, Ca2+, Mg2+, and silica. This factor represents rock-water interaction between the groundwater and the aquifer few reactions as shown in Eqs. (5 and 6) are listed below:
Reflecting the fact that chemical weathering process is also a predominant factor in the region, the fourth component (PCA-4) shows a 7% variance with an association between bicarbonate, fluoride, and silica, where HCO3− illustrates the impact of carbonate mineral dissolution. An experiment by Gao et al. (2019) illustrated that the increased fluoride contents in the microcosm flasks showed that microbial activity had induced fluoride release from the sediment into aqueous solutions. The experiment also showed that the fluoride culture kept growing despite the microbial population not increasing, while the nitrate showed a decreased trend during the first days of the experiment. In a natural groundwater-sediment system, the interaction between microbes and F-bearing sediments is most likely an ongoing process. Nitrate, acetate, and formate entirely vanish in the culture group but remain in the control group, indicating that the indigenous microorganisms in the culture experiment absorb them (Gao et al., 2019).
Denitrifying bacteria also utilize nitrate oxygen to create energy from organic carbon and release bicarbonate and nitrogen as a byproduct into the system (Babiker, 2004). Dissolved oxygen ranging from 1 to 6 mg/L favors the process of infiltrations, and thus the absence of Mn, and NH4+ also reduces the possibility of denitrification.
Factor score (FS)
Factor score analysis is a multivariate statistical method widely used to characterize the general connections between multiple observable variables in terms of a possibly smaller number of unobserved variables, referred to as factors, with minor information loss (Liang et al., 2020). The FS values of samples concerning the described factors (Table 5) show that the samples from SDQ-1, AB.M-2, SUL-3, and SUL-7 in F1 reflect the high effect of nitrate desert pools. The second factor score of samples KTN-2, BNQ-1, BYN-2, MSF-3, SUL-1, SUL-3, SUL-7, and ADL-1 reflects a high saline environment due to the predominance of Ca2+, Cl−, and Mg2+ which reflects hypersaline groundwater. The third factor represented in locations AB.M-1, AB.M-2, HTN-1, AND-1, BYN-2, MSF-3, SUL-3, ADL-1, and SRQ-4 indicating the predominance of chemical weathering. The FS for the fourth PCA represents samples KTN-2, AND-1, AND-2, BNQ-1, MSF-3, SUL-1, SUL-3, SUL-4, and SRQ-4, illustrating the influence of denitrifying bacteria associated with the release of HCO3- into groundwaters. However, it is notable that all four factors are active in SUL-3, and no significant factor is active in FIH, QBL-2, and JLB-1. The spatial distribution of all the factor scores regarding the association between each sample was conducted (Fig. 4).
Sources and distribution of nitrate
Further to have a general understanding of NO3− concentration and distribution around the study area, a contour map was generated (Fig. 5). It was observed that high nitrate concentration was found in the southern parts of the study area predominantly represented by PCA 1 and decreased gradually in the northeastern part. This might be due to the groundwater flow direction in Kuwait that moves from the southwest to the northeast towards the sea.
A study by Adnan Akber et al. (2006) reported that groundwater samples collected from Dammam and Kuwait aquifers contain nitrite-oxidizing and reducing bacteria. Similarly, in the present study, higher nitrate samples would contribute to microorganisms, and few samples have low values due to the consumption of NO3− by microbes. Within the groundwater, nitrate is the only dissolved inorganic nitrogen pool that can be detected. Given the rarity of mineral nitrate sources, the bulk of the nitrate came from microbial-degraded nitrogen fertilizers. The amounts of soluble reactive phosphorus were low, suggesting that phosphate was precipitated as insoluble apatite/fluorapatite (Gao et al., 2019).
The best conditions for nitrification are a pH of about 7 and oxygen (Keeney, 1970a, b). High temperature in arid regions due to high solar radiation favored by higher soil pH leads to the non-availability of NH3 in the soil, as it is lost to the atmosphere through volatilization (Wang et al., 2014). Studies have reported such conditions globally, and the presence of NH3 in soil indicated low air temperature and pH (Hu et al., 2008). Since the study area is located south of Kuwait’s Bay, the probability of oxidation of aqueous H2O2 and gaseous SO2 along with NH3 present in the atmosphere forms NH4HSO4 aerosols. This aerosol with higher relative humidity leads to acidic droplet formation. When the aerosol is associated with coastal CaCO3, pH increases loading and the volatilization of NH3, and the formation of CaSO4. Both cases are represented by the following reactions (Bidoglio & Stumm, 2013).
In general, nutrients and organic matter serve as potential energy sources for bacteria (Clark et al., 2003). Since nitrate is one of the nutrients being used as a food resource for a few microorganisms (Oskay & Yalcin, 2013), some samples contain low nitrate. Earlier studies have reported that the microbial population lowers nitrate concentrations (Mukhopadhyay et al., 2007). The variation of nitrate with respect to microbes also depends on the type of microbes present in the region. For instance, low nitrate is observed if the total viable counts are high.
Similarly, coliform bacteria are correlated to nitrate. Hence, nitrate could be high or low depending on the type of bacteria. Fecal coliform may reduce the nitrate, and it is also to be noted that higher nitrate indicates high total coliform bacteria (Vasudevan et al., 2021). It is also noted that cyanobacteria were reported to achieve nitrogen fixation in the warmer deserts (Gates et al., 2008). It was identified that the nitrogen derived by nitrification processes or due to nitrogen fixation facilitated by the microorganism might be transported either by surficial processes like eolian dust or by subsurface processes via infiltration to groundwaters in the arid regions. The nitrate in the playa sediments is associated with nitrogen-fixing bacteria (Ericksen, 1983). Water contains nitrogen in organic form decomposes into inorganic nitrogen either as free ammonia or ammonium salts. It further decomposes into nitrite and oxidizes to nitrate (Vasudevan et al., 2021).
Oxidation of NH4+could be another source of nitrate, and the NH4+ may be either derived from nitrogen-fixing bacteria or organic marine soils (Moreno-Castilla et al., 2003). Nitrification is considered one of the essential sources of nitrate in slightly wetter arid regions (Leatham et al., 1983). During nitrification of NH4+ through microbes, the oxygen atom is derived from both atmosphere and water molecules (Castignetti & Hollocher, 1984).
Desert NO3 −
Kuwait’s original soils are mainly non-saline, but when there is influence from seawater in coastal locations, significant salinity levels occur; varying amounts of gypsum and calcium carbonate add electrolytes to the soil solution (Omar & Shahid, 2013). The pH is buffered between mildly to moderately alkaline (7.4–8.3), which is higher than the ideal values (6.7–7.3), where most nutrients become accessible to plants (Tisdale et al., 1993). Alkaline pH, aerobic condition with less water content in the soil zone, low microbial population, and organic matter in the desert environment prevent denitrification and help the accumulated NO3− to be stable (Hartsough et al., 2001). Few studies in geographical conditions similar to Kuwait have also reported higher desert nitrate (Table 6). NO3− in the desert was derived from atmospheric sources through photochemical reaction and oxidation of N2 in the atmosphere. It is interesting to note that additional sources like plant recycling, weathering, and nitrogen fixation are also critical factors for higher NO3− concentration as observed in Mojave region (Michalski et al., 2004). Desert dunes have also been identified as geomorphic units favoring the accumulation of soil N2, especially the region with fixed dunes that accumulate NH4+. Thus, the nitrate could accumulate over thousands of years and be mobilized/ migrated to groundwater when there is a change in land use pattern and climate (Hong et al., 2014). The studies on the subsoil N in the arid shrublands and hyper-arid regions have indicated higher levels of N2 in the vadose zone (Walvoord et al., 2003).
A more extensive reservoir of nitrate in these regions, referred to as the “nitrate pool,” was put forth as a significant contributor of nitrate to groundwater (Al-Taani and Al-Qudah, 2013; Ma et al., 2012). The subsurface accumulation of NO3− in these arid regions varies with respect to microbial activity, growth of plants, physiography of land surface, and hydraulic properties of soil. It also varies concerning climatic conditions, temperate to semi-arid conditions at different degrees. The natural process in arid countries depends on the time scales and periods of intense rain events and hyper-arid conditions during geological time scales. The hard calcrete is the gatch layer preventing infiltration in several locations of Kuwait. The calcic and/or gypsic pan, often known as gatch, is prevalent in several Kuwaiti soils. A petrocalcic or petrogypsic horizon is a continuous pan produced from precipitated and cemented carbonate or gypsum that acts as a barrier to plant roots (Omar & Shahid, 2013). However, evaporation enrichment and leaching govern NO3− in the subsoil layer. Hence, the gatch layer plays a vital role in maintaining the NO3− at a certain depth, noting that as residence time increases at that layer, the nitrate will continue to increase.
Hazard Index
Groundwater is polluted due to the intrusion of seawater from south of Kuwait Bay. The calculation for the human health risk is assumed if the groundwater is directly consumed apart from agricultural product intake. This infers a possibility of risk that the consumption of direct groundwater may result in health issues but for the sea foods/vegetables, the consumption is based on the quantity and type of the product. In order to assess the possible risks of pollutants to human health, a process called health risk assessment essentially establishes a relationship between pollutant loads and human health (Ji et al., 2020). In this study, the health risk assessment was used to identify the negative impact of exposure to nitrate in groundwater on humans’ health. Therefore, Hazard Index (HQ) was calculated for the groundwater wells close to south Kuwait Bay and HQ values represent female and male adult’s values (Eq. 1). The estimated daily intake per unit weight through oral exposure pathway [mg/(kg day)] can be calculated by Eq. (2). Oral, dermal, and inhalational routes are the three major ways that a person might be exposed to pollutants. Typically, nitrate exposure occurs mostly via consumption (Soleimani et al., 2022) and it was considered for this study.
The HQ results are represented in Table 7. It was observed that out of nineteen sample locations, thirteen samples would have significant health effects on adult consumers, where the HQ value is above 1. The minimum HQ value for adult female and male is 0.446 and 0.407 respectively. whereas the maximum HQ value for adults female and male is 25.29 and 14.42 respectively. Therefore, it is inferred that the adult females will be more exposed to nitrate via ingestion than adult males in the current study.
Conclusion
It was concluded from the results that 13 out of 19 samples had high nitrate concentrations ranging from 55.6 to 803.9 mg/L. The higher concentrations of nitrate and sulfate reflect the anthropogenic influence in the study area. In addition, urbanization may increase nitrate concentration in groundwater due to increased sewer drains, usage of fertilizers in landscapes, and plantations in private homes and along the roads, where the water infiltrates below ground and facilitates nitrate contamination. The lithology in the study area, coupled with the groundwater flow direction, resulted in increased nitrate concentration in samples along the flow path. Moreover, the Spearman correlation between sulfate and nitrate illustrated a good positive correlation, indicating the sewage discharge either leakage from pipelines or septic tanks.
Nonetheless, the PCA results showed the anthropogenic impact compared to the geogenic process. In addition, the factor scores also reflected the significant role of anthropogenic activity in the study area. Overall, septic system and sanitary-sewer effluents, domestic animal wastes, penetration of runoff from roadways and parking lots, house and farm fertilizer use, and atmospheric deposition are all familiar sources of nutrients in urban settings. The health risk assessment scenario resulted in a total of thirteen sample locations that had an HQ value above 1, indicating health risk on adult humans. Further investigations are in process to characterize the leakage of subsurface sewerage networks and the role of geological structure in the oxidation of nitrogen used in enhanced oil recovery.
References
Abascal, E., Gómez-Coma, L., Ortiz, I., & Ortiz, A. (2021). Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Science of the Total Environment, 152233.
Akber, A., Mukhopadhyay, E., Al-Awadi, H., Al-Qallaf, A., Al-Haddad, E. A., & Bhandary, H. (2006). Investigations on distribution of nitrogen compounds in the groundwater of kuwait, WM002C, Hydrology Department, Water Resources Division, KISR.
Alex, R., Kitalika, A., Mogusu, E., & Njau, K. (2021). Sources of nitrate in ground water aquifers of the semiarid region of Tanzania. Geofluids, 2021, 1–20. https://doi.org/10.1155/2021/6673013
Ali, A., & Chidambaram, S. (2020). Assessment of trace inorganic contaminates in water and sediment to address its impact on common fish varieties along Kuwait Bay. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00559-6
Al-Rashed, M., & Sherif, M. (2001). Hydrogeological aspects of groundwater drainage of the urban areas in Kuwait City. Hydrological Processes, 15(5), 777–795. https://doi.org/10.1002/hyp.179
Al-Senafy, A., & Fadlelmawla, A. (2014). Impacts of submarine groundwater discharge on Kuwait Bay. WIT Transactions on Ecology and the Environment, 182, 165–173.
Al-Sulaimi, J. (1988). Calcrete and near-surface geology of Kuwait City and suburbs, Kuwait. Arabian Gulf. Sedimentary Geology, 54(4), 331–345. https://doi.org/10.1016/0037-0738(88)90040-1
Al-Sulaimi, J., & Al-Ruwaih, F. (2005). Geology and natural resources of Kuwait. Kuwait: Kuwait University, Authorship, Translation & Publication Committee.
Al-Sulaimi, J. S., & Pitty, A. F. (1995). Origin and depositional model of Wadi Al-Batin and its associated alluvial fan, Saudi Arabia and Kuwait. Sedimentary Geology, 97(3–4), 203–229.
Al-Taani, A. A., & Al-Qudah, K. A. (2013). Investigation of desert subsoil nitrate in Northeastern Badia of Jordan. Science of the Total Environment, 442, 111–115.
Aranibar, J. N., Villagra, P. E., Gomez, M. L., Jobbágy, E., Quiroga, M., Wuilloud, R. G., ... & Guevara, A. (2011). Nitrate dynamics in the soil and unconfined aquifer in arid groundwater coupled ecosystems of the Monte desert, Argentina. Journal of Geophysical Research: Biogeosciences, 116(G4).
ASTM. (2009). American Standard Test method for the determination of dissolved alkali and alkaline earth cations and ammonium in water and wastewater by ion chromatography. D6919-09. Retrieved December 20, 2021, from https://webstore.ansi.org/standards/astm/astmd691909
Babiker, I. (2004). Assessment of groundwater contamination by nitrate leaching from intensive vegetable cultivation using geographical information system. Environment International, 29(8), 1009–1017. https://doi.org/10.1016/s0160-4120(03)00095-3
Bain, R., Cronk, R., Wright, J., Yang, H., Slaymaker, T., & Bartram, J. (2014). Fecal contamination of drinking-water in low-and middle-income countries: a systematic review and meta-analysis. PLoS Medicine, 11(5), e1001644.
Bexfield, L. M., Toccalino, P. L., Belitz, K., Foreman, W. T., & Furlong, E. T. (2019). Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States. Environmental Science & Technology, 53(6), 2950–2960.
Bidoglio, G., & Stumm, W. (Eds.). (2013). Chemistry of aquatic systems: Local and global perspectives (Vol. 5). Springer Science & Business Media.
Boring, L. R., Swank, W. T., Waide, J. B., & Henderson, G. S. (1988). Sources, fates, and impacts of nitrogen inputs to terrestrial ecosystems: Review and synthesis. Biogeochemistry, 6, 119–159.
Carman, G. J. (1996). Structural elements of onshore Kuwait. GeoArabia, 1(2), 239–266.
Castignetti, D., & Hollocher, T. C. (1984). Heterotrophic nitrification among denitrifiers. Applied and Environmental Microbiology, 47(4), 620–623.
Clark, A., Turner, T., Dorothy, K. P., Goutham, J., Kalavati, C., & Rajanna, B. (2003). Health hazards due to pollution of waters along the coast of Visakhapatnam, east coast of India. Ecotoxicology and Environmental Safety, 56(3), 390–397.
Davidova, I., Hicks, M. S., Fedorak, P. M., & Suflita, J. M. (2001). The influence of nitrate on microbial processes in oil industry production waters. Journal of Industrial Microbiology and Biotechnology, 27(2), 80–86.
Dhanu Radha, S. V. V., Sabarathinam, C., Al-Ayyadhi, N., Al-Ajeel, F. K., Al-Qallaf, H., & Akber, A. (2022). Spatial and temporal variation of dissolved CO2 in rainwater from an arid region with special focus on its association with DIC and pCO2. Environmental Earth Sciences, 81(4), 1–19.
Dolfing, J., & Hubert, C. R. (2017). Using thermodynamics to predict the outcomes of nitrate-based oil reservoir souring control interventions. Frontiers in Microbiology, 8, 2575.
Ebensperger, A., Maxwell, P., & Moscoso, C. (2005). The lithium industry: its recent evolution and future prospects. Resources Policy, 30(3), 218–231.
Edmunds, W. M. (2003). Renewable and non-renewable groundwater in semi-arid and arid regions. In Developments in water science (Vol. 50, pp. 265-280). Elsevier.
Environment Public Authority (EPA). (2010). Waste management atlas of Kuwait.
Ericksen, G. E. (1983). The Chilean Nitrate Deposits: The origin of the Chilean nitrate deposits, which contain a unique group of saline minerals, has provoked lively discussion for more than 100 years. American Scientist, 71(4), 366–374.
Fernando, W. A. M., Ilankoon, I. M. S. K., Syed, T. H., & Yellishetty, M. (2018). Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review. Minerals Engineering, 117, 74–90.
Forster, S. S. D., Cripps, A. C., & Smith-Carington, A. (1982). Nitrate leaching to groundwater. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 296(1082), 477–489.
Ganyaglo, S., Banoeng-Yakubo, B., Osae, S., Dampare, S., & Fianko, J. (2010). Water quality assessment of groundwater in some rock types in parts of the eastern region of Ghana. Environmental Earth Sciences, 62(5), 1055–1069. https://doi.org/10.1007/s12665-010-0594-3
Gao, X., Luo, W., Luo, X., Li, C., Zhang, X., & Wang, Y. (2019). Indigenous microbes induced fluoride release from aquifer sediments. Environmental Pollution, 252, 580–590.
Gates, A. J., Richardson, D. J., & Butt, J. N. (2008). Voltammetric characterization of the aerobic energy-dissipating nitrate reductase of Paracoccus pantotrophus: exploring the activity of a redox-balancing enzyme as a function of electrochemical potential. Biochemical Journal, 409(1), 159–168.
Haller, L., McCarthy, P., O’Brien, T., Riehle, J., & Stuhldreher, T. (2013). Nitrate pollution of groundwater. 2014: Alpha Water Systems INC.
Hamilton, W. R., Woolley, A. R., & Bishop, A. C. (1983). Minerals, rocks, and fossils. Country Life Books.
Hartsough, P., Tyler, S. W., Sterling, J., & Walvoord, M. (2001). A 14.6 kyr record of nitrogen flux from desert soil profiles as inferred from vadose zone pore waters. Geophysical Research Letters, 28(15), 2955–2958.
He, S., Wu, J., Wang, D., & He, X. (2022a). Predictive modeling of groundwater nitrate pollution and evaluating its main impact factors using random forest. Chemosphere, 290, 133388. https://doi.org/10.1016/j.chemosphere.2021.133388
He, S., Li, P., Su, F., Wang, D., & Ren, X. (2022b). Identification and apportionment of shallow groundwater nitrate pollution in Weining Plain, northwest China, using hydrochemical indices, nitrate stable isotopes, and the new Bayesian stable isotope mixing model (MixSIAR). Environmental Pollution, 298, 118852. https://doi.org/10.1016/j.envpol.2022.118852
Hong, B., Gasse, F., Uchida, M., Hong, Y., Leng, X., Shibata, Y., ... & Wang, Y. (2014). Increasing summer rainfall in arid eastern-Central Asia over the past 8500 years. Scientific Reports, 4(1), 1–10.
Hu, M., Wu, Z., Slanina, J., Lin, P., Liu, S., & Zeng, L. (2008). Acidic gases, ammonia and water-soluble ions in PM2. 5 at a coastal site in the Pearl River Delta, China. Atmospheric Environment, 42(25), 6310–6320.
Jain, D., Chaudhary, P., Varshney, N., & Janmeda, P. (2020). Carcinogenic effects of N-nitroso compounds in the environment. Environment Conservation Journal, 21(3), 25–41. https://doi.org/10.36953/ECJ.2020.21304
Jeong, J., Jeen, S. W., Hwang, H. T., & Lee, K. K. (2020). Changes in geochemical composition of groundwater due to CO2 leakage in various geological media. Water, 12(9), 2597.
Ji, Y., Wu, J., Wang, Y., Elumalai, V., & Subramani, T. (2020). Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain. China. Exposure and Health, 12(3), 469–485. https://doi.org/10.1007/s12403-020-00357-6
Jin, Z., Zhu, Y., Li, X., Dong, Y., & An, Z. (2015). Soil N retention and nitrate leaching in three types of dunes in the Mu Us desert of China. Scientific Reports, 5(1), 1–8.
Jobbagy, E. G., & Jackson, R. B. (2001). The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry, 53(1), 51–77.
Keeney, D. (1970a). Nitrates in plants and waters1,2. Journal of Milk and Food Technology, 33(10), 425–432. https://doi.org/10.4315/0022-2747-33.10.425
Keeney, D. R. (1970b). Nitrates in plants and waters. Journal of Milk and Food Technology, 33(10), 425–432.
Kieft, T. L., Soroker, E., & Firestone, M. K. (1987). Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biology and Biochemistry, 19, 119–126. https://doi.org/10.1016/0038-0717(87)90070-8
Kjolholt, J., Stuer-Lauridsen, F., Skibsted Mogensen, A., & Havelund, S. (2003). The elements in the second rank-lithium. Copenhagen, Denmark: Miljoministeriet.
Laftouhi, N., Vanclooster, M., Jalal, M., Witam, O., Aboufirassi, M., Bahir, M., & Persoons, É. (2003). Groundwater nitrate pollution in the Essaouira Basin (Morocco). Comptes Rendus Geoscience, 335(3), 307–317. https://doi.org/10.1016/s1631-0713(03)00025-7
Leatham, S., Huckins, L. N., & Jacobson, R. L. (1983). Nitrate in Nevada playas. Available from the National Technical Information Service, Springfield VA 22161 as PB 83-265025, Price codes: A 07 in paper copy, A 01 in microfiche. Publication, (41086).
Li, P., & Qian, H. (2018). Water resources research to support a sustainable China. International Journal of Water Resources Development, 34(3), 327–336. https://doi.org/10.1080/07900627.2018.1452723
Li, P., Wu, J., Qian, H., Zhang, Y., Yang, N., Jing, L., & Yu, P. (2016). Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Exposure and Health, 8, 331–348. https://doi.org/10.1007/s12403-016-0193-y
Li, P., Qian, H., & Wu, J. (2018). Conjunctive use of groundwater and surface water to reduce soil salinization in the Yinchuan Plain, North-West China. International Journal of Water Resources Development, 34(3), 337–353. https://doi.org/10.1080/07900627.2018.1443059
Li, P., He, X., & Guo, W. (2019). Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Human and Ecological Risk Assessment, 25(1–2), 11–31. https://doi.org/10.1080/10807039.2018.1553612
Liang, C., Wang, C., Wang, S., Chang, T., & Chen, J. (2020). Application of factor analysis for characterizing the relationships between groundwater quality and land use in Taiwan’s Pingtung Plain. Sustainability, 12(24), 10608. https://doi.org/10.3390/su122410608
Liu, C. W., Lin, K. H., & Kuo, Y. M. (2003). Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Science of the Total Environment, 313(1–3), 77–89.
Lyons, W. B., Welch, S. A., Gardner, C. B., Sharifi, A., AghaKouchak, A., Mashkour, M., ... & Akhani, H. (2020). The hydrogeochemistry of shallow groundwater from Lut Desert, Iran: The hottest place on Earth. Journal of Arid Environments, 178, 104143.
Ma, J., Wang, Y., Zhao, Y., Jin, X., Ning, N., Edmunds, W. M., & Zhou, X. (2012). Spatial distribution of chloride and nitrate within an unsaturated dune sand of a cold-arid desert: Implications for paleoenvironmental records. CATENA, 96, 68–75.
McMahon, P. B., Böhlke, J. K., & Christenson, S. C. (2004). Geochemistry, radiocarbon ages, and paleorecharge conditions along a transect in the central High Plains aquifer, southwestern Kansas, USA. Applied Geochemistry, 19(11), 1655–1686.
McNally, W. H., & Mehta, A. J. (2004). Sediment transport and deposition in estuaries (sample chapter). In Encyclopedia of Life Support Systesm (EOLSS): Coastal Zones and Estuaries. Retrieved from http://www.eolss.net/sample-chapters/c09/E2-06-01-04.pdf
Michalski, G., Böhlke, J. K., & Thiemens, M. (2004). Long term atmospheric deposition as the source of nitrate and other salts in the Atacama Desert, Chile: New evidence from mass-independent oxygen isotopic compositions. Geochimica et Cosmochimica Acta, 68(20), 4023–4038.
Moreno-Castilla, C., Bautista-Toledo, I., Ferro-Garcıa, M. A., & Rivera-Utrilla, J. (2003). Influence of support surface properties on activity of bacteria immobilised on activated carbons for water denitrification. Carbon, 41(9), 1743–1749.
Mukhopadhyay, A., Akber, A., Azrag, E., Al-Awadi, E., Al-Hadad, A., & Al-Qallaf, H. (2008). Distribution and source of Nitrogen compounds in the groundwater of Kuwait. Nova Science.
Mukhopadhyay, A., Al-Haddad, A., Al-Otaibi, M., & Al-Senafy, M. (2007). Occurrence of hydrogen sulfide in the groundwater of Kuwait. Environmental Geology, 52(6), 1151–1161.
Omar, S. A., Al-Yacoubi, A., & Senay, Y. (1981). Geology and groundwater hydrology of the state of Kuwait. Journal of the Gulf and Arabian Peninsula Studies, 1, 5–67.
Omar, S. A., & Shahid, S. A. (2013). Reconnaissance Soil survey for the state of Kuwait. In Developments in soil classification, land use planning and policy implications (pp. 85–107). Springer, Dordrecht.
Oskay, M., & Yalcin, H. T. (2013). Screening of yeast strains for pectinolytic activity: Effects of different carbon and nitrogen sources in submerged fermentations. Journal of Biological Sciences, 15(3), 89–96.
Panda, B., Chidambaram, S., Snow, D., Singh, D. K., & Ramanathan, A. L. (2022). Source apportionment and health risk assessment of nitrate in foothill aquifers of Western Ghats. South India. Ecotoxicology and Environmental Safety, 229, 113075.
Peterjohn, W. T., & Schlesinger, W. H. (1990). Nitrogen loss from deserts in the southwestern United States. Biogeochemistry, 10(1), 67–79.
Phillips, F. M. (1994). Environmental tracers for water movement in desert soils of the American southwest. Soil Science Society of America Journal, 58, 15–24.
Pisciotta, A., Cusimano, G., & Favara, R. (2015). Groundwater nitrate risk assessment using intrinsic vulnerability methods: A comparative study of environmental impact by intensive farming in the Mediterranean region of Sicily, Italy. Journal of Geochemical Exploration, 156, 89–100. https://doi.org/10.1016/j.gexplo.2015.05.002
Qin, Y., Li, Y., Bao, H., Liu, F., Hou, K., Wan, D., & Zhang, C. (2012). Massive atmospheric nitrate accumulation in a continental interior desert, northwestern China. Geology, 40(7), 623–626.
Rahman, A., Mondal, N., & Tiwari, K. (2021). Anthropogenic nitrate in groundwater and its health risks in the view of background concentration in a semi arid area of Rajasthan, India. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-021-88600-1
Ren, X., Li, P., He, X., Su, F., & Elumalai, V. (2021). Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Archives of Environmental Contamination and Toxicology, 80(1), 74–91. https://doi.org/10.1007/s00244-020-00772-5
Rodriguez-Galiano, V. F., Luque-Espinar, J. A., Chica-Olmo, M., & Mendes, M. P. (2018). Feature selection approaches for predictive modelling of groundwater nitrate pollution: An evaluation of filters, embedded and wrapper methods. Science of the Total Environment, 624, 661–672.
Samayamanthula, D. R., Bhatti, M. E., Al-Enazi, N. F., Rashid, Z. A. J., Al-Kandari, S. A., & Jacob, H. (2021). Determination of Tri halo methanes in indoor swimming pools, Kuwait and development of health risk index. International Journal of Environmental Science and Technology, 19(8), 7575–7588.
Shah, K. A., & Joshi, G. S. (2017). Evaluation of water quality index for River Sabarmati, Gujarat. India. Applied Water Science, 7(3), 1349–1358.
SMEWW. (2017). Standard methods for the examination of water and waste water (23rd ed.). Denver, CO, USA: American Water Works Association. Retrieved December 23, 2021, from https://engage.awwa.org/PersonifyEbusiness/
Soleimani, H., Nasri, O., Ghoochani, M., Azhdarpoor, A., Dehghani, M., Radfard, M., & Heydari, M. (2022). Groundwater quality evaluation and risk assessment of nitrate using Monte Carlo simulation and sensitivity analysis in rural areas of Divandarreh County, Kurdistan province. Iran. International Journal of Environmental Analytical Chemistry, 102(10), 2213–2231.
Soomro, F., Rafique, T., Michalski, G., Ali, S. A., Naseem, S., & Khan, M. U. (2017). Occurrence and delineation of high nitrate contamination in the groundwater of Mithi sub-district, Thar Desert. Pakistan. Environmental Earth Sciences, 76(10), 355.
Tisdale, S. L., Nelson, W. L., Beaton, J. D., & Havlin, J. L. (1993). Soil fertility and fertilizers. New Jersey: Prentice Hall.
Uhlman, K., & Artiola, J. (2011). Nitrate contamination potential in Arizona groundwater: Implications for drinking water wells. Arizona: The University of Arizona Cooperative Extention.
U.S. EPA. (2014). Human Health Evaluation Manual. Supplemental Guidance: Update of Standard Default Exposure Factors-OSWER Directive, 9200(1–120), 6.
Vasudevan, U., Gantayat, R. R., Chidambaram, S., Prasanna, M. V., Venkatramanan, S., Devaraj, N., Nepolian, M., & Ganesh, N. (2021). Microbial contamination and its associations with major ions in shallow groundwater along coastal Tamil Nadu. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00712-1
Vigneron, A., Bishop, A., Alsop, E. B., Hull, K., Rhodes, I., Hendricks, R., & Tsesmetzis, N. (2017). Microbial and isotopic evidence for methane cycling in hydrocarbon-containing groundwater from the Pennsylvania region. Frontiers in Microbiology, 8, 593.
Walvoord, M. A., Phillips, F. M., Stonestrom, D. A., Evans, R. D., Hartsough, P. C., Newman, B. D., & Striegl, R. G. (2003). A reservoir of nitrate beneath desert soils. Science, 302(5647), 1021–1024.
Wang, H., & Zhang, Q. (2019). Research advances in identifying sulfate contamination sources of water environment by using stable isotopes. International Journal of Environmental Research and Public Health, 16(11), 1914.
Wang, X., Lu, X., Li, F., & Yang, G. (2014). Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: focusing on ammonia inhibition. PloS One, 9(5), e97265.
Wang, Y., & Li, P. (2022). Appraisal of shallow groundwater quality with human health risk assessment in different seasons in rural areas of the Guanzhong Plain (China). Environmental Research, 207, 112210. https://doi.org/10.1016/j.envres.2021.112210
Ward, M. H., Jones, R. R., Brender, J. D., De Kok, T. M., Weyer, P. J., Nolan, B. T., & Van Breda, S. G. (2018). Drinking water nitrate and human health: An updated review. International Journal of Environmental Research and Public Health, 15(7), 1557.
Wei, M., Wu, J., Li, W., Zhang, Q., Su, F., & Wang, Y. (2022). Groundwater geochemistry and its impacts on groundwater arsenic enrichment, variation, and health risks in Yongning County, Yinchuan Plain of northwest China. Exposure and Health, 14(2), 219–238. https://doi.org/10.1007/s12403-021-00391-y
(WHO), World Health Organisation. (2017). Guidelines for drinking water quality, p. 631, 4th edition, Incorporating the 1st addendum. Retrieved November 25, 2021, from https://www.who.int/publications/i/item/9789241549950
Worldpopulationreview. (2021). Kuwait Population.
Wu, J., Li, P., Wang, D., Ren, X., & Wei, M. (2020). Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Human and Ecological Risk Assessment, 26(6), 1603–1621. https://doi.org/10.1080/10807039.2019.1594156
Yang, P., Yuan, D., Ye, X., Xie, S., Chen, X., & Liu, Z. (2013). Sources and migration path of chemical compositions in a karst groundwater system during rainfall events. Chinese Science Bulletin, 58(20), 2488–2496.
Zaady, E. (2005). Seasonal change and nitrogen cycling in a patchy Negev Desert: a review. Arid Land Research and Management, 19(2), 111–124.
Zaady, E., Groffman, P. M., & Shachak, M. (1996). Litter as a regulator of N and C dynamics in macrophytic patches in Negev desert soils. Soil Biology and Biochemistry, 28(1), 39–46.
Zhang, X., Qian, H., Chen, J., & Qiao, L. (2014). Assessment of groundwater chemistry and status in a heavily used semi-arid region with multivariate statistical analysis. Water, 6(8), 2212–2232.
Zhang, Q., Qian, H., Xu, P., Li, W., Feng, W., & Liu, R. (2021). Effect of hydrogeological conditions on groundwater nitrate pollution and human health risk assessment of nitrate in Jiaokou Irrigation District. Journal of Cleaner Production, 298, 126783.
Zhou, Z., Ansems, N., & Torfs, P. (2015). A global assessment of nitrate contamination in groundwater. International Groundwater Resources Assessment Center. Internship report, 4.
Acknowledgements
The authors would like to extend their appreciation to the International Atomic Energy Agency (IAEA) for the in-kind contribution to the study through RAS7034. The support of the Kuwait Institute for Scientific Research’s (KISR’s) management (WM0114S) was pivotal in carrying out the various tasks of the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent for publication
Since this study is not attempting to republish or publish any third party or author’s previously published material, this section does not apply.
Conflict of interest
The authors declare no competing interests.
Research involving human participants and/or animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsabti, B., Sabarathinam, C. & SVV, D.R. Identification of high nitrate concentration in shallow groundwater of an arid region: a case study of South Kuwait’s Bay. Environ Monit Assess 195, 143 (2023). https://doi.org/10.1007/s10661-022-10698-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10698-1