Abstract
An extensive study has been carried out to evaluate the occurrence and spatial distribution of high nitrate (NO3 −) concentrations in the groundwater of Mithi sub-district of district, Tharparkar, Sindh Province, Pakistan. Groundwater is the only consistent source of drinking water in the study area which is available at a depth between 3 and 125 m and is fetched by domestic animals from hand-dug wells. A total of 243 groundwater sample were collected to analyze for nitrate ion along with other physicochemical parameters. Overall groundwater quality reflects the influence of evaporation and silicate mineral weathering. Nitrate concentrations range up to 1610 mg/L with mean and median values of 240 and 162 mg/L, respectively, and almost 88.5% (n = 215) of the groundwater samples contain nitrate concentrations higher than the limit of 50 mg/L set by WHO for drinking water. The variations in nitrate contents in the groundwater are illustrated on regional map, showing high concentrations across the entire study area. High nitrate concentrations have been positively correlated with potassium, sodium and sulfate ions, indicating their possible genetic affiliations. The strength of association of nitrate is moderate with respect to potassium (r = .416, p = 1.3E−11) and sulfate (r = .419, p = 9.2E−12) and low with respect to sodium (r = .293, p = 3.5E−06). Agriculture is very limited in the study area due to the limited availability of water and is unlikely to be the source of the nitrate. Livestock manure is an important and most noticeable source of nitrate as most of the dug-wells serve as common water source for humans and livestock. Furthermore, the direct inclusion of manure-contaminated soil in water source also contributes to further exacerbating the problem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Limits to global water resources have received due attention in recent years, and water availability is at a real threat, particularly in desert areas which are characterized by higher evaporation rates as compared to precipitation. These regions receive small amount of rainfall at unpredictable frequencies, and due to unreliable recharge rates and the limited availability of surface water, groundwater resources have become highly important for sustaining human population in these areas (Parsons and Abrahams 2009; Scanlon et al. 2006; Walker 2012). Groundwater aquifers in deserts are often overlain by the highly permeable, sandy-textured soils, making them susceptible to the possible introduction of variety of surface pollutants. This increases the risk of contaminants from the surface being flushed into groundwater during recharge events. Nitrate is one of such pollutants and is prevalent in groundwater around the world, especially in countries with intensive agricultural and large urban centers (Majumdar and Gupta 2000; Nas and Berktay 2006; Ngatcha and Daira 2010; Spalding and Exner 1993; Stadler et al. 2008; Zhang et al. 1996). Nitrate is often found in higher concentration in desert aquifers, possibly due to sparse vegetation, surficial discharge of livestock and human waste, microbial activities and aerobic conditions (Gates et al. 2008; Walvoord et al. 2003). Fixation of biological N and the nitrification process may also contribute significant amount of nitrate in the environment (Jones Jr et al. 1995). Atmospheric deposition is one other source of nitrate that may accumulate in the regions with little rainfall (Walvoord et al. 2003). Large deposits of nitrate mined from Atacama Desert, Chile, have been known to have accumulated by long-term deposition of nitrate from the atmosphere (Michalski et al. 2004), and similar is the case of high nitrate concentrations in Mojave Desert, California (Graham et al. 2008). High levels of nitrate have also been found in the Kalahari Desert in Southern Africa (Heaton 1984; Schwiede et al. 2005; Stadler et al. 2012; Stone and Edmunds 2014) and Kumtag Desert in China (Qin et al. 2012).
Nitrate is one of the most stable forms of fixed nitrogen and is an important part of the nitrogen cycle (WHO 2003). However, at high concentration, this chemical form of nitrogen may cause adverse health and environmental effects. The World Health Organization (WHO) considers a limit of 50 mg/L as the safe limit of nitrate in drinking water, while the United States Environmental Protection Agency (USEPA) recommends 10 mg/L nitrate as nitrogen (44.2 mg/L as nitrate) (EPA 1995; WHO 2003). Nitrate enters in the water system either by surface runoff or by leaching through soil surface via percolating water (Garwood and Ryden 1986).
Elevated nitrate concentrations in drinking water may be harmful to human health, especially for infant babies causing “blue baby syndrome.” When nitrate is consumed, it is reduced to nitrite by the endogenous bacteria that oxidizes Fe2+ to Fe3+ (ferrous to ferric) in hemoglobin (Knobeloch et al. 2000), and in its presence, hemoglobin is called methemoglobin having poor oxygen-binding capacity (Hatfield and Follett 2007; Knobeloch et al. 2000). Children under the age of 6 months are most prone to this problem owing to the non-availability of reductase enzyme that converts methemoglobin back to hemoglobin (Hatfield and Follett 2007; Knobeloch et al. 2000). Higher nitrate has also been found to be related to different types of cancers (WHO 2003), as around 5% of nitrate in the body is bacterially converted to nitrite at different sites (WHO 2003). Under acidic conditions in the stomach, nitrite reacts with amines and amides to form N-nitroso compounds (NOCs) which are known to be animal carcinogens (Hernández-Ramírez et al. 2009; Ward 2009). Nitrate has also been inferred to cause non-Hodgkin lymphoma and birth defects (Brender et al. 2013; Kilfoy et al. 2010; Manassaram et al. 2007; Ward et al. 2006). These issues are of concern if nitrate-contaminated groundwater is the only available drinking water source as is the case in the Thar Desert in Pakistan.
Nitrate poisoning in livestock has been intensively studied confirming livestock losses due to consumption of elevated nitrate in feed or groundwater (Davidson et al. 1941; Michalski et al. 2010). As in the case of humans, nitrate reduction to nitrite also causes methemoglobinemia in ruminants; however, it manifests in the form of cyanosis and hypoxia, the fatal disorders which may be instantly life-threatening for ruminants (Cockburn et al. 2013; Lee and Beauchemin 2014), and pregnant ones have been reported to abort due to lack of oxygen (CDC 1996; Manassaram et al. 2007). Drinking water for cattle has been considered safe for consumption when nitrate is below 50 mg/L, but is potentially harmful and even lethal when higher levels are consumed (Beede 1992). Thus, in regions like Thar Desert where livestock rely on groundwater for drinking, elevated nitrate in groundwater may also threaten local economy as local population solely depend on rearing of cattle and livestock.
In the light of above information, the present work has been carried out as a reconnaissance study in Mithi sub-district of Thar Desert in Sindh Province of Pakistan, to assess the endemic problem of high nitrate concentration in groundwater. This has become necessary, especially in the context of recent periods of drought in the study area which have caused a number of infant deaths (Mansoor 2014; Samoon 2016).
Study area
The Thar Desert is the ninth largest and one of the most densely populated arid deserts of the world. According to the census of 1998, the region has a population of more than one million with a population density of about 46 people per square kilometer (DCR 2000) that is mainly concentrated in villages that are scattered throughout the desert region. The desert extends toward Punjab Province in the northeast and across the Indo-Pakistan border eastward, spreading over an area of about 22,000 km2.
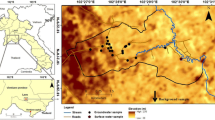
The Mithi sub-district is approximately located between N 24° 10′ and 25° 00′ and E 69° 24′ and 70° 36′ in the lower middle part of Thar Desert (Fig. 1). It is spread over an area of 5340 km2 and has a population of 241,548 with 2.7% average annual growth rate and population density of 45.2 people km2 according to the census of 1998 (District Census Report of Tharparkar District 2000). Mithi Town, situated N 24° 44′ and E 69° 51′, is the headquarters of the Tharparkar district and the sub-district of the same name. The sub-district is topographically higher in the northeastern part of the Thar Desert, and its elevation ranges from sea level in the south to more than 120 m above sea level in the northeast around the Khawaria area.
The livelihoods of the local inhabitants are dependent on agriculture and livestock rearing. The soil in the interdunal flat land is highly fertile and supports plant growth and quite ideal for grazing. Annual rainfall in the region is between 200 and 250 mm in a wet year (Ahmad et al. 2004). However, the precipitation rate is very variable, and periods of drought lasting for up to 4 years commonly occur in the region. This is a limiting factor for all agricultural activities in the area (Zaigham 2003).
Groundwater is the only reliable source of water which is found at depths ranging from 5 m to more than 150 m and has mainly been obtained from hand-dug wells, present throughout the desert. These wells serve as a common water source for both humans and their livestock. Domestic animals such as camel and donkey are often used to fetch water from these wells using ropes and buckets. These dug-wells are usually improperly lined and also devoid of casing caps above the ground level that render them prone to the introduction of contaminants that are washed in from the surface.
Materials and methods
A total of 243 groundwater samples were collected randomly from the entire study area. The sampling strategy involved the collection of groundwater samples from wells primarily used for drinking by the larger population in each selected village. Groundwater samples were collected from wells directly above the outlet from the water table using a stainless steel container attached to a fiber rope. Sample were filtered and stored in a 1.5-L polyethylene sampling bottle, pre-washed with 1% dilute nitric acid and subsequently rinsed with deionized water (Clesceri et al. 1998). Groundwater samples were analyzed at the site for pH, Eh, electrical conductivity (EC), dissolved oxygen (DO) and temperature, and the depth of wells was determined by a rope used for fetching groundwater from wells. Most of the wells in the study area are dug-wells: that is basically a hole, with a diameter of 1 m and a depth that reaches up to 1.5 m below the water table, but often less. Standard methods for groundwater analyses recommended by the American Public Health Association (APHA) were used, and the concentration of nitrate ion was determined by ion-selective electrode method (Clesceri et al. 1998). The statistical interpretation of the results of chemical analyses was carried out using MS Excel software for data interpretation, and groundwater mapping for spatial distributions has been done with the help of ArcGIS 10.3.1 using an inverse distance weighted (IDW) interpolation technique.
Results and discussion
TDS content of groundwater
A review of groundwater analysis has shown high concentration of major cations and anions (Table 1) which are probably derived by the combination of different factors like rock weathering, evaporation saline intrusion and leaching of animal wastes. For convenience in description, groundwater samples have been grouped into four categories according to their TDS content and the extent to which their TDS levels are within or in excess of the WHO guideline value of 1000 mg/L. A total of 12.3% (n = 30) groundwater samples were found to have TDS levels within WHO maximum permissible limit of 1000 mg/L, whereas 28.4% (n = 69) had TDS levels in the range 1001−3000 mg/L; 30.0% (n = 73) had TDS levels in the range 3001−5000 mg/L; and 29.2% (n = 71) groundwater samples had TDS levels above 5000 mg/L (Table 2). In more than 87.7% (n = 213) of groundwater samples, TDS concentrations were found to be more than 1000 mg/L and found to be unfit for drinking.
On the basis of TDS values, the entire region may be characterized as having brackish to moderately saline groundwater. Figure 2 indicates that the northern part of the area is almost entirely dominated by saline water (TDS = 3000–10,000), whereas groundwater salinity is generally much lower in the southern part of the region (TDS = 1000–3000 mg/L). Since the southern part is much closer to the Arabian Sea, it could be more prone to salt water intrusion which would result in higher TDS values. However, the data collected in this study have not suggested that this is taking place.
The observed distribution of groundwater salinities may be related to the distribution of rainfall, as the southern part of the region generally receives more rainfall than the northern part of the area. This is likely to cause higher groundwater recharge rates in the southern part of the area, resulting in lower TDS values. Furthermore, the groundwater of Thar Desert is likely to be influenced by water–rock interactions (Naseem et al. 2010; Rafique et al. 2008, 2009, 2013, 2015a, b). Since the southern part of the area comprises of relatively shallower aquifers, therefore, it may be under lesser influence of rock weathering due to lesser residence time, an effect causing low mineral leaching in the groundwater.
Nitrate content of groundwater
Nitrate concentrations in groundwater in the region range between 3.4 and 1610 mg/L, with mean and median values of 240 and 162 mg/L, respectively (Table 1). These results include some of the highest nitrate concentrations (>500 mg/L) that have been measured in the region and are of concern from a human health perspective.
Some of the highest nitrate concentrations that have been measured in village water supplies include the villages of: Ukraro #210 (1610 mg/L); Godanghri #204 (1482 mg/L); Lonihar # 43 (958 mg/L); Asabo # 5 (920 mg/L); Mithi Town # 84, 89, 82 (844, 526, 513 mg/L); Garho # 126 (812 mg/L); Pilro # 166 (751 mg/L), Islamkot Town # 108 (739 mg/L); Morano # 155 (706 mg/L); Pabuhar # 117 (674 mg/L); Mokhar Junejo # 92 (661 mg/L); Jindi jo Tar # 87 (609 mg/L); Mithrio Soomro # 170 (601 mg/L); Veri Bheel # 158 (578 mg/L); Gorano # 180 (566 mg/L); Thare jo Tar # 80 (552 mg/L); Bhave jo Tar # 98 (552 mg/L); Khario Ghulam Shah # 122 (548 mg/L); Matto jo Tar # 162 (532 mg/L); Sanghar # 77 (530 mg/L); Ade jo Tar # 125 (528 mg/L); and Miro jo Tar # 156 (526 mg/L).
The investigations that were undertaken in this study have indicated that nitrate levels in more than 88% of the samples (i.e., in 215 samples) exceeded the WHO recommended limit of 50 mg/L for drinking water (Fig. 3) (WHO 1994), and many of these samples also contain more than 1000 mg/L TDS (Fig. 2). Around 28.4% (n = 69) samples contain nitrate concentration in the range of 201–400 mg/L (Fig. 3), roughly five times higher than the WHO maximum recommended limit, a level that is considered to be unsafe for long-term consumption by humans and cattle.
The spatial distribution of nitrate concentration indicates that high nitrate concentrations are widely distributed in groundwater across the study area (Fig. 1). Rare zones with low nitrate concentrations (shown in green color in Fig. 1) are scattered mainly in the northwestern part of the study area, in the close vicinity of the irrigated Indus flood plains. Low nitrate concentrations in groundwater in these scattered zones may be due to dilution by irrigation water and/or uptake of soil nitrate by the plants. A low nitrate region was also found in the southernmost part of the area and may be associated with shifting sand dunes which blow away feces of herds discharged on the surface.
Nitrate concentrations in groundwater were found to have a modest correlation with potassium ions (r = .416, p = 1.3E−11), and a weak positive correlation of nitrate has been found with sodium (r = .293, p = 3.5E−06) (Table 3), indicating a possible association among the ions. Potassium is an important constituent of animal manure and is chiefly present in urine, while sodium is found in higher concentration in desert soils (Eghball et al. 2002). Both potassium and sodium ions form saltpeter with nitrate ions in the arid environment and are readily dissolved in the aqueous media. A similar correlation (r = .419, p = 9.2E−12) was also observed between nitrate and sulfate ions in the groundwater.
Well depth also plays an important role in nitrate transport from the surface to the water table. Generally, shallow wells are more prone to nitrate contamination than the deep wells because in shallow wells, the water table is closer to the surface and potential nitrate sources such as fertilizers and septic systems (Larson and Henley 1966; Spalding and Exner 1993; Townsend and Young 1995; Young 2010). In this study, nitrate concentrations at different depths of sampling wells have not shown any significant pattern (Fig. 4). Although groundwater is much deeper in the northern part of the study area and much shallower toward the south, yet high nitrate levels have been found irrespective of depths of sampling wells. Statistically, no correlation has been found between nitrate concentration in the groundwater and the depth of sampling wells, maybe due to higher nitrate concentration in majority of the samples. In some other studies which have reported significant correlation between nitrate and well depth, the proposed sources have been the agriculture activities, intense use of fertilizers and on-site septic tank system (Babiker et al. 2004; Hudak 1999; Nkotagu 1996). This is not the case with Mithi sub-district of Thar Desert area where agriculture is extremely minimal due to unavailability of adequate water sources and installation of on-site septic tank system is not a practice either. Therefore, fertilizer and septic tanks may be disregarded as sources of nitrate pollution, and henceforth, no relation has been found between well depth and nitrate.
Livestock rearing is the major occupation of the local inhabitants. According to an agricultural census, the livestock population is more than .35 million (Zaigham 2003). Drinking water for cattle is considered safe for consumption when nitrate is less than 44 mg/L. A concentration of 45–132 mg/L is also assumed to be safe if diet is low in nitrate (Waldner and Looper 2007). Nitrate becomes potentially detrimental for livestock at 133–220 ppm level when consumed for long periods, and cattle are at risk of death when its levels are between 220 and 661 ppm. Over 661 ppm water is considered unfit for consumption by cattle as it could cause serious health effects (Waldner and Looper 2007). Around 28% samples have shown nitrate levels in the range of 201–400, and more than 17% of the samples contain highly detrimental level of nitrate in excess of 400 mg/L. This potentially threatens livestock viability in the study area which is the main source of livelihood for Thari people. Livestock mortality caused by the evapo-concentration of nitrate produced by nitrification of manure surrounding a desert watering hole has been previously noted (Michalski et al. 2010).
Nitrate is highly mobile, and soil of the Thar Desert is a highly permeable, well-sorted sandy soil; therefore, nitrate can be easily leached to groundwater within percolating water. Where nitrate leaching from oxidation of organic nitrogen in animal manure is important, direct introduction of animal manure is also as important in this part of the world as many of the dug-wells have been seen with no protective casing on top which can cause the windblown activities to transport contaminated soil to the water body easily (Fig. 5a–d). It has also been observed that the rope with which the water is fetched from dug-wells is highly contaminated with animal excreta and this also causes the direct inclusion of nitrate to the water body. Such direct introduction of the pollutants to the groundwater is possibly the main reason behind no regular pattern in correlation of nitrate with other water quality parameters.
Conclusion
The groundwater of study area has been found to be severely contaminated by the presence of high nitrate concentrations with a mean value of 240 mg/L, and it threatens the already limited and sparse resources of drinking water in the area. About 88.5% (n = 215) of the total of 243 samples have been shown to have nitrate concentrations greater than the WHO limit of 50 mg/L for drinking purpose, and in some samples, it is even greater than 500 mg/L which indicated the severity of nitrate contamination in the area. Anthropogenic activities, like utilization of animals to lift water and improper lining of wells, are considered to be the principal causes of high nitrate concentrations found in the study area. As whole area is covered with extensive and thick dunned sand and cattle discharge plenty of waste near the water sources, the wind blown activities transport the contaminants directly into the wells which further contributes to aggravating the problem in the area.
References
Ahmad S, Hussain Z, Qureshi AS, Majeed R, Saleem M (2004) Drought mitigation in Pakistan: current status and options for future strategies. International Water Management Institute, Colombo
Babiker IS, Mohamed MAA, Terao H, Kato K, Ohta K (2004) Assessment of groundwater contamination by nitrate leaching from intensive vegetable cultivation using geographical information system. Environ Int 29:1009–1017
Beede DK (ed) (1992) Water for dairy cattle. In: Large Dairy Herd Management, Management Service edn. American Dairy Science Association, Champaign
Brender JD et al (2013) Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the National Birth Defects Prevention Study. Environ Health Perspect 121:1083
CDC (1996) Spontaneous abortions possibly related to ingestion of nitrate-contaminated well water—LaGrange County, Indiana, 1991–1994 vol 45. Atlanta
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, vol 20. American Public Health Association, Washington
Cockburn A et al (2013) Nitrite in feed: from animal health to human health. Toxicol Appl Pharmacol 270:209–217
Davidson WB, Doughty JL, Bolton JL (1941) Nitrate poisoning of livestock. Can J Comp Med Vet Sci 5:303
District Census Report of Tharparkar District (2000). Government of Pakistan, Islamabad
Eghball B, Wienhold BJ, Gilley JE, Eigenberg RA (2002) Mineralization of manure nutrients. J Soil Water Conserv 57:470–473
Garwood EA, Ryden JC (1986) Nitrate loss through leaching and surface runoff from grassland: effects of water supply, soil type and management. In: Van der Meer HG, Ryden JC, Ennik GC (eds) Nitrogen fluxes in intensive grassland systems. Martinus Nijhoff, Dordrecht, pp 99–113
Gates JB, Bohlke JK, Edmunds WM (2008) Ecohydrological factors affecting nitrate concentrations in a phreatic desert aquifer in northwestern China. Environ Sci Technol 42:3531–3537
Graham RC, Hirmas DR, Wood YA, Amrhein C (2008) Large near-surface nitrate pools in soils capped by desert pavement in the Mojave Desert. Calif Geol 36:259–262
Hatfield JL, Follett RF (2007) Relationship of environmental nitrogen metabolism to human health. In: Hatfield JL, Follett RF (eds) Nitrogen in the environment: sources, problems, and management, 2nd edn. Elsevier Science, Amsterdam, pp 71–104
Heaton THE (1984) Sources of the nitrate in phreatic groundwater in the western Kalahari. J Hydrol 67:249–259. doi:10.1016/0022-1694(84)90244-0
Hernández-Ramírez RU et al (2009) Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int J Cancer 125:1424–1430. doi:10.1002/ijc.24454
Hudak PF (1999) Chloride and nitrate distributions in the Hickory aquifer, Central Texas, USA. Environ Int 25:393–401. doi:10.1016/S0160-4120(99)00016-1
Jones JB Jr, Fisher SG, Grimm NB (1995) Nitrification in the hyporheic zone of a desert stream ecosystem. J N Am Benthol Soc 14(2):249–258
Kilfoy BA et al (2010) Risk of non-Hodgkin lymphoma and nitrate and nitrite from the diet in Connecticut women. Cancer Causes Control 21:889–896
Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (2000) Blue babies and nitrate-contaminated well water. Environ Health Perspect 108:675
Larson TE, Henley L (1966) Occurrence of nitrate in well waters. Illinois State Water Survey, Champaign
Lee C, Beauchemin KA (2014) A review of feeding supplementary nitrate to ruminant animals: nitrate toxicity, methane emissions, and production performance. Can J Anim Sci 94:557–570
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders. Indian J Environ Health 42:28–39
Manassaram DM, Backer LC, Moll DM (2007) A review of nitrates in drinking water: maternal exposure and adverse reproductive and developmental outcomes. Cien Saude Colet 12:153–163
Mansoor H (2014) 311 Thar children died in 11 months: The Daily DAWN DEC 15, 2014. https://www.dawn.com/news/1150898. Accessed 4 May 2017
Michalski G, Bohlke JK, Thiemens M (2004) Long term atmospheric deposition as the source of nitrate and other salts in the Atacama Desert, Chile: new evidence from mass-independent oxygen isotopic compositions. Geochim Cosmochim Acta 68:4023–4038
Michalski G, Earman S, Dahman C, Hershey RL, Mihevc T (2010) Multiple isotope forensics of nitrate in a wild horse poisoning incident. Forensic Sci Int 198:103–109
Nas B, Berktay A (2006) Groundwater contamination by nitrates in the city of Konya, (Turkey): a GIS perspective. J Environ Manag 79:30–37
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78:1313–1321
Ngatcha B, Daira D (2010) Nitrate pollution in groundwater in two selected areas from Cameroon and Chad in the Lake Chad basin. Water Policy 12:722–733
Nkotagu H (1996) Origins of high nitrate in groundwater in Tanzania. J Afr Earth Sci 22:471–478
Parsons AJ, Abrahams AD (2009) Geomorphology of desert environments. Springer Science & Business Media, Berlin
Qin Y, Li Y, Bao H, Liu F, Hou K, Wan D, Zhang C (2012) Massive atmospheric nitrate accumulation in a continental interior desert, northwestern China. Geology 40:623–626
Rafique T, Naseem S, Bhanger MI, Usmani TH (2008) Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environ Geol 56:317–326
Rafique T, Naseem S, Usmani TH, Bashir E, Khan FA, Bhanger MI (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J Hazard Mater 171:424–430
Rafique T, Naseem S, Usmani TH, Bhanger MI, Shirin K (2013) Impact of seawater on distribution of fluoride and other ions in groundwater of Diplo area, Thar Desert Pakistan. Water Environ Res 85:579–586
Rafique T, Ahmed I, Soomro F, Khan MH, Shirin K (2015a) Fluoride levels in urine, blood plasma and serum of people living in an endemic fluorosis area in the Thar Desert, Pakistan. J Chem Soc Pak 37(6):1223–1230
Rafique T, Naseem S, Ozsvath D, Hussain R, Bhanger MI, Usmani TH (2015b) Geochemical controls of high fluoride groundwater in Umarkot sub-district, Thar Desert, Pakistan. Sci Total Environ 530:271–278
Samoon H (2016) Nine more children die in Thar, drought toll hits 100. The Daily DAWN 21st Jan 2016. https://www.dawn.com/news/1234455/nine-more-children-die-in-thar-drought-toll-hits-100. Accessed 4 May 2017
Scanlon BR, Keese KE, Flint AL, Flint LE, Gaye CB, Edmunds WM, Simmers I (2006) Global synthesis of groundwater recharge in semiarid and arid regions. Hydrol Process 20:3335–3370
Schwiede M, Duijnisveld WHM, Bottcher J (2005) Investigation of processes leading to nitrate enrichment in soils in the Kalahari Region, Botswana. Phys Chem Earth 30:712–716
Spalding RF, Exner ME (1993) Occurrence of nitrate in groundwater-a review. J Environ Qual 22:392–402
Stadler S et al (2008) Understanding the origin and fate of nitrate in groundwater of semi-arid environments. J Arid Environ 72:1830–1842
Stadler S, Talma AS, Tredoux G, Wrabel J (2012) Identification of sources and infiltration regimes of nitrate in the semi-arid Kalahari: regional differences and implications for groundwater management. Water SA 38:213–224
Stone AEC, Edmunds WM (2014) Naturally-high nitrate in unsaturated zone sand dunes above the Stampriet Basin, Namibia. J Arid Environ 105:41–51
Townsend MA, Young DP (1995) Factors affecting nitrate concentrations in ground water in Stafford County, Kansas current research in earth sciences, Kansas Geological Survey. Bulletin 238:1–9
U.S. Environmental Protection Agency (1995) Drinking water regulations and health advisories. Office of Water, Washington, DC
Waldner DN, Looper ML (2007) Water for dairy cattle. In: Cooperative Extension Service. New Mexico State University, pp 1–5
Walker J (2012) Hot deserts: engineering, geology and geomorphology: engineering group working party report. Geological Society of London, London
Walvoord MA, Phillips FM, Stonestrom DA, Evans RD, Hartsough PC, Newman BD, Striegl RG (2003) A reservoir of nitrate beneath desert soils. Science 302:1021–1024
Ward MH (2009) Too much of a good thing? Nitrate from nitrogen fertilizers and cancer. Rev Environ Health 24:357–363
Ward MH, Cerhan JR, Colt JS, Hartge P (2006) Risk of non-Hodgkin lymphoma and nitrate and nitrite from drinking water and diet. Epidemiology 17:375–382
WHO (1994) Fluorides and oral health: report of the WHO expert committee on oral health status and fluoride use. vol 846. World Health Organization
WHO (2003) Nitrate and nitrite in drinking-water: background document for development of WHO guidelines for drinking-water quality. World Health Organization, Geneva http://apps.who.int/iris/handle/10665/75380
Young CR (2010) Extent of denitrification in Northport groundwater. Stony Brook University, New York
Zaigham NA (2003) Strategic sustainable development of groundwater in Thar desert of Pakistan. Water resources in the South: Present scenario and future prospects, commission on science and technology for sustainable development in the South. Islamabad, Pakistan: Commission on science and technology for sustainable development in the South (COMSATS), vol 3. Publications on Science and Technology
Zhang WL, Tian ZX, Zhang N, Li XQ (1996) Nitrate pollution of groundwater in northern China Agriculture. Ecosyst Environ 59:223–231
Acknowledgements
The authors are thankful to anonymous reviewer for comprehensively reviewing the manuscript and helpful suggestions. The authors are highly thankful to the Pakistan Science Foundation for financial support under Project No. PSF/RES/SPCSIR/Env(86).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soomro, F., Rafique, T., Michalski, G. et al. Occurrence and delineation of high nitrate contamination in the groundwater of Mithi sub-district, Thar Desert, Pakistan. Environ Earth Sci 76, 355 (2017). https://doi.org/10.1007/s12665-017-6663-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6663-0