Abstract
Fishes have been widely used as a representative to estimate the health of an aquatic ecosystem. In the present study, Labeo rohita was selected for biomarker study against decabromodiphenyl ether (BDE-209), a persistent organic pollutant (POP), as it is a widely used Indian carp. The results suggested significant effects on the optimum metabolism of Labeo rohita. After 48 to 72 h of exposure, most of the biomarkers such as lactate dehydrogenase (LDH), creatine kinase (CK), serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and hepatosomatic index (HSI) increased drastically indicating the higher index of tissue and liver damage. On the contrary, succinic dehydrogenase (SDH), acetylcholinesterase (AChE), and alkaline phosphatase (ALP) showed a reverse trend suggesting the shifting of fish metabolism towards anaerobic respiration mode because of induced stress. Increased catalase (CAT) activity was also observed, which indicated increased abundance of reactive hydroxyl species and therefore a possible oxidative stress in fishes. It is further suggested to understand and examine the biotransformation characteristics and degradation pathways of polybrominated diphenyl ether (PBDE)s, which would be useful to comprehend their environmental fate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fishes have been widely used as a representative to estimate the health of aquatic ecosystem and toxicology of associated biota whereof enzymatic activities such as lactate dehydrogenase (LDH), succinic dehydrogenase (SDH), creatine kinase (CK), and alkaline phosphatase (ALP) activity act as the biomarkers for water pollution (Hook et al., 2014). In general, biomarker indicates any particular stress which affects the normal functioning of an organism such as reproduction, physiological developments, and adaptability in a given environment. Therefore, any change in the normal mechanisms of the organism reflects the presence of contamination in environment. The measurement mainly occurs in body fluids, cells, or tissues indicating biochemical or cellular modifications due to the presence and magnitude of toxicants, and due to host response (Parente & Hauser-Davis, 2013). In water pollution monitoring program, measuring biomarkers in aquatic bioindicator animals can be used as reliable tool for designing environmental monitoring, surveillance, and hazard assessment or for planning further remediation measures for the water bodies (Kumari & Khare, 2018). Biomarkers have recently become an integral component of environmental monitoring programmes in several countries, having an advantage over chemical analysis in demonstrating whether or not an organism has been meaningfully exposed. For some classes of chemicals listed as persistent organic pollutants, the detection limit is very low but still they bring deleterious physiological changes and in such cases, biomarkers can play an important role by determining whether the physiology of the exposed organism is significantly different from normal or not (Kumari & Khare, 2018). In one of the studies, it was inferred that the mammalian and fishery systems show same toxicology and adaptive responses to oxidative stress (Prieto et al., 2006). Conclusively, fishery models in addition to mammalian models can be further useful for knowing the different mechanisms of oxidative stress. Biochemical alterations in aquatic organisms in cellular structures and tissue organisation are commonly used to indicate the potential hazards in response to contamination (Zhao et al., 2018). Industrial wastewaters are a major source of water contamination as it contains variety of pollutants such as suspended solids, chlorides, heavy metals, and pesticides. Surface discharge of such wastewaters without treatment contaminates the water bodies and is exposed to aquatic biota and therefore has a significant impact on fish biochemical activity and metabolism (Marathe et al., 2021). There have been numerous studies on different organic pollutants including pesticide toxicology of fishes, indicating diverse effects including inhibition of AChE (acetylcholinesterase) activity, oxidative damage, mutagenesis and carcinogenicity, and histopathological and developmental changes (Kumari et al., 2011; Kumari et al., 2014; Topal et al., 2017; Khare et al., 2019). Similarly, persistent organic pollutants (POPs) are a group of chemicals of very high environmental concern and therefore need special attention as they are categorised as persistent and bio-accumulative and have very high potentials to bio-magnify in food chain and have long-range transport potentials (Guo et al., 2019). POPs are also reported to have several health effects in humans like cancers, birth defects, dysfunctional immunity and reproductive systems and are now becoming a major global issue (Ashraf, 2017). Likewise, polybrominated diphenyl ethers (PBDEs) are considered as the pollutants of high concern and are listed in Annex A by Stockholm Convention which suggests total elimination of the chemical from the environment. Presently, there are 209 congeners of PBDEs, which are widely used as flame retardants in various polymer-based commercial and household products, such as electrical, electronic equipment, paint, textiles, plastics in casings of televisions, personal computer, and toys (Sindiku et al., 2015). India has recently banned in the year 2020, the trade, use, import, and export of seven chemicals including commercial Penta-BDE (tetrabromodiphenyl ether and pentabromodiphenyl ether), and commercial Octa-BDE (hexabromodiphenyl ether and heptabromodiphenylether) and has established concentration limits for all PBDEs in certain electrical goods (Sharkey et al., 2020). Currently, there is no production or stockpile information available on PBDEs in India (Chetry, 2018).
The presence of PBDEs has also been detected in water bodies and other matrices such as fishes, human milk, and aquatic birds in different parts of the world indicating its ubiquitous presence and therefore the degree of threat (Kumari et al., 2014). Previous studies conducted in foreign countries have also shown the presence of different PBDE congeners in fishes, wild chinook salmon (Oncorhynchus tshawytscha), and wild common carp (Cyprinus carpio) wherein congeners such as 47, 49, and 99 showed higher percentage (Browne et al., 2009; Montory et al., 2010; Noyes et al., 2010; Sloan et al., 2010). The fishes showed higher level of debrominations for the congeners. In addition, decabromodiphenyl ether, BDE-209, also showed the removal of meta-substituted bromine atoms in common wild carp (Stapleton et al., 2004; 2006). Apart from this, a higher level of debromination was also observed for the congeners, namely BDEs 99, 153, 183, 203, 208, and 209 in three fish species viz., rainbow trout (Oncorhynchus mykiss), common carp (Cyprinus carpio), and Chinook salmon (O. tschwatcha in the study of Roberts et al. (2011). There are numerous studies examining the effect of different pollutants on fish biomarkers. For instance, serum glutamine pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), acetylcholinesterase, superoxide dismutase (SOD), SDH-, and LDH-based biomarker studies have been conducted against variety of pollutants such as pesticides, heavy metals, and pharmaceuticals (Chen et al., 2012; Khare et al., 2019; Monteiro et al., 2013). Very few studies have also examined the effect of different PBDE congeners on different enzymatic biomarkers such as AChE, glutathione-S-transferase (GST), SOD,and catalase and observed significant alterations in fishes like goldfish (Carassius auratus) (Feng et al., 2013a, b). Still, the data is very limited on the impact of co-existing PBDEs on fishes, and as per our knowledge, no studies involving the use of enzymatic biomarkers of fishes are available in Indian context. L. rohita is one of the most important commercial and cultivable India’s major carps and extremely sensitive to the presence of organic pollutants which may significantly damage certain physiological and biochemical processes. Therefore, the present study assesses the ecological risks of PBDEs (BDE-209) for fish using various biometric and enzymatic biomarkers. The present study shall provide biologically relevant data for different toxicological and risk assessment studies on various enzymatic alterations in fishes via PBDE exposure in Indian context.
Methods and materials
Selection of experimental fish
Labeo rohita, weight 34.4 ± 09.87 g; length 13.4 cm ± 1.30 collected from College of Fishery Science, Telankhedi, Nagpur, were selected for the experiment. The fishes were later acclimatised under lab conditions for 14 days before the start of experiment according to the methodology of Kumari et al. (2011). Three replications were made for both control fish tank and experimental fish tank and each tank contained 5 fishes. The fish species is selected because it is one of the major Indian carps and important sources of food in India (Bais, 2018).
Polybrominated diphenyl ether- BDE 209
Analytical grade and certified PBDE-209, a deca-PBDE with 98% purity, CAS number 1163–19-5 (Sigma-Aldrich) was purchased and used in the study.
Rationale for selection of cod liver oil for dietary exposure of BDE-209
The PBDE compounds have very low solubility in water, i.e. < 0.0001 to 0.0005 mg/L at room temperatures (at 25 °C) and therefore limit its redistribution (US EPA, 2010; Al-Omran, 2018; Wang et al., 2021). Harmon (2015) also stated that the most relevant approach to study the effects of PBDEs on fish and biota is the exposure through contaminated food since they are not particularly soluble in water. Therefore, cod liver oil was used in our study to ensure homogeneous distribution of BDE-209 in feed used for experiment. Noyes et al. (2011) and Sarkar et al. (2015) also exposed PBDE to fishes by mixing in cod liver oil. Rochman et al. (2014) and Xie et al. (2014) also adopted the similar mode of experimentation to determine the adverse effects of PBDE on fish metabolism. Cod liver oils are extracted from fishes only and are considered to be the rich source of vitamin D and to supply fat and additional vitamins. The purity of the product is maintained at extremely high levels (Case et al., 2011) and product with similar range of purity was used in the present study showing negligible chances of any sort of contamination.
Dietary exposure
The fishes were exposed to BDE-209 in all the three tanks through dietary exposure (ingestion) of 9.8 ± 0.16 μg of BDE-209/g wet weight (ww) of food at 5% of their body weight per day. The BDE-209 was mixed in a mixture of 25 g of blood worm and 25 g of fish food in cod liver oil (20 ml). For control fishes, a control diet (fish food, blood worm, and cod liver oil) containing no BDE-209 was given. Dietary exposure was made on the basis of the study of Noyes et al. (2011) where 9.8 ± 0.16 μg of BDE-209/g wet weight (ww) of food was the actual concentration of PBDE 209 to which the fishes were exposed. Since the study of Noyes et al. (2011) showed bioaccumulation of BDE-209 in the fish body along with debromination to other by products such as BDE-179, BDE-188, BDE-201, and BDE-202 and nona BDE congeners (BDE-206, BDE-207, and BDE-208) based on GC/ECNI-MS analysis at a concentration of 9.8 ± 0.16 μg/g ww of food at 5% of their body weight per day; it was assumed that BDE-209 readily bioaccumulates in fish body and also debrominates to lower congeners. Therefore, our study primarily focussed on determining the effect of BDE-209 exposure to the enzymatic activity in the fish body and traces out the immediate response.
Experimental design
After the acclimatisation, a short-term (up to 96 h) toxicity test was performed. The conditions of the treated fishes were monitored regularly up to 96 h. The fish’s serum and tissue samples were analysed after 24, 48, 72, and 96 h of exposure to BDE-209. The blood samples were collected with cardiac puncture method. After dissection, different tissues such as muscles, gills, liver, and brain were collected and weighed carefully. The tissue was homogenised at 10% w/v concentration in 0.65 M phosphate buffer (PBS). Later, spectrophotometric analysis was performed for different assays using UV spectrophotometer (Shimadzu; Model- UV-1800) equipped with temperature control system (Khare et al., 2019).
Biometric and enzymatic parameters
Biometric parameter, i.e. condition factor (Ponderal Index) and hepato-somatic index, was calculated by the formula mentioned in the methodology of Salam and Davies (1994) and Kumari (2006) as follows:
Measurement of enzymatic parameters included acetylcholinesterase activity (AchE), lactate dehydrogenase activity (LDH), succinic dehydrogenase activity (SDH), alkaline phosphatase activity (ALP), serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT) and catalase activity in serum except for LDH and SDH which was measured in the muscle and liver respectively.
-
AchE activity was measured as per the methodology of Ellman et al. (1961). Ten microliters of sample (serum, homogenate from gills, brain, and liver) was mixed with 1.5 ml of working agent and optical density was measured at 405 nm for 1.5 min at every 30 s of interval in UV–visible spectrophotometer.
-
LDH activity was measured as mentioned in the method of Young and Koplovitz (1995) and Elliot and Wilkinson (1961). The muscle tissue was homogenised in phosphate buffer (pH 7.4) then centrifuged for 10 min at 10,000 rpm. The supernatant was pooled for further analysis. Twenty-five-microliter sample was mixed with 1 ml of working reagent and incubated for 1 min at 37 °C. Optical density was measured for the next 2 min at the interval of 1 min.
-
SDH activity was evaluated based on the method of Khare et al. (2019) where the known weight of liver was homogenised in the known amount of phosphate buffer. A total of 0.5 ml of 5% tissue homogenate was mixed with 0.25 ml of 0.1 M sodium succinate, 0.25 ml of 0.065 M potassium phosphate buffer (pH-7.4), and 0.25 ml of 0.3% aqueous triphenyl tetrazolium chloride (TTC). This mixture was incubated at 33 °C for 1 h. A blank without homogenate and a control without substrate was also run simultaneously. The extraction of red coloured formazan was performed with 5 ml of acetone followed by the measurement of optical density at 485 nm via UV–visible spectrophotometer.
-
For ALP, 0.02 ml of sample was mixed with 1 ml of working reagent and optical density was measured taking distilled water as a blank at 405 nm for 3 min at the interval of 1 min (Bowers and McComb 1975).
-
For, SGPT and SGOT, 0.l ml of serum sample was mixed with 0.8 ml and 0.2 ml of working reagents 1 and 2 respectively. This mixture was incubated at 37 °C for 1 min. Optical density was measured at 340 nm for 3 min at an interval of 1 min (Reitman & Franckel, 1957).
-
Catalase activity was determined according to the method of Khare et al. (2019). The known quantity of various tissues, namely gills, brain, liver, and muscle, was homogenised in phosphate buffer. Later, 0.1 ml of hydrogen peroxide, 1.95 ml phosphate buffer, and 0.05 ml were added to the tissue extract followed by measurement of absorbance at 240 nm. The activity was expressed as mmol−1H2O2 min−1 mg−1 protein.
QC/QA
The sampled organs were washed with distilled water and the apparatus was cleaned with ethanol to avoid the risk of contamination. Due care was taken to avoid any delay while tissue sampling and processing. The samples and the kit were maintained at room temperature prior to analysis to ensure accurate results. During the analysis, the temperatures prescribed by the test kit were maintained with the help of temperature control system attached to UV–visible spectrophotometer (Shimadzu; Model-UV-1800). The QC/QA included analysis of sample in triplicates as well as running of blank. All the necessary precautions were taken to reduce the risk of contamination at all stages of sample collection, processing, and analysis by maintaining consistent QC/QA techniques.
Statistical analysis
The statistical analysis was performed using Minitab 2017. All the data were expressed as mean ± standard deviation (mean ± SD). The graphs were made using MS Excel 2016. Significant differences between control and experimental groups were determined and compared using one-way ANOVA followed by Tukey HSD test at 95% confidence level (alpha = 0.05).
Result and discussion
In this study, various enzymatic parameters were selected to be studied along with biometric and behavioural changes as a function of BDE-209. A suit of different enzymatic biomarkers, aminotransaminase - biomarkers of liver and heart, cholinesterase - biomarker of neuron function, lactic and succinic dehydrogenases - biomarker of oxygen stress, alkaline phosphatase and creatine phosphokinase - biomarker of tissue damage, and catalase - biomarker of oxidative stress were selected; considering the responses obtained, these biomarkers may be used as a tool in bio-monitoring program to monitor ecotoxicity risk of PBDE group of chemicals which has not been studied in detail and there is no study related to this aspect in Indian conditions till date.
Behavioural changes in the exposed fishes
The behavioural study indicated a preliminary response to BDE-209 exposure. Fish exposed to BDE-209 showed significant behavioural abnormalities initially such as mucus secretions, lethargic movements, and accumulation near aerators in fish tanks. The efficiency of oxygen intake was significantly reduced in such settings, which was indicated by increased breathing rate and more frequent gathering of fish near the aerators gulping fresh air. Due to respiratory incumbency, there was a gradual weariness, lethargy, and a loss of balance. They soon became sedentary at the bottom of the tank, and their bellies began to rise after a while. Similar behavioural responses were reported in C. punctatus while exposed to mercuric chloride, malathion, and organophosphate (Pandey et al., 2005; Pandey et al., 2011). The alteration in swimming activity of fish is often assessed as a response to toxicity; this indicates significant damage to nervous system. Later, as the duration of the exposure increased, more evident changes were observed, such as redness over the fins and blood patches on the anus parts. At the time of dissection, pulpy liver, almost in dissolved form, was also observed. Similar observations were also made in the studies of Sivaperumal and Sankar (2011), Kumari et al. (2014), and Khare et al. (2019) in L. rohita and Catla catla in response to the exposure of different xenobiotics.
Biometric parameter
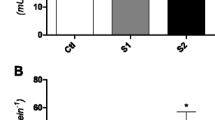
Condition factor indicates about the fish well-being. The exposure of PBDE (BDE-209) has significantly affected the optimum metabolism of L. rohita which resulted in decreased physiological condition as represented from the calculated values of condition factor (p < 0.0001) in comparison to control fishes (Fig. 1). There was a slight decrease in the condition factor in the present study; however, the values were very low (about 1.0) as compared with the study of Kumari et al. (2014) in which the fishes exposed to chromium (VI) has CF above 1.2 at 48, 72, and 96 h. The reason could be the selection of different pollutants. Contrarily, increased HSI (p < 0.0001) was also observed in the present study up to 48 h of exposure as compared to controls which may be due to the increased capacity of liver to bio-transform xenobiotics and therefore the enhanced the metabolism in liver. However, after 72 h, the HSI started decreasing which may be due to the biotransformation of BDE-209 inside the fish body into the hydrophilic compounds (for example, less brominated PBDEs) which showed negligible impact and got excreted out of body; as a result, the species was adapted to the existing environment and therefore recovered normal metabolism of the body (Fig. 1). In many studies, biometric parameters have been used as stress monitoring biomarkers to assess the presence of various pollutants (Huuskonen & Lindström-Seppä, 1995; Kumari et al., 2014). Noyes et al. (2011) also showed altered histopathological effects such as increased hypertrophy and liver phenotypic alterations in the fishes (fathead minnows) in the presence of BDE-209.
Enzymatic parameters
Cholinesterase is one of the essential enzymes for the proper functioning of the nervous system. AChE enzyme breaks the substrate acetylcholine into acetic acid and choline and mediates the transmission of the impulses. There was a slight decrease in AChE activity up to 24 h. However, after 24 h, the activity decreased nearly by twofold indicating severe impact of BDE-209 on neurotransmission activity of brain (Fig. 2). The observed inhibition in AChE activity is due to the accumulation of acetylcholine (AChE), a neurotransmitter at synaptic junction. The suppression of AChE activity is very well reflected through reduced locomotory motion of the fishes which is a major behavioural change (Rao et al., 2005). It was therefore inferred that BDE-209 has an affinity for fish AChE thereby affecting its secretion and locomotory behaviour in fishes. Reduced AChE levels are also reported to cause enhanced activation of cholinergic neurons, which can cause tremors, convulsions, and irregular or lethargic swimming (Almeida et al., 2010; Fernández-Vega et al., 2002). Similar observations with respect to lethargic movements were also observed in the present study. It is also stated that, when AChE activity declines, it causes the termination of acetylcholine receptor functioning and nerve membranous posterior functioning, putting organisms in an excited state for a long time and causing maladjustments and mortality as well in most cases (Han et al., 2010). Xie et al. (2014) also showed decreased AChE activity with increased dose of BDE-209 in gold fishes. He further stated that the mixture of different congeners had more pronounced inhibitory effect on AChE activity in comparison to individual compound exposure. Significant decrease in AChE activity was also reported by Khare et al. (2019) while investigating the synergistic effect of pesticide, carbayl, and methyl parathion on Catla catla. Similar observations were also made by Nephale et al. (2021) in fish Clarias gariepinus in the presence of various organic pollutants. Lu et al. (2013) assessed the effects of PBDE compounds (BDE-47 and BDE-99) on Carassius auratus (gold fish) and found altered AChE and catalase activity after 24–48 h. He further stated that AChE activity in brains tends to decrease with time and is also dose-dependent. It was also observed that after 48 h, the AChE activity began to increase slightly in the present study indicating the recovery of normal metabolism in exposed fishes and short-term effect of PBDE-exposure on AChE activity.
LDH activity acts as an indicator of anaerobiosis, which might be beneficial for the fishes to tolerate hypoxia under stressed condition due to toxicant exposure. LDH activity was significantly increased in exposed fishes (Fig. 3) indicating anaerobic mode of metabolism and impairment of aerobic pyruvate (Kumari & Sinha, 2010; Kumari, 2006). There was a significant difference between the LDH activity (p < 0.0001) of control fish and fish that were exposed for 24 h, and after 72 h, the activity increased drastically; this could be justified with the fact of debromination of PBDE to lower congeners after 72 h of exposure posing significant threat (Roberts et al., 2011). Similar trend in LDH activity was reported by Khare et al. (2019), when Catla catla were exposed to methyl parathion and carbayl. Similarly, increased LDH activity was also reported by Nephale et al. (2021) when specific biomarker study was conducted in fish Clarias gariepinus. Therefore, studies suggest that LDH can be used as the biomarker for biotransformation activity of various pollutants. Increased LDH activity was also shown by Kodavanti et al. (2005) in rat neuron cultures in response to exposure to PBDE compound (BDE-47). On the contrary, the SDH activity (p < 0.0001) decreased tremendously after 24 h of exposure and the decrease in the activity continued till 96 h, contrary to the increase in LDH activity due to anaerobic mode of respiration because of toxicant (BDE-209)-induced stress condition (Fig. 3). Similarly, the decrease in SDH activity was also observed in spotted seabass (Lateolabrax maculatus) and Cyprinus carpio; however, the changes were monitored in response to abiotic parameters such as water temperatures and dietary protein/energy ration (Lu et al., 2019; Vodianitskyi et al., 2021). One study investigated the effect of Bisphenol S in fresh water fish, Oreochromis Mossambicus, and they found a decreased SDH activity like in this present study (Anjali et al., 2021). SDH is the only mitochondrial metabolic enzyme. The decreased SDH activity indicates its inhibition at mitochondrial level which, as an alternative, enhances the other pathways for carbohydrate metabolism (Anjali et al., 2021; Kumari & Sinha, 2010; Srivastava et al., 2016).
ALP is hydrolase enzyme responsible for removing phosphate group from various types of molecules like protein, nucleotides, and alkaloids. In the present study, ALP activity (p < 0.0001) in serum was decreased by many folds as compared to control samples (Fig. 4). However, after 72 h, slight reversal in the activity was observed which could be due to the release of enzymes directly into the bloodstream. The results were analogous to the pronouncement of Kumari et al. (2015) wherein authors studied the effect in the presence of heavy metals. One of the most recent studies also showed the effect of bisphenol A, naphthalene, and butachlor on metabolic and antioxidant enzymes of goldfish and found altered ALP activity like in this present study (Ebrahimzadeh et al., 2021). Significantly higher alterations were observed in the ALP activity of exposed fishes in comparison to controls. Similar response was also observed by Alonso et al. (2010) wherein BDE-99 exposure significantly altered the ALP activity in adult male rats. Likewise, the enzyme CK catalyses the conversion of creatine and consumes adenosine triphosphate (ATP) to create phosphocreatine (PCr) and adenosine diphosphate (ADP). CK is an enzyme assayed in blood as a marker of myocardial infraction or severe muscular breakdown (Silverman et al., 1976). Induced CK activity (p < 0.0001) was observed in just 24 h of exposure; after 48 h, a marked increase in activity was observed indicating significant muscular damage due to toxicity induced by BDE-209 (Fig. 4). Oxidative stress along with renal dysfunction was also reported by Milovanovic et al. (2018) in Wistar rats after subacute exposure to BDE-209 and various alterations in biochemical parameters by Banaee et al. (2014) which was associated with the reduction in fish muscles (Alburnus mossulensis) exposed to sub-lethal concentrations of fenpropathrin.
SGOT and SGPT play important role in the protein metabolism. It is well-known that the increase in SGPT activity is an index to liver damage (Hsu et al., 2003). Significant alteration was observed in SGOT (p < 0.0001) and SGPT (p < 0.0001) in serum (Fig. 5). The SGOT and SGPT activity decreased in comparison to controls; however, it increased after 72 h showing delayed response of this chemical on these biomarkers. Similar trends were also reported in the study of Ebrahimzadeh et al. (2021) in which the evaluation of toxicity was based on 21 days of exposure of bisphenol A, naphthalene, and butachlor to gold fish. Studies have confirmed that the decreased SGOT and SGPT activity is due to cell injury (Gabriel et al., 2012; Muralidharan, 2014). On the contrary, in liver and muscles, the SGOT and SGPT activity was found to be increased in the presence of polyaromatic hydrocarbons in Ramnogaster arcuate and Pangasianodon (Kumar et al., 2020; Ronda et al., 2019). The increased expression is because of stress conditions in fishes or due to cell injury which demands high energy; as a result, aminotransferases mediated interconversion of carbohydrate and protein metabolism occurs, increasing the expression of SGPT and SGOT (Kumar et al., 2020; Ronda et al., 2019). Thus, it can be concluded that such enzymatic activity can be successfully used as indicator of fish health. The decrease in catalase activity was also observed which is probably due to formation of highly reactive oxygen species (hydroxyl species) in the presence of toxicants which indicates the oxidative stress in the fishes. CAT is a H2O2 scavenger which prevents its formation and thus protects from the deleterious effect caused by oxidative damage (Lukaszewicz-Hussain, 2010; Talas et al., 2008). In the present study, the catalytic activity increased in liver, gills, muscle, and brain after 24 h. After 48 h of exposure, increasing trends were noticed and then after 72 h, drastic increase in the CAT activity in brain (p < 0.0001) was observed. Whereas, in muscle, the catalase activity (p < 0.0001) showed minor alterations till 48 h but increased significantly during 72 h. Significant alteration in CAT activity in gills (p < 0.001) was observed during 24 and 48 h whereas consistent increasing trend was observed in the liver (p < 0.0001) in the present study (Fig. 6). Similar results were also observed by Kumari et al. (2014) in which catalase activity was tested against chromium (IV). Li et al. (2011) also observed a decrease in the activity in brain under the effect of different pollutants (carbamazepine) in rainbow trout. Slaninova et al. (2014) also showed increased CAT activity in the liver, gills, and kidney in L. rohita in the presence of Malathion. One study specific to BDE-209 also indicated significant alterations in CAT activity in the liver after BDE-209 exposure in gold fish (Xie et al., 2014). On the contrary, the decreased CAT activity was observed in L. rohita in gill and liver; however, the comparison was made in between reference and water polluted with heavy metals wherein the cause of decreased activity in L. rohita was suggested to be the overproduction of SOD (Mahamood et al., 2021). Lu et al. (2013) also showed significantly altered CAT activity in gold fish exposed to BDE-47 and BDE-99; however, the alterations were highly significant at higher doses in comparison to lower doses and also, there was not any linearity in between the activity and the time (i.e. the time dependence was not apparent). The decreased CAT activity was also observed by Iglesias et al. (2021) in the brain of Gambusia affinis on exposure with BDE-209.
Raldúa et al. (2008) also evaluated multiple biochemical and histological indicators in field-exposed fish and discovered that PBDE concentrations were linked not only to high activity of phase I and II metabolic enzymes, oxidative stress in the liver, but also to neurotoxicity in the brain. Other than these, prominent effects on digestive enzyme (protease, amylase, and lipase) activities were also observed by Chang et al. (2021) in fishes exposed to BDE 209. Furthermore, Table 1 shows numerous studies where different fishes were exposed to BDE-209 and degradation products were obtained in the long-term study. These studies further stated that fishes have the ability to absorb BDE-209 majorly from their diet which later leads to metabolism and debromination to lower congeners like hexa- and hepta-BDEs and methoxylated and hydroxylated PBDEs as well. However, most of the studies are long-term study spanning 14 days–5 months and have mostly involved the findings on the level of debromination and its effects; therefore, direct comparisons were quite not feasible. Since, in the present study, exposure has been given from 24 to 96 h only and after every 24, a set of fishes was sacrificed to assess the immediate enzymatic biomarkers of BDE-209 exposure, and therefore, measuring the concentration in different parts of the fishes on long-term basis was out of the scope of the study at present.
The comparison of control with exposed fishes in the present study showed very clear differences in various enzymatic levels because of direct exposure of fishes to BDE-209 via food; therefore, a clear association between exposure and enzymatic alterations was well identified in this study, and thus, the identified biomarkers can be used in future monitoring program of PBDEs-linked exposure assessment. In addition, no such study has been published in Indian context; therefore, this kind of study will be helpful in scientific domain to understand the ill effect of PBDEs either individual or in combination with different congeners on different biota especially fishes which would be beneficial to conduct the risk assessment for environmental scenarios explaining the concerns for top predators and humans.
Conclusion
The studies focusing on toxic effect of PBDE on fishes are limited in India, which is very important as Indian carps like L. rohita, Catla catla, and other fishes are an essential part of Indian diet and fresh water food chains. In this study, the PBDE (BDE-209) had severely affected the optimum metabolism of L. rohita which resulted in decrease physiological condition such as condition factor. The LDH activity was significantly increased indicating hypoxic condition. Similarly, alterations in normal enzymatic activities of SDH, SGOT, SGPT, ALP, CK, and CAT were also observed indicating higher stress conditions in the surrounding water due to BDE-209 exposure; however, no mortality was observed. We can conclude that fishes may be considered as a sensitive bio-indicator to the pollution in the water bodies and the enzymatic biomarkers can be remarkably used for monitoring of pollution in water. Moreover, it is important to understand the biotransformation characteristics of BDE-209 to comprehend its environmental fate and get a clear picture of its deleterious effects, which will be further useful for conducting risk assessment and health hazards studies. The application of biomarker is quite feasible in laboratory condition, but application to the field situation becomes quite challenging because of factors such as uptake, absorption, and chemical speciation and accumulation in tissue through food chain. Hence, application of biomarker at laboratory level becomes primary and the most important step towards succession of application of biomarker at field level. Extensive research is required to study the individual as well as synergistic effect of various pollutants in laboratory condition prior applying to the actual field.
Few important points should be considered while using the same strategy developed in lab conditions to diagnose the health of aquatic ecosystem in field condition. A suite of sensitive multiple biomarkers that encompasses specificity to different contaminant classes should be used detecting both short-term responses as well as long-term ecologically relevant endpoints to provide a weight-of-evidence approach for establishing relationships between environmental stressors and ecological effects. The experimental designs must focus in multiple biomarker approach with greater sample size, long-term biomonitoring, and knowledge about organisms’ ecological aspects which will enable a better data interpretation on the environmental quality, as well as the interference of non-polluting factors in the biomarker responses at a field level.
Data availability
All data generated or analysed during this study are included in this published article.
References
Almeida, J. R., Oliveira, C., Gravato, C., Guilhermino, L. (2010). Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax L. exposed to the organophosphate pesticide fenitrothion. Ecotoxicology. Nov;19(8), 1369–81. https://doi.org/10.1007/s10646-010-0523-y. Epub 2010 Aug 5. PMID: 20686920.
Alonso, V., Linares, V., Bellés, M., Albina, M. L., Pujol, A., Domingo, J. L., & Sánchez, D. J. (2010). Effects of BDE-99 on hormone homeostasis and biochemical parameters in adult male rats. Food and Chemical Toxicology, 48(8–9), 2206–2211.
Al-Omran, L. S. (2018). Physiochemical Properties and Environmental Levels of Legacy and Novel Brominated Flame Retardants. In F. Zafar, & E. Sharmin (Eds.), Flame Retardants. IntechOpen. https://doi.org/10.5772/intechopen.79823
Anjali, V. R., Shehna Mahim, S., Reshmi, S., et al. (2021). Bisphenol S induced metabolic disruption in a freshwater fish, oreochromis mossambicus J. of Aquat Biol & Fisheries, 9:68–75Ashraf MA (2017) Persistent organic pollutants (POPs): A global issue, a global challenge, Environmental Science and Pollution 24, 4223–4227. https://doi.org/10.1007/s11356-015-5225-9
Ashraf, M. A. (2017). Persistent organic pollutants (POPs): a global issue, a global challenge. Environmental Science and Pollution Research, 24(5), 4223–4227.
Bais, B. (2018). Fish Scenario in India with Emphasis on Indian Major Carps Int J Avian Wildl Biol, 3, 409–411.
Banaee, M., Sureda, A., Zohiery, F., Hagi, B. N., & Garanzini, D. S. (2014). Alterations in biochemical parameters of the freshwater fish. Alburnus Mossulensis, Exposed to Sub-Lethal Concentrations of Fenpropathrin Int J Aquat Biol, 2(2), 58–68.
Bowers Jr, G. N., & McComb, R. B. (1975). Measurement of total alkaline phosphatase activity in human serum. Clinical chemistry 2113, 1988–1995.
Browne, E. P., Stapleton, H. M., Kelly, S. M., Tilton, S. C., & Gallagher, E. P. (2009). In vitro hepatic metabolism of 2, 20, 4, 40, 5-pentabromodiphenyl ether (BDE 99) in Chinook salmon (Onchorhynchus tshawytscha). Aquatic Toxicology, 92(4), 281–287.
Case, L. P., Daristotle, L., Hayek, M. G., & Raasch, M. F. (2011). Chapter 26 - Common nutrition myths and feeding practices. Canine and Feline Nutrition (3rd ed.) https://doi.org/10.1016/B978-0-323-06619-8.10026-X
Chang, X., Kang, M., Feng, J., Zhang, J., & Wang, X. (2021). Effects of BDE-209 exposure on growth performance, intestinal digestive enzymes, and intestinal microbiome in common carp (Cyprinus carpio L.). Aquaculture and Fisheries. https://doi.org/10.1016/j.aaf.2021.05.005
Chen, L. G., Yu, K., Huang, C. J., Yu, L. Q., Zhu, B. Q., Lam, P. K. S., Lam, J. C. W., & Zhou, B. S. (2012). Prenatal transfer of polybrominated diphenyl ethers (PBDEs) results in developmental neurotoxicity in zebrafish larvae. Environ Sc & Tech., 46, 9727–9734.
Chetry, B. (2018). Persistent organic pollutants (POPs) in India: Country situation report. Toxic Link, New Delhi, India.
Ebrahimzadeh, M., Heidari, B., Nazarhaghighi, F., & Valipour, A. (2021). Physiological responses of the goldfish (Carassius auratus) during subacute exposure to organic pollutants. Bulletin of Environ Contamin and Toxicol, 106(5), 773–778.
Elliott, B. A., & Wilkinson, J. H. (1961). Serum" α-Hydroxybutyric dehydrogenase" in myocardial infarction and in liver disease. The Lancet, 277(7179), 698–699.
Ellman, G. L., Courtney, K. D., Andres Jr, V., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7(2), 88–95.
Feng, M., Li, Y., Qu, R., Wang, L., & Wang, Z. (2013b). Oxidative stress biomarkers in freshwater fish Carassius auratus exposed to decabromodiphenyl ether and ethane, or their mixture. Ecotoxicology, 22(7), 1101–1110.
Feng, M., Qu, R., Wang, C., Wang, L., & Wang, Z. (2013a). Comparative antioxidant status in freshwater fish Carassius auratus exposed to six current-use brominated flame retardants: a combined experimental and theoretical study. Aquatic toxicology, 140, 314–323.
Fernández-Vega, C., Sancho, E., Ferrando, M. D., & Andreu, E. (2002). Thiobencarb-induced changes in acetylcholinesterase activity of the fish Anguilla anguilla. Pesticide Biochemistry and Physiology, 72(1), 55–63.
Gabriel, U. U., Akinrotimi, O. A., & Ariweriokuma, V. S. (2012). Changes in metabolic enzymes activities in selected organs and tissue of Clarias gariepinus exposed to cypermethrin. J Environ Eng Technol, 1, 13–19.
Guo, W., Pan, B., Sakkiah, S., Yavas, G., Ge, W., Zou, W., & Hong, H. (2019). Persistent organic pollutants in food: contamination<br>sources, health effects and detection methods. International Journal of Environmental Research and Public Health, 16(22), 4361.
Han, Z. X., Lv, C. X., & Zheng, Z. R. (2010). Toxicological effects of PBDEs on Carassius aurats. Chinese J. Geochem., 29, 217–222.
Harmon, S. M. (2015). The toxicity of persistent organic pollutants to aquatic organisms. In Comprehensive Analytical Chemistry, 67, 587–613. Elsevier. https://doi.org/10.1016/B978-0-444-63299-9.00018-1
Hook, S. E., Gallagher, E. P., & Batley, G. E. (2014). The role of biomarkers in the assessment of aquatic ecosystem health. Integrated Environ Assess and Mgmt, 10(3), 327–341.
Hsu, T. L., Chiang, Y., Wang, W.-K., Chao, P.-T., Bao, J.-G., & Wang, Y. Y. L. (2003). Pulse analysis as a possible real-time biomarker complementary to SGPT and SGOT for monitoring acute hepatotoxicity toxicology. Mechanisms and Methods, 13(3), 181–186.
Huuskonen, S., & Lindström-Seppä, P. (1995). Hepatic cytochrome P4501A and other biotransformation activities in perch (Perca fluviatilis): The effects of unbleached pulp mill effluents. Aquatic Toxicology, 31(1), 27–41.
Iglesias, J. M. P., Gonzalez, P. S., Calderon, M. R., Natale, G. S., & Almeida, C. A. (2021). Comprehensive evaluation of the toxicity of the flame retardant (decabromodiphenyl ether) in a bioindicator fish (Gambusia affinis). https://doi.org/10.21203/rs.3.rs-500736/v1
Khare, A., Chhawani, N., & Kumari, K. (2019). Glutathione reductase and catalase as potential biomarkers for synergistic intoxication of pesticides in fish. Biomarkers, 3;24(7),666–76.
Kodavanti, P. R. S., Ward, T. R., Ludewig, G., Robertson, L. W., & Birnbaum, L. S. (2005). Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicological Sciences, 88(1), 181–192. https://doi.org/10.1093/toxsci/kfi289
Kumar, N., Krishnani, K. K., & Singh, N. P. (2020). Effect of zinc on growth performance and cellular metabolic stress of fish exposed to multiple stresses. Fish Physiol and Biochem., 46(1), 315–329.
Kumari, K. (2006). Effect of some pesticides on the biochemical changes in the frog, Rana tigrina and the toad, Bufo melanostictus (Unpublished doctoral dissertation) Patna University, India.
Kumari K., & Khare, A. (2018). Integration of biomarker approach in pollution monitoring programme of aquatic ecosystem. In: Varjani S., Parameswaran B., Kumar S., Khare S. (eds) Biosynthetic technology and environmental challenges. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7434-9_18
Kumari, K., & Sinha, R. C. (2010). Biochemical changes in the toad, Bufo melanostictus as a function of methyl parathion: ascorbic acid as a biomarker of oxidative stress. Вісник Харківського національного університету імені ВН Каразіна. Серія: Біологія, (12), 90–97.
Kumari, K., Dange, S., Khare, A., Goyal, S. K., & Wate, S. R. (2015). Application of biomarker approach for rapid assessment of aquatic pollution. World Ocean Science Congress, 5–8 February, Kerala India.
Kumari, K., Khare, A., & Dange, S. (2014). The applicability of oxidative stress biomarkers in assessing chromium induced toxicity in the fish Labeo rohita. BioMed research inter.
Kumari, K., Ranjan, N., & Sinha, R. C. (2011). Multiple biomarker response in the fish, Labeo rohita due to hexavalent chromium. In 2011 2nd International Conference on Biotechnology and Food Science). International Proceedings of Chemical, Biological and Environmental Engineering, 7, 155–158.
Kuo, Y. M., Sepulveda, M. S., Sutton, T. M., Ochoa-Acuna, H. G., Muir, A. M., Miller, B., & Hua, I. (2010). Bioaccumulation and biotransformation of decabromodiphenyl ether and effects on daily growth in juvenile lake whitefish (Coregonus clupeaformis). Ecotoxicology, 19(4), 751–760.
Li, Z. H., Zlabek, V., Velisek, J., Grabic, R., Machova, J., Kolarova, J., ... & Randak, T. (2011). Acute toxicity of carbamazepine to juvenile rainbow trout (Oncorhynchus mykiss): effects on antioxidant responses, hematological parameters and hepatic EROD. Ecotoxicology and Environmental Safety, 74(3), 319–327.
Lu, G. H., Qi, P. D., & Chen, W. (2013). Integrated biomarker responses of Carassius auratus exposed to BDE-47, BDE-99 and their mixtures.
Lu, K. L., Cai, L. S., Wang, L., Song, K., Zhang, C. X., & Rahimnejad, S. (2019). Effects of dietary protein/energy ratio and water temperature on growth performance, digestive enzymes activity and non‐specific immune response of spotted seabass (Lateolabrax maculatus) Aquaculture Nutrition, 26(6), 2023–2031.
Lukaszewicz-Hussain, A. (2010). Role of oxidative stress in organophosphate insecticide toxicity–Short review. Pesticide Biochemistry and Physiology, 98(2), 145–150.
Mahamood, M., Javed, M., Alhewairini, S. S. et al. (2021). Labeo rohita, a bioindicator for water quality and associated biomarkers of heavy metal toxicity. NPJClean Water, 4, 17. https://doi.org/10.1038/s41545-021-00107-4
Marathe, D., Singh, A., Raghunathan, K., Thawale, P., & Kumari, K. (2021). Current available treatment technologies for saline wastewater and land-based treatment as an emerging environment-friendly technology: A review. Water Environment Research, 93(11), 2461–2504. https://doi.org/10.1002/wer.1633
Milovanovic, V., Buha, A., Matovic, V., Curcic, M., Vucinic, S., Nakano, T., & Antonijevic, B. (2018). Oxidative stress and renal toxicity after subacute exposure to decabrominated diphenyl ether in Wistar rats. Environmental Science and Pollution Research International, 25(8), 7223–7230. https://doi.org/10.1007/s11356-015-5921-5 Epub 2015 Dec 16 PMID: 26676538.
Monteiro, D. A., Rantin, F. T., & Kalinin, A. L. (2013). Dietary intake of inorganic mercury: Bioaccumulation and oxidative stress parameters in the neotropical fish Hoplias malabaricus. Ecotoxicology, 22, 446–456.
Montory, M., Habit, E., Fernandez, P., Grimalt, J. O., & Barra, R. (2010). PCBs and PBDEs in wild Chinook salmon (Oncorhynchus tshawytscha) in the Northern Patagonia. Chile Chemo, 78(10), 1193–1199.
Muralidharan, L. (2014). Chronic toxic impacts of fenthion on the profiles of enzymes in the freshwater fish Cyprinus carpio (Linn). International journal of fisheries and aquatic studies, 1:51–56.
Nephale, L. E., Moyo, N. A., & Rapatsa, M. M. (2021). Use of biomarkers in monitoring pollution status of urban rivers, Limpopo, South Africa. Environmental Science and Pollution Research, 1–13.
Noyes, P. D., Hinton, D. E., & Stapleton, H. M. (2011). Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicological Sc., 122(2), 265–274.
Noyes, P. D., Kelly, S. M., Mitchelmore, C. L, & Stapleton, H. M. (2010). Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquatic Toxicology, 97(2), 142–150.
Pandey, A. K., Nagpure, N. S., Trivedi, S. P., Kumar, R., Kushwaha, B., & Lakra, W. S. (2011). Investigation on acute toxicity and behavioral changes in Channa punctatus (Bloch) due to organophosphate pesticide profenofos. Drug and Chemical Toxicology, 34(4), 424–428.
Pandey, S., Kumar, R., Sharma, S., Nagpure, N. S., Srivastava, S. K., & Verma, M. S. (2005). Acute toxicity bioassays of mercuric chloride and malathion on air-breathing fish Channa punctatus (Bloch). Ecotoxicology and Environmental Safety, 61, 114–120.
Parente, T., & Hauser-Davis, R. A. (2013). The use of fish biomarkers in the evaluation of water pollution. Pollution and Fish Health in Tropical Ecosystems Chapter, 7, 164–181.
Prieto, A., Jos, A., Pichardo, S., Moreno, I., & Camean, A. (2006). Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp). Aquatic Toxicology, 77(3), 314–321.
Rao, J. V., Begum, G., Pallela, R., Usman, P. K., & Rao, R. N. (2005). Changes in behavior and brain acetylcholinesterase activity in mosquito fish, Gambusia affinis in response to the sub-lethal exposure to chlorpyrifos. International Journal of Environmental Research and Public Health, 2(3–4), 478–483. https://doi.org/10.3390/ijerph2005030013
Raldúa, D., Padrós, F., Solé, M., Eljarrat, E., Barceló, D., Riva, M. C., & Barata, C. (2008). First evidence of polybrominated diphenyl ether (flame retardants) effects in feral barbel from the Ebro River basin (NE, Spain). Chemosphere, 73, 56–64.
Reitman, S., & Franckel, S. (1957). A colorimetric method for the determination of serum glutamic oxalo acetic and glutamic pyruvic transaminase. American Journal of Clinical Pathology, 28, 56–63.
Roberts, S. C., Noyes, P. D., Gallagher, E. P., & Stapleton, M. (2011). Species-specific differences and structure− activity relationships in the debromination of PBDE congeners in three fish species. Environmental Science & Technology, 45(5), 1999–2005.
Rochman, C. M., Kurobe, T., Flores, I., & Teh, S. J. (2014). Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Science of the Total Environment, 493, 656–661.
Ronda, A. C., Oliva, A. L., Orazi, A. A. H., & MM, Marcovecchio JE,. (2019). Biomarker responses to polycyclic aromatic hydrocarbons in the native fish Ramnogaster arcuata. South America Int J of Environ Res., 13(1), 77–89.
Salam, A., & Davies, P.M.C. (1994). Body composition of northern pike (Esoxlucius L.) in relation to body size and condition factor. Fisheries Research, 19(3–4), 193–204.
Sarkar D, Chowdhury JP, Singh SK (2015). Effect of polybrominated diphenyl ether (BDE-209) on testicular steroidogenesis and spermatogenesis through altered thyroid status in adult mice. Gen Comp Endocrinol, 2016 Dec 1;239, 50-61. https://doi.org/10.1016/j.ygcen.2015.11.009. Epub 2015 Nov 19. PMID: 26602377.
Sharkey, M., Harrad, S., Abou-Elwafa Abdallah, M., Drage, D. S., & Berresheim, H. (2020). Phasing-out of legacy brominated flame retardants: The UNEP Stockholm Convention and other legislative action worldwide. Environment International, 144.
Silverman, L. M., Mendell, J. R., Sahenk, Z., & Fontana, M. B. (1976). Significance of creatine phosphokinase isoenzymes in duchenne dystrophy. Neurology, 26(6), 561–561.
Sindiku, O., Babayemi, J., Osibanjo, O., Schlummer, M., Schluep, M., Watson, A., & Weber, R. (2015). Polybrominated diphenyl ethers listed as Stockholm Convention POPs, other brominated flame retardants and heavy metals in e-waste polymers in Nigeria. Environmental Science and Polish Research, 22(19), 14489–14501.
Sivaperumal, P., & Sankar, T. V. (2011). Toxic effects of methyl parathion on antioxidant enzymes and acetylcholinesterase activity in freshwater fish, Labeo rohita.
Slaninova, A., Modra, H., Hostovsky, M., Sisperova, E., Blahova, J., Matejova, I., Vicenova, M., Faldyna, M., Zelnickova, L., Tichy, F., & Svobodova, Z. (2014). Effects of subchronic exposure to N, N-diethyl-m-toluamide on selected biomarkers in common carp (Cyprinus carpio L.). BioMed research international, 2014. https://doi.org/10.1155/2014/828515
Sloan, C., Anulacion, B., Bolton, J., Boyd, D., Olson, O., Sol, S., Ylitalo, G., & Johnson, L. (2010). Polybrominated diphenyl ethers in outmigrant juvenile Chinook salmon from the Lower Columbia River and Estuary and Puget Sound. Washington Arch Environ Contam Toxicol., 58(2), 403–414.
Srivastava, P., Singh, A., & Pandey, A. K. (2016). Pesticide’s toxicity in fishes: Biochemical, physiological and genotoxic aspects. Biochemical and Cellular Archives, 16(2), 199–218.
Stapleton, H. M., Alaee, M., Letcher, R. J., & Baker, J. E. (2004). Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environmental Science and Technology, 38(1), 112–119.
Stapleton, H. M., Brazil, B., Holbrook, R. D., Mitchelmore, C. L., Benedict, R., Konstantinov, A., & Potter, D. (2006). In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environmental Science and Technology, 40(15), 4653–4658.
Talas, Z. S., Ozdemir, I., Yilmaz, I., & Gok, Y. (2008). Antioxidant effects of novel synthetic organoselenium compound in rat lung and kidney. Ecotoxicology and Environmental Safety, 72(3), 916–921.
Topal, A., Atamanalp, M., Oruç, E., & Erol, H. S. (2017). Physiological and biochemical effects of nickel on rainbow trout (Oncorhynchus mykiss) tissues: Assessment of nuclear factor kappa B activation, oxidative stress and histopathological changes. Chemosphere, 166, 445–452.
USEPA. US Environmental Protection Agency. (2010). An Exposure Assessment of Poly-brominated Diphenyl Ethers. Available from: http://www.epa.gov/ncea
Vodianitskyi, O. M., Potrokhov, O. S., Zinkovskyi, O. G., Khudiiash, Y. M., Prychepa, M. V. (2021). Effects of increasing water temperature and decreasing water oxygen concentration on enzyme activity in developing carp embryos (Cyprinus carpio). Fisheries & Aquatic Life, 29(1).
Wang, Y., Zhang, Q., Chen, S., Cheng, L., Jing, X., Wang, X., ... & Rao, Q. (2021). Determination of polybrominated diphenyl ethers in water samples using effervescent-assisted dispersive liquid-liquid icroextraction with solidification of the aqueous phase. Molecules, 26(5), 1376. https://doi.org/10.3390/molecules26051376
Xie, Z., Lu, G., & Qi, P. (2014). Effects of BDE-209 and its mixtures with BDE-47 and BDE-99 on multiple biomarkers in Carassius auratus. Environmental toxicology and pharma, 38(2), 554–561.
Young, G. D., & Koplovitz, I. (1995). Acute toxicity of cyclohexylmethylphosphonofluoridate (CMPF) in rhesus monkeys: serum biochemical and hematologic changes. Archives of Toxicology, 69(6), 379–383.
Zeng, Y. H., Luo, X. J., Chen, H. S., Yu, L. H., Chen, S. J., & Mai, B. X. (2012). Gastrointestinal absorption, metabolic debromination, and hydroxylation of three commercial polybrominated diphenyl ether mixtures by common carp. Environmental Toxicology and Chemistry, 31(4), 731–738.
Zhao, S. J., Guo, S. N., Zhu, Q. L., Yuan, S. S., & Zheng, J. L. (2018). Heat-induced oxidative stress and inflammation involve in cadmium pollution history in the spleen of zebrafish. Fish & Shellfish Immunology, 72, 1–8.
Acknowledgements
The corresponding author thankfully acknowledges Science and Engineering Research Board (SERB), Government of India, Delhi for the financial assistance under Early Career Research Award Scheme. The authors are thankful to the Director, CSIR-NEERI, Nagpur for providing all the laboratory facilities.
Funding
Science and Engineering Research Board.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, K., Singh, A., Swamy, S. et al. Use of enzymatic biomarkers of Labeo rohita to study the effect of polybrominated diphenyl ether (BDE- 209) via dietary exposure in laboratory conditions. Environ Monit Assess 194, 499 (2022). https://doi.org/10.1007/s10661-022-09963-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-09963-0