Abstract
Previous studies have examined the presence, distribution, and concentrations of toxic contaminants in two major waterways in the Pacific Northwest: the lower Columbia River and Estuary (LCR&E) and Puget Sound, Washington. However, those studies have not reported on the levels of polybrominated diphenyl ethers (PBDEs) in juvenile Chinook salmon (Onchorynchus tshawytscha). Populations of Chinook salmon from the LCR&E and Puget Sound are declining, and some stocks are currently listed as “threatened” under the Endangered Species Act. Bioaccumulation of contaminants, including PBDEs, by juvenile Chinook salmon in the LCR&E and Puget Sound is of concern due to the potential toxicity of the contaminants and associated sublethal effects in fish. In this article, we present the concentrations of PBDEs measured in gutted bodies and stomach contents of outmigrant juvenile Chinook salmon collected at six sites in the LCR&E and four sites in Puget Sound. For comparison, we also analyzed gutted bodies of juvenile Chinook salmon from eight hatcheries in the LCR&E as well as samples of the hatchery fish feeds. The mean ∑PBDE concentrations measured in bodies of juvenile Chinook salmon from the different sites ranged from 350 to 2800 ng/g lipid weight, whereas those in stomach contents ranged from less than the quantitation limit (<2 ng/g wet weight) to 39 ng/g wet weight. The levels of PBDEs in the hatchery fish were significantly lower than those measured in the salmon samples collected from the LCR&E and Puget Sound. These results show that outmigrant juvenile Chinook salmon in the LCR&E and Puget Sound have been exposed to PBDEs in the environment and that these chemicals are bioaccumulating in their tissues; thus, the potential effects of PBDEs on these salmon should be further investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Columbia River and Puget Sound are two major aquatic systems in the Pacific Northwest (USA) that support a number of commercially and recreationally important species of fish. These systems provide habitat for several species of salmon, including populations or genetic stocks such as the West Cascades fall Chinook salmon (Onchorynchus tshawytscha) from the Lower Columbia River, Upper Willamette River Chinook salmon, and Puget Sound Chinook salmon, all of which are fish stocks that are currently listed as “threatened” under the Endangered Species Act (ESA) (Good et al. 2005). Various natural and anthropogenic factors are contributing to the decline of these Chinook salmon populations (National Research Council 1996). Potential threats, including exposure to toxic contaminants, are being assessed in the Lower Columbia River and Estuary (LCR&E) and in Puget Sound in order to identify and address the actions needed to promote the recovery of Chinook salmon (Fresh et al. 2005; NMFS 2007).
Contaminants, including polybrominated diphenyl ethers (PBDEs), are of concern as a possible threat to ESA-listed salmon because of their potential toxicity to fish at low concentrations (Jenssen et al. 2004; Tomy et al. 2004). PBDEs have been extensively used as flame retardants in electronic components, plastics, textiles, and other products (Rahman et al. 2001). There have been three commercial formulations of these compounds produced—penta-BDE, octa-BDE, and deca-BDE. The penta-BDE and octa-BDE technical products were produced in the United States until 2004, when the manufacturer voluntarily phased out production of these mixtures, whereas deca-BDE is currently still produced and used in the United States. PBDEs are considered ubiquitous in the environment (Hites 2004), and are dispersed via air, water and sewage sludge following their release from industrial waste or from products containing PBDEs during their use or after their disposal (Hale et al. 2003; Rahman et al. 2001). For example, penta-BDE was primarily used to prevent flammability of polyurethane foam (PUF) used in furniture, carpet pads, and motor vehicles. In addition to historical releases of PBDEs during the production of technical mixtures and manufacture of products containing PBDEs, PBDEs apparently continue to enter the environment in the vaporized state, on particles to which they have resorbed or on fragments of disintegrated PUF (Hale et al. 2002; Harrad et al. 2004; Rudel et al. 2003) and plastic (Suzuki et al. 2009; Webster et al. 2009). These persistent compounds have low water solubility, high binding affinity to particles, and a tendency to accumulate in sediments (de Wit 2002). Due to their lipophilicity, PBDEs can readily bioaccumulate in exposed organisms (Burreau et al. 1997; Stapleton et al. 2004c).
Previous analyses of suspended sediments, passive water samplers (e.g., semipermeable membrane devices), and resident fish collected from the LCR&E have shown the presence of PBDEs (Johnson et al. 2006; Morace 2006). PBDEs have also been measured in waterways that discharge into Puget Sound (Hart Crowser Inc. et al. 2007) as well as in fish (PSAT 2007) and marine mammals (Krahn et al. 2007; Ross 2006) that are known to reside in waters of Puget Sound for a portion of their lives. Other toxic contaminants, such as polychlorinated biphenyls (PCBs), chlorinated pesticides, and polycyclic aromatic hydrocarbons have previously been reported in various environmental samples collected from Puget Sound and the LCR&E (Feist et al. 2005; Hinck et al. 2006; McCarthy and Gale 2001; West et al. 2001, 2008), including juvenile Chinook salmon (Johnson et al. 2007a, 2007b; Olson et al. 2008; Stehr et al. 2000). Moreover, some of these contaminants (e.g., PCBs) were measured in some Chinook salmon at concentrations that might cause sublethal effects in these animals.
The objectives of this study were to measure PBDE concentrations in threatened juvenile salmon from various riverine and estuarine sites in the Pacific Northwest to determine the extent of their exposure to this emerging contaminant of concern and to measure the levels of PBDEs in juvenile salmon and feed from hatcheries in this region to determine whether they might be exposed to these compounds during hatchery rearing. To our knowledge, this study is the first to report PBDE concentrations and congener proportions measured in gutted bodies and stomach contents of juvenile Chinook salmon collected in the LCR&E and Puget Sound and to evaluate PBDE exposure in salmon from different threatened stocks.
Methods
Sample Collection
Juvenile Chinook salmon were collected by beach seine from six sites in the LCR&E (Fig. 1) at three to five time points at each site during the spring and summer of 2005. The Warrendale site is located on the Columbia River just downstream of the Bonneville Dam, in a minimally developed area. The Portland site is on the Willamette River in downtown Portland, which is in a heavily urbanized and industrialized area. The Willamette/Columbia confluence site (from here on referred to as the Confluence) is in an urbanized area south of Vancouver, Washington. Several Environmental Protection Agency (EPA) Superfund sites are located in this area, as well as a number of industries, including aluminum and steel plants, chemical plants, pulp and paper mills, and electronics plants. The Columbia City site is semirural, with some local industry associated with wood products (e.g., pulp mills, wood treatment facility, lumber mills). The Beaver Army Terminal site is a semirural site near Quincy, Oregon, downstream of Longview, Washington. Local industries include pulp and paper mills, a lumber company, a wood treatment facility, and gypsum and aluminum cable plants. The Point Adams site is in a semirural area near Hammond, Oregon. Local industries include a fish processing plant and lumber mill. Juvenile Chinook salmon were not found at all sites during every month of sampling due to different outmigration times. Additionally, juvenile Chinook salmon were collected by beach seine from four sites in Puget Sound, Washington in 2006 (Fig. 2). The Duwamish Waterway and Elliott Bay sites are adjacent to heavily industrialized and urbanized areas in Seattle, and the Elliott Bay site is also adjacent to a major sewage outfall. The Snohomish River site is adjacent to a sewage treatment plant and a landfill, and local industry includes a pulp mill. The Skagit Delta site is in an agricultural and semirural area, downstream from the city of Mount Vernon. Juvenile Chinook salmon and samples of fish feed were obtained from eight hatcheries on the lower Columbia River and its tributaries (Fig. 1). These hatcheries are thought to be major contributors to Chinook salmon populations in the Lower Columbia, based on release data and previous genetic analyses of juvenile salmon collected from sites within the estuary (Johnson et al. 2007b).
At each location, 8–45 individual juvenile Chinook salmon were collected for necropsy at each sampling time point. Chinook salmon bodies were measured (to the nearest millimeter) and weighed (to the nearest 0.1 g) and then sacrificed by a blow to the head. Stomach contents were removed from each fish and composited. Fin clips from the LCR&E Chinook salmon were also collected and preserved in 70% ethanol for determination of genetic stock of origin. Bodies, stomach contents, and fish feed samples were stored at –80°C prior to chemical analysis.
Genetic Analyses
To examine differences in levels of PBDEs among populations of Chinook salmon from the LCR&E, the genetic stock of origin was determined for the Chinook salmon using microsatellite analyses as described in the study by Narum et al. (2008). An existing Chinook salmon microsatellite genetic baseline for the Columbia River Basin was used to determine the stock composition and origin of salmon individuals captured in the LCR&E. Chinook salmon were assigned to their most likely baseline population of origin based on their multilocus microsatellite and then summed over the appropriate genetic Reporting Group (RG), which are pools of genetically similar populations generally corresponding to Evolutionarily Significant Units (ESUs). Genetic RGs identified for the subyearling Chinook salmon, which were our target species, were as follows: West Cascades fall Chinook salmon from the Lower Columbia River, Spring Creek fall Chinook salmon from the Lower to Middle Columbia River, Upper Willamette River Chinook salmon, Deschutes/Snake River fall Chinook salmon, and Upper Columbia River summer/fall Chinook salmon. Some of these RGs included fish of hatchery origin, but due to the high degree of historical mixing, straying, and introgression among wild and hatchery populations, individual wild and hatchery fish could not be reliably distinguished in the genetic analyses (Myers et al. 2006). Although not all of the salmon for which genetic information was obtained were analyzed for PBDEs, the data on the entire set of fish are reported to provide a more accurate representation of stock distribution at the sampling sites.
Analyses for PBDEs and Percent Lipid Content
For Chinook salmon samples collected from the LCR&E and the hatcheries, bodies of the same genetic RG were composited prior to analysis. Because of limited funds for chemical analyses, not all fish collected for genetic RG determination were analyzed for PBDEs and lipid content. Stomach content samples were also composites. For the Chinook salmon collected from the Puget Sound sites, individual bodies were analyzed rather than composite samples because of the limited number of fish collected. Only seven samples of stomach contents were analyzed because of the limited amount of material available from the Puget Sound fish. Fish feed from the LCR&E hatcheries were analyzed in lieu of hatchery fish stomach contents.
Samples of homogenized bodies and stomach contents were extracted and analyzed for PBDEs using a modification of a previously described method (Sloan et al. 2005). For the surrogate standard, 75 ng of PCB 103 were added to each sample prior to extraction. Samples were extracted using automated, pressurized solvent extraction, and the extracts were cleaned up using gravity-flow columns containing silica gel and alumina followed by high-performance size-exclusion liquid chromatography. A high-performance liquid chromatography (HPLC) internal standard (75 ng of tetrachloro-meta-xylene) was added to each sample prior to clean up to quantify the surrogate standard, and a final internal standard (30 ng of tetrachloro-ortho-xylene) was added, after the extract was concentrated, to quantify the HPLC standard. A low-resolution quadrupole gas chromatograph/mass spectrometer (GC/MS) was used to measure the concentrations of PBDE congeners 28, 47, 49, 66, 85, 99, 100, 153, 154, and 183 [congener numbers are based on the numbering system used for PCBs by Ballschmiter et al. (1992)]. For each bromination level, two ions were monitored and their combined response was used for analyte quantification. The ion masses (daltons) monitored are as follows: 406 and 408 for the tri-BDE (BDE 28), 484 and 486 for the tetra-BDEs (BDEs 47, 49, and 66), 564 and 566 for the penta-BDEs (BDEs 85, 99, and 100), 642 and 644 for the hexa-BDEs (BDEs 153 and 154) and 562 and 564 for the hepta-BDE (BDE 183). The GC/MS was calibrated using four multilevel calibration standards ranging from 2.5 pg/μL to 1.0 ng/μL, and a surrogate standard was used to calculate the analyte concentrations. The lower limit of quantitation (LOQ) for each congener was based on the analyte’s area in the lowest-level calibration standard; that is, the LOQ is the concentration that an analyte would have in a given sample if it had an area equal to the analyte’s area in the lowest-level GC/MS calibration standard analyzed concurrently. The signal-to-noise ratio for each congener in the lowest-level calibration standard was 25 or greater. LOQs ranged from 0.20 to 0.71 ng/g wet weight for bodies and from 0.4 to 4.0 ng/g wet weight for stomach contents, depending on the mass of sample available for analysis. Summed (∑) PBDE concentrations reported are the sum of the concentrations of the 10 congeners listed earlier, with congener concentrations below the LOQ (<LOQ) treated as zero.
Total percent lipid in samples of bodies was determined using thin-layer chromatography/flame ionization detection as previously described (Ylitalo et al. 2005). A 1-μL aliquot of concentrated extract (subsampled prior to cleanup) was spotted on a Type SIII Chromorod, which was then developed in a chromatography tank containing 60:10:0.02 hexane:diethyl ether:formic acid (v:v:v) for 24 min prior to being scanned. Total percent lipid values were calculated using the sum of the concentrations of five lipid classes measured: wax esters/sterol esters, triglycerides, free fatty acids, cholesterols and polar lipids (e.g., phospholipids).
Quality Assurance for PBDEs and Percent Lipid
Quality assurance (QA) guidelines for measuring PBDEs and percent lipid were followed as described by Sloan et al. (2006). The Standard Reference Material (SRM) 1947 from the National Institute of Standards and Technology and a method blank sample were analyzed with each batch of up to 15 body or stomach content samples. Each analyte was below the LOQ in all method blanks (n = 14). In all SRM 1947 samples (n = 14), at least six of the seven congeners having certified values were within 30% of either end of the 95% confidence interval for the certified value. In addition, ~5% of the samples were analyzed in duplicate and percent differences of the analyte concentrations greater than 1 ng/g wet weight ranged from 0% to 17%, except for one sample with percent differences of 24–54%. The recoveries of the surrogate standard ranged from 60% to 122%. Four of the SRM 1947 samples were analyzed in batches containing only stomach contents and were not analyzed for percent lipid because stomach contents were not analyzed for lipid; thus, percent lipid values for only 10 SRM 1947 samples were reported. Percent lipid measured in the SRM 1947 samples (n = 10) met the QA criterion that the percent lipid values were within 35% of either end of the 95% confidence interval for the reference value. The duplicate samples analyzed for the percent lipid had percent differences of the percent lipid values ranging from 9% to 15%, except for one sample with a percent difference of 36%.
Statistical Analyses
Mean concentrations of ∑PBDEs (ng/g lipid) in Chinook salmon bodies were compared among the sampling sites and the genetic reporting groups using one-way analysis of variance (ANOVA) and the Tukey–Kramer Honestly Significant Difference (HSD) post hoc test (Zar 1984) with a significance level set at p < 0.05. Due to large differences in the variances about the means, the PBDE data were log-transformed to reduce the differences in the variances prior to performing the statistical analyses (Zar 1984). To include all data, all “<LOQ” results were set to zero and the statistical analyses were performed on log(concentration + 1) (Zar 1984). All statistical analyses were completed using JMP Statistical Software (SAS Institute, Inc., Cary, NC).
Results and Discussion
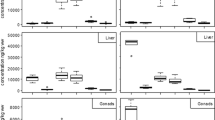
The PBDEs were found in juvenile Chinook salmon bodies collected from all sites in the LCR&E and Puget Sound. The ∑PBDE concentrations were variable within and among the collection sites (Table 1). Mean ∑PBDEs measured in bodies from Portland, Columbia City, Snohomish, and Beaver Army Terminal were statistically significantly higher than those in bodies from Warrendale, and all mean concentrations in bodies from the LCR&E and Puget Sound sites were significantly higher than those in the hatchery salmon (Table 1). PBDEs were <LOQ in bodies from six out of the eight hatcheries. The concentrations measured in the bodies from Puget Sound sites were generally within the range of those measured in bodies from the LCR&E. Concentrations in bodies from the Skagit Delta (n = 12) ranged from 83 to 1000 ng/g lipid weight, except for one fish that had an exceptionally high concentration of 13,000 ng/g lipid weight. Concentrations in bodies from the Duwamish River and Elliott Bay, which are urban sites, were expected to be among the highest values, but they were among the sites with relatively moderate mean concentrations.
The concentrations of PBDEs measured in the salmon stomach contents indicate the levels of recent dietary exposure to PBDEs. Similar to the results for bodies, the concentrations measured in the stomach content samples varied within and among the sites (Table 2). Portland, the Confluence, Columbia City, and Snohomish River showed the highest mean ∑PBDE concentrations in stomach contents; however, only one sample of composited stomach contents from the Confluence site had a relatively high concentration (110 ng/g wet weight), and the remaining stomach contents samples from this site had 10 ng/g or less. PBDEs were <LOQ in the single stomach content composite from the Duwamish River; however, the LOQs of the individual congeners were high (~2 ng/g wet weight each) due to the small mass of the sample (<0.3 g) for this site. The hatchery fish feed (Table 2) had relatively low concentrations compared to the stomach contents of salmon from most sites in the LCR&E. Statistical comparisons were not made among stomach content samples due to the limited number of samples from some sites.
Numerous studies have measured PBDE concentrations in various fish species across North America (Ackerman et al. 2008; Batterman et al. 2007; Brown et al. 2006; Chernyak et al. 2005; desJardins Anderson and MacRae 2006; de Wit 2002; Dodder et al. 2002; Hale et al. 2001; Ikonomou et al. 2006; Johnson-Restrepo et al. 2005; Law et al. 2006; Rayne et al. 2003; Rice et al. 2002; Streets et al. 2006; Zhu and Hites 2004). In a recent study by the Washington State Department of Ecology (Johnson et al. 2006), PBDEs were measured in composite fillet samples of several fish species, not including Chinook salmon, collected statewide from lakes and rivers during 2004–2005, including the Lower Columbia River. The authors reported ∑PBDE concentrations of 530, 850, and 1200 ng/g lipid weight (8.4, 17, and 31 ng/g wet weight) in single samples of composited fillets of peamouth (Mylocheilus caurinus), northern pikeminnow (Ptychocheilus oregonenis), and largescale sucker (Catostomus macrocheilus), respectively, collected from the Columbia River downstream of Beaver Army Terminal. Interestingly, even though these concentrations were measured in fillets of adult fish, they are in the same range as those reported in bodies of juvenile salmon in the current study.
Genetic analyses indicated that a variety of fall Chinook salmon stocks were present at the LCR&E sampling site (Fig. 3). Overall, Lower Columbia River fall salmon and Middle Columbia River fall salmon were most common, and Deschutes/Snake River fall salmon were observed least frequently. Stock distribution varied from site to site, and not all stocks were found at each site. Mean ∑PBDE concentrations were highest in salmon bodies belonging to the Upper Willamette River RG and lowest in salmon from the Deschutes/Snake River RG (Table 3). With the exception of salmon belonging to the Deschutes/Snake River RG, mean concentrations were higher in salmon from all the RGs than in juvenile salmon collected from Lower Columbia River hatcheries (p < 0.05); however, no significant differences were observed among fish from the various RGs.
In Fig. 4, mean concentrations in juvenile salmon from the four stocks collected at the Warrendale site (the rural area upstream of the urban centers of Portland, Oregon and Vancouver, Washington) were compared to those in salmon from the same stocks collected at all other sites on the Lower Columbia River (below Warrendale) combined. Results of two-way ANOVA indicated that although the genetic RG to which the fish belonged had no significant effect on whole-body PBDE concentration (p = 0.3283), fish collected from sites below Warrendale had significantly higher PBDE levels than those from the Warrendale site (p = 0.0029).
The presence of PBDEs in the stomach contents show that the Pacific Northwest outmigrant juvenile Chinook salmon in this study are exposed to PBDEs through their diet, which consists primarily of terrestrial and aquatic insects. Other potential exposure routes include intake of contaminated sediment and suspended particles during feeding and, to a lesser extent, contaminated water (de Boer et al. 2003). Body burdens of individual BDE congeners in fish reflect the concentrations to which the fish are exposed plus the fish’s ability to assimilate and depurate the congeners. Figures 5 and 6 show the mean relative proportions of the individual PBDE congeners measured in the salmon bodies and in the stomach contents, respectively, based on collection sites, as well as in the penta-BDE commercial product “DE-71” (La Guardia et al. 2006). We found that BDEs 47 and 99 were the predominant congeners in the LCR&E and Puget Sound Chinook salmon and stomach contents, followed by BDEs 100, 153, and 154 when found at measurable concentrations. The remaining congeners were generally near or below the LOQ. For the Skagit Delta stomach contents, the only congener that was above the LOQ was BDE 47. BDEs 47, 99, and 100 are the major congeners in the penta-BDE commercial products and were not found by La Guardia et al. (2006) at measurable concentrations in octa or deca commercial PBDE products. PBDE congener distributions could be altered in the environment by various abiotic factors (Hale et al. 2006) or anaerobic microbial debromination (Tokarz et al. 2008). Some differences in congener proportions, particularly with respect to the percentage of BDE 99, were seen among the sites in this study for both stomach contents and bodies. These differences could be due to differences in the congener distributions to which the salmon prey were exposed. They could also be due to differences among the various species of prey in their ability to metabolize certain congeners because the salmon consumed varying proportions of the invertebrate species among the sites.
Mean PBDE congener distributions in gutted bodies of juvenile Chinook salmon collected from Puget Sound, WA and the LCR&E, and in Technical Product DE-71 (La Guardia et al. 2006). The proportions of each congener are shown as the mean percentage of the summed PBDE concentrations, and the error bars show the standard deviations. Numbers of samples are in parentheses
Mean PBDE congener distributions in stomach contents of juvenile Chinook salmon collected from Puget Sound, Washington and the LCR&E, and in Technical Product DE-71 (La Guardia et al. 2006). The proportions of each congener are shown as the mean percentage of the summed PBDE concentrations, and the error bars show the standard deviations. Numbers of samples are in parentheses
Uptake efficiencies of individual BDE congeners in fish have been found to vary (Burreau et al. 1997). Body burdens can also reflect biotransformation of certain congeners by some species (Stapleton et al. 2004b, 2004c). Carp (Cyprinus carpio) and other species have exhibited a lack of bioaccumulation of BDE 99 in some field studies (Hale et al. 2001; Johnson et al. 2006). Unlike the results of these studies, BDE 99 was found to be a prominent congener measured in the fish in the current study, and thus it apparently is not selectively biotransformed to a great extent by juvenile Chinook salmon. Browne et al. (2009) found that microsomal fractions from adult Chinook salmon liver slowly debrominated BDE 99 to BDE 49 (average conversion of 3.1% by 16 h) but not to BDE 47, and the debromination of BDE 99 was inefficient compared to that by carp microsomal fractions. Other studies have also measured BDE 99 in several fish species, albeit at various proportions to the other congeners measured (de Wit 2002; desJardins Anderson and MacRae 2006; Hites 2004; Johnson et al. 2006). Previous studies have shown that BDE 209 can debrominate to lower brominated congeners but had limited bioavailability (<1%) and was not biotransformed to measurable concentrations of BDEs 47, 99, or 100 by rainbow trout (Oncorhynchus mykiss) (Kierkegaard et al. 1999) or carp (Stapleton et al. 2004a).
The toxicity of PBDEs has been investigated in several studies, as noted in the reviews by de Wit (2002) and Ross et al. (2008). Effects most regularly reported include alteration in thyroid hormone levels or thyroid function and neurodevelopmental defects. Such effects have been reported in fish as well as mammals (e.g., Lema et al. 2007; Muirhead et al. 2006), although generally at doses higher than those typically observed in the environment, and not specifically in juvenile Chinook salmon. Additionally, there is some inconsistency in the effects observed in different studies, possibly because dioxinlike chemical impurities (i.e., brominated dibenzodioxins and dibenzofurans) present in commercial PBDE mixtures are responsible for some of the toxicity (Kuiper et al. 2008). A few studies have observed PBDE effects in fish, including one salmonid species (lake trout), at environmentally relevant concentrations. For example, thyroid hormone alterations have been observed in juvenile turbot (Schophtalamus maximus) exposed to water-borne BDE 47 at concentrations of 5 ppb in the water column (Jenssen et al. 2004), in juvenile lake trout (Salvelinus namaycush) exposed to a mixture of 13 PBDE congeners in the diet at total concentrations as low as ~23 ng/g dry weight of dried food (Tomy et al. 2004), and in adult flounder with a BDE-47 concentration in muscle of 51 ng/g wet weight or above (Kuiper et al. 2008), although a comparable effect was not observed in zebrafish, the other test species in this study (Kuiper et al. 2008). Killifish (Fundulus heteroclitus) embryos incubated in aqueous solutions of as little as 1 ppb of DE-71 mixture developed behavioral changes suggesting neurodevelopmental toxicity (Timme-Laragy et al. 2006). Raldúa et al. (2008) found evidence of oxidative stress in wild barbels (Barbus graellsii) with PBDE body burdens similar to those we measured in salmon from Portland, the Confluence, Columbia City, and Snohomish River; however, exposure to other contaminants as well as other environmental stressors (e.g., low oxygen levels in water) might have also contributed to the oxidative stress of the barbels in that study. Although the current study did not examine toxicological effects of PBDEs in juvenile Chinook salmon, the levels measured in some fish and stomach contents were comparable to those associated with the biological effects in some of the studies cited earlier. This suggests that PBDEs might be contributing to reduced health and fitness in outmigrant juvenile Chinook salmon in the LCR&E and Puget Sound, particularly in combination with exposure to the other environmental contaminants that have been detected in salmon from these areas [e.g., PCBs, organochlorine pesticides (Johnson et al. 2007a, 2007b; LCREP 2007; Olson et al. 2008)]. Still, the reported effects of low-level exposure to PBDEs in fish are quite subtle and not always consistently observed, so the ultimate effect of these compounds on juvenile salmon survival is uncertain.
In summary, the results of the current study show that juvenile Chinook salmon collected from urban and nonurban sites in the LCR&E and Puget Sound are bioaccumulating PBDEs in their tissues and might be at risk for sublethal effects of these compounds, although the actual impact of PBDEs on salmon health is yet to be determined. In the LCR&E, where genetic information was available on sampled fish, PBDE accumulation was observed in salmon from several threatened stocks, with the greatest exposure occurring in fish collected in and down stream of the urban area of Portland, Oregon. The concentrations of ∑PBDEs measured in stomach contents indicate that the salmon are exposed to PBDEs through their diet. Furthermore, the hatcheries do not appear to be a principal source of the PBDE contamination found in the fish. Fish in the LCR&E and Puget Sound are also exposed to other contaminants, such as polycyclic aromatic hydrocarbons, PCBs, and chlorinated pesticides; thus PBDEs and other contaminants could have additive or synergistic toxicological effects. These potential threats on the survival and productivity of salmon stocks might be important for consideration in fishery recovery planning.
References
Ackerman LK, Schwindt AR, Massey Simonich SL, Koch DC, Blett TF, Schreck CB, Kent ML, Landers DH (2008) Atmospherically deposited PBDEs, pesticides, PCBs and PAHs in western U.S. national park fish: Concentrations and consumption guidelines. Environ Sci Technol 42:2334–2341
Ballschmiter K, Bacher R, Mennel R, Fischer R, Riehle U, Swerev M (1992) Determination of chlorinated biphenyls, chlorinated dibenzodioxins and chlorinated dibenzofurans by GC-MS. J High Resolut Chromatogr 15:260–270
Batterman S, Chernyak S, Gwynn E, Cantonwine D, Jia C, Begnoche L, Hickey JP (2007) Trends of brominated diphenyl ethers in fresh and archived Great Lakes fish (1979–2005). Chemosphere 69:444–457
Brown FR, Winkler J, Visita P, Daliwal J, Petreas M (2006) Levels of PBDEs, PCDDs, PCDFs, and coplanar PCBs in edible fish from California coastal waters. Chemosphere 64:276–286
Browne E, Stapleton H, Shannon K, Tilton S, Gallagher E (2009) In vitro hepatic metabolism of 2, 2’, 4, 4’, 5-pentabromodiphenyl ether (BDE 99) in Chinook salmon (Onchorhynchus tshawytscha). Aquat Toxicol 92:281–287
Burreau S, Axelman J, Broman D, Jakobsson E (1997) Dietary uptake in pike (Esox lucius) of some polychlorinated biphenyls, polychlorinated naphthalenes and polybrominated diphenyl ethers administered in natural diet. Environ Toxicol Chem 16:2508–2513
Chernyak SM, Rice CP, Quintal RT, Begnoche LJ, Hickey JP, Vinyard BT (2005) Time trends (1983–1999) for organochlorines and polybrominated diphenyl ethers in rainbow smelt (Osmerus mordax) from Lakes Michigan, Huron and Superior, USA. Environ Toxicol Chem 24:1632–1641
de Boer J, Wester P, van der Horst A, Leonards PEG (2003) Polybrominated diphenyl ethers in influents, suspended particulate matter, sediments, sewage treatment plant and effluents and biota from the Netherlands. Environ Pollut 122:63–74
de Wit CA (2002) An overview of brominated flame retardants in the environment. Chemosphere 46:583–624
desJardins Anderson T, MacRae JD (2006) Polybrominated diphenyl ethers in fish and wastewater samples from an area of the Penobscot River in Central Maine. Chemosphere 62:1153–1160
Dodder N, Strandberg B, Hites R (2002) Concentrations and spatial variations of polybrominated diphenyl ethers and several organochlorine compounds in fishes from the Northeastern United States. Environ Sci Technol 36:146–151
Feist GW, Webb MAH, Gundersen DT, Foster EP, Schreck CB, Maule AG, Fitzpatrick MS (2005) Evidence of detrimental effects of environmental contaminants on growth and reproductive physiology of white sturgeon in impounded areas of the Columbia River. Environ Health Perspect 113:1675–1682
Fresh KL, Casillas E, Johnson LL, Bottom DL (2005) Role of the estuary in the recovery of Columbia River Basin salmon and steelhead: an evaluation of the effects of selected factors on salmonid population viability. NOAA Tech. Memo. NMFS-NWFSC-69. US Department of Commerce, Washington, DC
Good TP, Waples RS, Adams P (Eds) (2005) Updated status of Federally listed ESUs of West Coast salmon and steelhead. NOAA Tech. Memo. NMFS-NWFSC-66. US Department of Commerce, Washington, DC
Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG (2003) Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int 229:771–779
Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor MT (2006) Brominated flame retardant concentrations and trends in abiotic media. Chemosphere 64:181–186
Hale RC, La Guardia JJ, Harvey E, Mainor M (2002) Potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere 46:729–735
Hale RC, La Guardia JJ, Harvey E, Mainor TM, Duff WH, Gaylor MO (2001) Polybrominated diphenyl ether flame retardants in Virginia freshwater fishes (USA). Environ Sci Technol 35:4585–4591
Harrad S, Wijesekera R, Hunter S, Halliwell C, Baker R (2004) Preliminary assessment of U.K. human dietary and inhalation exposure to polybrominated diphenyl ethers. Environ Sci Technol 38:2345–2350
Hart Crowser, Inc., Washington Department of Ecology, US Environmental Protection Agency, Puget Sound Partnership (2007) Phase 1: initial estimate of toxic chemical loadings to Puget Sound. Ecology Publication No. 07-10-079. Olympia, WA
Hinck JE, Schmitt CJ, Blazer VS, Denslow ND, Bartish TM, Anderson PJ, Coyle JJ, Dethloff GM, Tillitt DE (2006) Environmental contaminants and biomarker responses in fish from the Columbia River and its tributaries: spatial and temporal trends. Sci Total Environ 366:549–578
Hites H (2004) Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol 38:945–956
Ikonomou MG, Fernandez MP, Hickman ZL (2006) Spatio-temporal and species-specific variation in PBDE levels/patterns in British Columbia’s coastal waters. Environ Pollut 140:355–363
Jenssen G, Tyrhaug IB, Sormo EG, Andersen OD (2004) Effects of PBDE-47 on thyroid and steroid hormone status in juvenile turbot (Schophtalamus maximus). Organohalogen Compounds 66:3078–3081
Johnson A, Seiders K, Deligeannis C, Kinney K, Sandvik P, Era-Miller B, Alkire D (2006) PBDE flame retardants in Washington rivers and lakes: Concentrations in fish and water, 2005–06. Washington State Department of Ecology, Olympia, WA
Johnson LL, Ylitalo GM, Arkoosh MR, Kagley AN, Stafford C, Bolton JL, Buzitis J, Anulacion BF, Collier TK (2007a) Contaminant exposure in outmigrant juvenile salmon from Pacific Northwest Estuaries of the United States. Environ Monit Assess 124:167–194
Johnson LL, Ylitalo GM, Sloan CA, Lundrigan T, Anulacion BF, Kagley AN, Arkoosh MR, Larson K, Siipola M, Collier TK (2007b) Persistent organic pollutants in outmigrant juvenile Chinook salmon from the Lower Columbia Estuary, USA. Sci Total Environ 374:342–366
Johnson-Restrepo B, Kannan K, Addink R, Adams DH (2005) Polybrominated diphenyl ethers and polychlorinated biphenyls in a marine foodweb of Coastal Florida. Environ Sci Technol 39:8243–8250
Kierkegaard A, Lennart B, Tjärnlund U, de Wit CA, Jansson B (1999) Dietary uptake and biological effects of decabromodiphenyl ether in rainbow trout (Oncorhynchus mykiss). Environ Sci Technol 33:1612–1617
Krahn MM, Hanson MB, Baird RW, Boyer RH, Burrows DG, Emmons CK, Ford JK, Jones LL, Noren DP, Ross PS, Schorr GS, Collier TK (2007) Persistent organic pollutants and stable isotopes in biopsy samples (2004/2006) from Southern Resident killer whales. Marine Pollut Bull 54:1903–1911
Kuiper RV, Vethaak AD, Cantón RF, Anselmo H, Dubbeldam M, van den Brandhof E-J, Leonards PEG, Wester PW, van den Berg M (2008) Toxicity of analytically cleaned pentabromodiphenylether after prolonged exposure in estuarine European flounder (Platichys flesus), and partial life-cycle exposure in fresh water zebrafish (Danio rerio). Chemosphere 73:195–202
La Guardia MJ, Hale RC, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 40:6247–6254
Law K, Halldorson T, Danell R, Stern G, Gewurtz S, Alaee M, Marvin C, Whittle M, Tomy G (2006) Bioaccumulation and trophic transfer of some brominated flame retardants in a Lake Winnipeg (Canada) food web. Environ Toxicol Chem 25:2177–2186
LCREP (Lower Columbia River Estuary Partnership) (2007) Lower Columbia River estuary ecosystem monitoring: Water quality and salmon sampling report. Prepared by the Lower Columbia River Estuary Partnership, Portland, OR
Lema SC, Schultz IR, Scholz NL, Incardona J, Swanson P (2007) Cardiac arrhythmia, spinal defects and changes in cerebrospinal fluid flow in fish larvae following embryonic exposure to 2, 2’, 4, 4’-tetrabromodiphenyl ether (PBDE 47). Aquat Toxicol 82:296–307
McCarthy KA, Gale RW (2001) Evaluation of persistent hydrophobic organic compounds in the Columbia River Basin using semipermeable-membrane devices. Hydrol Processes 15:1271–1283
Morace JL (2006) Water-quality data, Columbia River Estuary, 2004–05. US Geological Survey Data Series 213. US Geological Survey, Washington, DC
Muirhead EK, Skillman AD, Hook SD, Schulz IR (2006) Oral exposure of PBDE-47 in fish: toxicokinetics and reproductive effects in Japanese medaka (Oryzias latipes) and fathead minnows (Pimephales promelas). Environ Sci Technol 40:523–528
Myers JM, Busack C, Rawding D, Marshall AR, Teel DJ, Van Doornik DM, Maher MT (2006) Historical population structure of Pacific salmonids in the Willamette River and lower Columbia River basins. NOAA Tech. Memo NMFS-NWFSC-73. US Department of Commerce, Washington, DC
Narum SR, Schultz TL, Van Doornik DM, Teel D (2008) Localized genetic structures persist in wild populations of Chinook salmon in the John Day River despite gene flow from outside sources. Trans Am Fish Soc 137:1650–1656
National Research Council, Committee on Protection, Management of Pacific Northwest Anadromous Salmonids (1996) Upstream: Salmon and society in the Pacific Northwest. National Academy Press, Washington, DC
NMFS (National Marine Fisheries Service) (2007) Puget Sound Salmon Recovery Plan. Prepared for NMFS by Shared Strategy Development Committee. Shared Strategy for Puget Sound, Seattle, WA
Olson OP, Johnson LL, Ylitalo GM, Rice CA, Cordell J, Collier TK, Steger J (2008) Fish habitat use and chemical contaminant exposure at restoration sites in Commencement Bay, Washington. NOAA Tech. Memo. NMFS-NWFSC-88. US Department of Commerce, Washington, DC
PSAT (Puget Sound Action Team) (2007) Puget Sound update: Ninth report of the Puget Sound Ambient Monitoring Program. Puget Sound Action Team, Olympia, WA
Rahman F, Langford KH, Scrimshaw MD, Lester JN (2001) Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ 275:1–17
Raldúa D, Parósm F, Solé M, Eljarrat E, Barceló D, Riva MC, Barata C (2008) First evidence of polybrominated diphenyl ether (flame retardants) effects in feral barbel from the Ebro River basin (NE, Spain). Chemosphere 73:56–64
Rayne S, Ikonomou M, Antcliffe B (2003) Rapidly increasing polybrominated diphenyl ether concentrations in the Columbia River System from 1992 to 2000. Environ Sci Technol 37:2847–2854
Rice C, Chernyak S, Begnoche L, Quintal R, Hickey J (2002) Comparisons of PBDE composition and concentration in fish collected from the Detroit River, MI and Des Plaines River, IL. Chemosphere 49:731–737
Ross PS (2006) Fireproof killer whales (Orcinus orca): flame-retardant chemicals and the conservation imperative in the charismatic icon of British Columbia, Canada. Can J Fish Aquat Sci 63:224–234
Ross PS, Couillard CM, Ikonomou MG, Johannessen SC, Lebeuf M, Macdonald RW, Tomy GT (2008) Large and growing environmental reservoirs of Deca-BDE present an emerging health risk for fish and marine mammals. Marine Pollut Bull 58:7–10
Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG (2003) Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37:4543–4553
Sloan CA, Brown DW, Pearce RW, Boyer RH, Bolton JL, Burrows DG, Herman DP, Krahn MM (2005) Determining aromatic hydrocarbons and chlorinated hydrocarbons in sediments and tissues using accelerated solvent extraction and gas chromatography/mass spectrometry. In: Ostrander GK (ed) Techniques in aquatic toxicology, vol 2. CRC Press, Boca Raton, FL, pp 631–651
Sloan CA, Brown DW, Ylitalo GM, Buzitis J, Herman DP, Burrows DG, Yanagida GK, Pearce RW, Bolton JL, Boyer RH, Krahn MM (2006) Quality assurance plan for analyses of environmental samples for polycyclic aromatic compounds, persistent organic pollutants, fatty acids, stable isotope ratios, lipid classes, and metabolites of polycyclic aromatic compounds. NOAA Tech. Memo. NMFS-NWFSC-77, US Department of Commerce, Washington, DC
Söderström G, Sellström U, de Wit CA, Tysklind M (2004) Photolytic debromination of decabromodiphenyl ether (BDE 209). Environ Sci Technol 38:127–132
Stapleton JM, Alaee M, Letcher RJ, Baker JE (2004a) Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ Sci Technol 38:112–119
Stapleton JM, Letcher RJ, Baker JE (2004b) Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio). Environ Sci Technol 38:1054–1061
Stapleton JM, Letcher RJ, Juliana JL, Baker JE (2004c) Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio). Environ Toxicol Chem 23:1939–1946
Stehr CM, Brown DW, Hom T, Anulacion BF, Reichert WL, Collier TK (2000) Exposure of juvenile Chinook and chum salmon to chemical contaminants in the Hylebos Waterway of Commencement Bay, Tacoma, Washington. J Aquat Ecosyst Stress Recovery 7:215–227
Streets SS, Henderson SA, Stoner AD, Carlson DL, Sicik MF, Swackhamer DL (2006) Partitioning and bioaccumulation of PBDEs and PCBs in Lake Michigan. Environ Sci Technol 40:7263–7269
Suzuki G, Akiko K, Sakai S-I, Takigami H (2009) Existence state of bromine as an indicator of the source of brominated flame retardants in indoor dust. Environ Sci Technol 43:1437–1442
Timme-Laragy AR, Levin ED, Di Guilio RT (2006) Developmental and behavioral effects of embryonic exposure to the polybrominated diphenylether mixture DE-71 in the killifish (Fundulus heteroclitus). Chemosphere 62:1097–1104
Tokarz JA III, Ahn M-Y, Leng J, Filley TR, Nies L (2008) Reductive debromination of polybrominated diphenyl ethers in anaerobic sediment and a biomimetic system. Environ Sci Technol 42:1157–1164
Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT (2004) Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush). Environ Sci Technol 38:1496–1504
Webster TF, Harrad S, Millette JR, Holbook RD, Davis JM, Stapleton HM, Allen JG, McClean MD, Ibarra C, Abdalla MA-E, Covaci A (2009) Identifying transfer mechanisms and sources of decabromodiphenyl ether (BDE 209) in indoor environments using environmental forensic microscopy. Environ Sci Technol 43:3067–3072
West J, O’Neill S, Lippert G, Quinnell S (2001) Toxic contaminants in marine and anadromous fishes from Puget Sound, Washington: results of the Puget Sound Ambient Monitoring Program Fish Component 1989–1999. Washington Department of Fish and Wildlife, Olympia, WA
West JE, O’Neill SM, Ylitalo GM (2008) Spatial extent, magnitude, and patterns of persistent organochlorine pollutants in Pacific herring (Clupea pallasi) populations in the Puget Sound (USA) and Strait of Georgia (Canada). Sci Total Environ 394:369–378
Ylitalo GM, Yanagida GK, Hufnagle LC Jr, Krahn MM (2005) Determination of lipid classes and lipid content in tissues of aquatic organisms using a thin layer chromatography/flame ionization detection (TLC/FID) microlipid method. In: Ostrander GK (ed) Techniques in aquatic toxicology, vol 2. CRC Press, Boca Raton, FL, pp 227–237
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice Hall, Englewood Cliffs, NJ
Zhu L, Hites R (2004) Temporal trends and spatial distributions of brominated flame retardants in archived fishes from the Great Lakes. Environ Sci Technol 38:2779–2784
Acknowledgments
We thank Richard Boyer, Ron Pearce, Karen Tilbury, and Gladys Yanagida for assistance with the chemical analyses, Jon Buzitis, Dan Lomax, Bill Mowitt, Mark Myers, and Maryjean Willis for the fish collection, Jill Leary, Jennifer Morace, Greg Fuhrer, Deborah Marriot, and Scott McEwen for study design and project management for LCR&E sampling, and Cathy Laetz and David Baldwin for reviewing this manuscript. This work was supported by the Bonneville Power Administration and the Lower Columbia River Estuary Partnership.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sloan, C.A., Anulacion, B.F., Bolton, J.L. et al. Polybrominated Diphenyl Ethers in Outmigrant Juvenile Chinook Salmon from the Lower Columbia River and Estuary and Puget Sound, Washington. Arch Environ Contam Toxicol 58, 403–414 (2010). https://doi.org/10.1007/s00244-009-9391-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-009-9391-y