Abstract
The health of a marine ecosystem can effectively be monitored by studying the levels of biomarkers in a representative species. A change in background level of a biomarker indicates exposure to a specific type of pollutants. It also identifies bioavailability and the organism response to the causative agent among the compounds present in the surrounding water body. Yellowfin seabream (Acanthopagrus latus), a local variety of fish, was examined for parent PAHs in the liver, its metabolites in bile by the GC-MS method as exposure biomarkers and cytochrome P4501A1 by assay of ethoxyresorufin-o-deethylase (EROD) in the liver as an effect biomarker. A comparison was made between fish collected in 2015 with the fish collected in 2005–2006 and stored at − 80 °C in the fish bank. The objective was to examine the extent of changes in the environmental quality of the Kuwait marine area and the status of fish health concerning oil-related pollutants since Arabian Gulf is surrounded by oil-producing countries. Interestingly, insignificant differences between the liver PAH content and EROD activity were observed in fish over the sampling periods. The fish efficiently metabolized PAHs and excreted hydroxy-metabolites in bile. The study suggested that environmental quality of the Kuwait marine area was not deteriorated to any serious extent in the last decade and biomarkers can be used effectively in assessing the thrust of sub-optimal levels of various contaminants present in the marine area on the resident biota.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the Arabian Gulf, oil pollution is ubiquitous although the concentration of its harmful component has always been found less in this area in comparison to other highly polluted sites in the world (Beg et al. 2015; Lyons et al. 2015; Al-Sarawi et al. 2015). However, the persistent contamination of oil in the marine environment requires careful watch on its concentration in water and its component especially PAHs in marine biota. It is well documented that both anthropogenic and natural sources contribute towards PAH pollution in the environment. Low molecular weight PAHs are more soluble and have high acute toxicity to the biota, with the increase in molecular weight solubility decreases but carcinogenicity increases (Salvo et al. 2016). PAHs with low aqueous solubility adhere to suspended particulate matter and by deposition their concentration increases in sediments. PAH accumulation in exposed fish and other biota depends on the metabolic ability of the organism (Kannan and Perrotta 2008). PAHs in fish are quickly disposed of, that is why low to moderate levels of accumulation was observed even in contaminated areas (Varanasi et al. 1990). Other organisms like planktons, crustaceans, and mollusks have a lower ability to metabolize PAHs which results in a greater accumulation in their tissues (Stegeman and Lech 1991).
In fish, metabolism of PAHs is well-studied; primarily, mixed function oxidases insert oxygen across the double bond in aromatic hydrocarbons forming epoxides and dihydrodiols, known as the phase 1 reaction of metabolism. Subsequently, in phase 2, the metabolites formed are conjugated with glucuronic acid, glutathione, or sulfate to form water-soluble conjugates. PAH metabolites in fish bile samples have been analyzed in a large number of studies as a measure of exposure (Krahn et al. 1993; Lin et al. 1996; Stroomberg et al. 1999; Aas et al. 2000; Ruddock et al. 2003). A rapid method utilizing fixed wavelength fluorescence (FF) has been advocated for the detection of PAH metabolites in the bile (Lin et al. 1996; Aas et al. 1998). Though FF screening method indicates PAH metabolites present in the bile, exact quantification of individual metabolites is not obtained (Aas et al. 2000). However, considering its simplicity, FF method is recommended for routine screening of the biological effects (ICES 2001).
Fish from Kuwait’s marine area have been previously analyzed for PAHs to define contamination and food safety (Literathy et al. 1992; Al-Yakoob et al. 1993; Fowler et al. 1993; Saeed et al. 1995; Sadiq et al. 2002). A study of the PAH body burden in three varieties of fish collected between 2004 and 2005 (Beg et al. 2009) revealed that more than 50% of tongue sole, 11–33% mullet, 15–20% yellowfin seabream contained PAHs higher than 600 ng/g dry tissue. PAHs in tissues were not correlated with the effect biomarker, ethoxyresorufin-o-deethylase (EROD). Torre et al. (2012) reported that PAHs in bile are better correlated with the effect biomarkers of PAH metabolism. Assessment of PAH metabolite concentrations in bile (rather than tissue levels) is used to indicate exposure (De Silva et al. 2006). After the 1991 Gulf war, sherry (Lethrinus kalloprerus) was collected during the Mt. Mitchell expedition from Arabian Gulf and analyzed for bile metabolites as an indicator of exposure to Kuwait crude oil (Krahn et al. 1993). Studies suggested that in detoxification process, several PAH metabolites produce carcinogenic metabolites in fish capable of causing hepatic lesions and neoplasm (Jhonson-Restrepo et al. 2008). In the present study, yellowfin seabream (Acanthopagrus latus) collected between 2005 and 2006 and stored at − 80 °C were examined along with samples collected in 2015 from the same Kuwait marine area. PAHs in fish liver and metabolites in bile were estimated as exposure biomarkers, and EROD activity in liver was determined as the effect biomarker. The objective was to determine if there is a difference in exposure biomarker PAHs and EROD in a representative fish species from Kuwait’s marine area after a gap of 10 years to assess if environmental quality had changes over this time period.
Materials and methods
Collection of fish
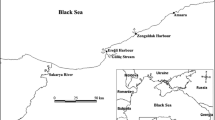
The fish yellowfin seabream (shaem, Acanthopagrus latus) was selected for this study because its stocks are considered to be local. It spawns in Kuwait waters, matures in its coast, and distributed throughout the marine area of Kuwait (Al-Yamani et al. 2004). As per Oslo and Paris (OSPAR) Commission recommendations, 12 fish of similar size, sex, and age are to be individually analyzed from each sampling station (OSPAR Commission 1999). The fish of the same sex were not available in a sampling campaign. Samples were collected by trawling (fish trawl net) over short hauls of 30-min duration in Kuwait Bay and Auha area around Global Positioning System (GPS) locations N 29° 26.69, E 48° 04.93 and N 29° 17.92, E 48° 23.60 respectively (Fig. 1). The sampling was done on the 13th and 16th of January 2015 in winters and 14th and 15th of July 2015 in summer months. Archived samples collected in 2005 and 2006 from the same locations were obtained from storage at – 80 °C for the analysis.
Condition index
The condition index (CI) and hepatosomatic index (HSI) of fish were calculated according to Sole et al. (2010) as given:
Processing of fish for the biomarker analysis
The collection, storage, and processing of fish were as described earlier (Beg et al. 2015). The fish soon after the collection were immersed in the liquid nitrogen and brought to the laboratory where stored in − 80 °C deep freezers. At the time of analysis, the fish were transferred to a refrigerator and allowed to thaw, then dissected. The bile was collected either by a syringe, or the gallbladder was taken out and ruptured in a dish to drain bile from which a measured volume of bile was taken for the analysis.
The liver was dissected, and tissue was divided for enzyme assay and chemical analysis. EROD was determined according to Stagg et al. (1995). A known quantity (300 mg each) was homogenized using Potter Elvehjem homogenizer in 3.0 ml buffer that contained 50 mM Tris-HCl buffer of pH 7.5, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), and 150 mM NaCl. The homogenate was centrifuged in refrigerated Eppendorf centrifuge for 20 min at 10,000×g. The supernatant was collected and stored in Eppendorf tubes for enzyme assay.
The reaction mixture for EROD assay contained 100 mM Tris-HCl buffer, pH 7.5, and 100 mM NaCl; 2 μM ethoxyresorufin dissolved in dimethyl sulfoxide (DMSO), an aliquot of enzyme preparation, and 0.5 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH) were added to start the reaction. The increase fluorescence was monitored for 3 min at 415-nm excitation and 580-nm emission wavelengths. The enzyme activity was calculated using molar absorbance in 100 mM Tris-HCl; pH 7.5 for resorufin at 561 nm was 23.2 mM cm−1 and expressed as picomoles of resorufin formed/min/mg protein.
Total protein was determined in tissue homogenates using the Lowry method (Lowry et al. 1951) with bovine serum albumin as standard.
Extraction and analysis of PAHs in liver
The method for the PAH analysis in the tissue was as reported earlier (Saeed et al. 1995). Liver tissue, 1-g wet weight, was weighed in a 7-ml mixture of chloroform: methanol (3:2). The mixture was ultrasonicated for 30 min, allowing the mixture to settle and filtered through a 0.45-μm filter, and the filtrate was collected. The residue was extracted two more times by ultrasonication for 15 min, each with 5 ml fresh solvent each time. Combined filtrates were reduced to about 2–3 ml on a rotary evaporator and then to dryness under a stream of nitrogen. For fatty samples, the residue was taken in 10 ml hexane in a round-bottom flask and added to 10 ml of 0.1 M potassium hydroxide (KOH) solution, the contents were refluxed for 2 h then transferred to a separatory funnel, and the aqueous layer was separated. The organic layer was washed three times with distilled water and dried over anhydrous sodium sulfate and the volume to 1 ml under the stream of nitrogen.
The cleanup was done by solid phase extraction (SPE) Silica cartridge containing 1-g silica in a 6-ml tube. The sample (1 ml) was loaded into the cartridge, and the sample was allowed to go into silica; 3 ml hexane passed through the cartridge, and the effluent was discarded. Subsequently, 3 ml of hexane:dichloromethane mixture (90:10) was passed, and effluent was collected, and the fraction was evaporated under nitrogen to dryness. Then, 1 ml of hexane containing 2 ppm of internal standards (d10-anthracene and d12-chrysene) was added.
The PAHs in tissue were analyzed as described earlier (Beg et al. 2009) by a Shimadzu GC-17A gas chromatograph using splitless injection on a 30-m HP5-ms column (0.25 mm i.d., 0.25-μm film thicknesses) and helium as carrier gas. The GC was coupled to a Shimadzu QP-5050A mass selective detector operated in electron impact (EI) mode using selected ion monitoring (SIM). The GC conditions were as follows: injector temperature, 290 °C; transfer line, 280 °C; initial oven temperature, 60 °C; initial hold, 1 min; ramp rate, 6 °C/min; final temp., 280 °C; final hold, 20 min; carrier gas, helium; and flow rate, 1.7 ml/min. Identification and quantification were carried out against five calibration standards of known concentration. A peak was positively identified if it was within ± 0.05 min of the retention time in the calibration standard and quantified if the S/N = 3, and the ratio of the ion to its qualifier ion was within ± 20% of the standard value.
Analysis of PAH metabolites in bile by GC/MS method
The method for deconjugation of bile metabolites was as described by van Schanke et al. (2001). Two hundred thirty microliters of HPLC grade water was added to 10-μl bile in a micro-centrifuge. To this was added 10 μl of β-glucuronidase and arylsulfatase enzyme solution containing 30 and 60 U/ml activity, respectively. The mixture was incubated in a shaking water bath at 37 °C for 45 min, and 250 μl chilled methanol was added to stop the reaction. The supernatant containing deconjugated metabolites was separated by centrifugation at 16,000×g for 10 min and stored in amber vials at 4 °C before analysis. Final extract in 0.5 ml was evaporated under nitrogen, and trimethylsilyl derivatives of OH-PAHs (TMS-O-PAHs) were prepared by the addition of 50 μl of bis(trimethylsilyl) trifluoroacetamide (BSTFA) in derivatization vial and heated at 60 °C for 60 min. After incubation, the reaction mixture was evaporated to dryness under a stream of nitrogen, and the derivatized residue was dissolved in 100 μl hexane and injected into an Agilent GC 6890 gas chromatograph with 5973 (MSD). Ion source temperature was kept at 280 °C. The derivatives were separated by a DB-5 capillary column coated with 5% phenyl methyl polysiloxane (30-m length × 0.25 mm i.d., 0.25-μm film thickness; J & W Scientific, Folsom, CA.) using helium as carrier gas at a flow rate of 1.0 ml/min. An autosampler was attached to GC and injections were performed in a splitless mode at 270 °C temperature. The oven temperature was initially programmed from 100 °C (2 min on hold) to 160 °C at a rate of 15 °C/min, then ramped at a rate of 10 °C/min to 295 °C (6 min on hold), and then increased at 9 °C/min to 300 °C, with a final hold time of 9 min. TMS-O-PAHs were scanned at selected ion monitoring mode. Fish bile samples were analyzed for 13 hydroxylated PAHs (OH-PAHs). Molecular ion cluster of {M+} mass to charge ratio (m/z) was 216.1 for 2,3-dihydroxynaphthalene and trans-1,2-dihydroxy-1,2-dihydronaphthalene; 254.0 for 2-hydroxyfluorene, 9-hydroxyfluorene, and 1-hydroxy-9-fluorenone; 266.0 for 1-hydroxy-phenanthrene, 2-hydroxyphenantharene, and 3-hydroxyphenantharene; 290 for 1-hydroxypyrene; 316 for 1-hydroxychrysene, 2-hydroxychrysene, 3-hydroxychrysene, and 4-hydroxychrysene; and 340 for 3-hydroxybenzo(a)pyrene.
Quality assurance/quality control
Analytical laboratories of Environment and Life Sciences Research Centre participate regularly in proficiency testing organized by the Environmental Research Associate (ERA, USA). The Chromatography laboratory has been recognized as the laboratory of excellence for achieving 100% acceptable data for PAHs in the April 2017 WP ERA proficiency testing round 267. For day to day analysis, analytical quality control measures were undertaken by including blanks and replicates and incorporating internal standards to check acceptable recovery during extraction and cleanup process. Samples for which extra bile was available with known amount of standards were added and recovery monitored.
Statistical analysis
The data were statistically analyzed using Minitab-15 statistical package and presented as means ± SD. Normality test was applied for variance check. One-way ANOVA test was used. Only P < 0.05 was accepted as significant.
Results
Yellowfin seabream collected between winter and summer of 2005–2006 were analyzed together with those collected in 2015 from Kuwait Bay and Auha area of Kuwait. The parameters used for comparison were morphometric measurement, condition factor (CF), hepatosomatic index (HIS), PAHs in the liver, PAH metabolites in the bile, and EROD activity in liver tissue. The data are presented in Table 1.
Morphometric measurements
Morphometric measurements showed that fish collected in summer months from both Auha and Kuwait Bay were larger in size and weight (P < 0.05) compared to winter with the exception from Auha (2005–2006 samples). The liver weight also showed a similar pattern as observed in morphometric measurements and the change was statistically significant (P < 0.05) summer and winter fish. However, comparing 2005–2006 and 2015, the only significant increase in the liver of fish was found in summer 2015 (P < 0.05). The condition index was significantly higher in fish from Auha in summer 2005–2006 and Kuwait Bay in summer 2015, compared to fish in winter. HSI was always higher in winter fish compared to summer fish; however, the difference was statistically significant (P < 0.05) only for fish from Kuwait Bay. In summer 2015, the fish obtained were bigger with just a few fish from each area were comparable to the winter sample regarding size and weight. It was attempted to group large-sized and small-sized fish separately to enable comparison with other samples. The CI and HSI were similar in fish from the two areas. The HSI in the lower weight fish was less than in higher weight fish. The fish collected from Kuwait Bay contained significantly higher protein in the liver (P < 0.05) in winter compared to summer from both sampling stations. In 2015, liver protein in fish was higher (P < 0.05) than in 2005–2006 fish, irrespective of the season and sampling area.
Ethoxyresorufin-o-deethylase
EROD activity fluctuated in the same range in the two sampling areas and seasons in 2005–2006 samples. In summer 2015, the fish captured were of bigger size but protein in liver and EROD activity were significantly (P < 0.05) less than that observed in winter months (Table 1). Since the enzyme activity is expressed on the basis of protein in the tissue, the lowering in EROD activity in summer is possibly due to the effect of season rather than the size of the fish.
PAHs in fish liver
Sixteen priority PAHs were analyzed in liver tissue of fish, and the data for the average values with standard deviation are presented in Table 1. The range of ∑PAHs in fish collected in summer of 2005 was 84.88 to 638.41 ng g−1 wet wt. from Auha area and 31.31 to 181.48 ng g−1 wet wt. from Kuwait Bay area. However, in winter 2006, lower concentrations of ∑PAHs were obtained compared to summer sampling and ranged from 17.50 to 79.95 ng g−1 wet wt. in fish from Auha area and from 24.22 to 86.72 ng g−1 wet wt. from Kuwait Bay. In winter 2015, the fish from Auha contained ∑PAHs 23.26 to 166.1 ng g−1 wet wt. and from Kuwait Bay 23.24 to 89.98 ng g−1 wet wt. In summer of 2015, ∑PAHs ranged from 20.68 to 94.64 from Auha area and from 16.12 to 347.5 from Kuwait Bay area.
In all the analyses, indenopyrene, dibenzanthracene, and benzoperylene were not detected in any sample examined. Benzo(b)fluoranthene was either not detected or detected mostly in fish from Auha area. Benzo(k)fluoranthene was detected in fish, but the frequency of detection varied from 25 to 80% on samples analyzed. The frequency of benzo(a)pyrene (BaP) in summer 2005 was 50% in fish from both the sampling areas; whereas in winter 2006, BaP was detected in 67 and 100% samples from Auha and Kuwait Bay area. In winter of 2015, BaP was detected in 67% fish from Auha and 50% in fish from Kuwait Bay area; whereas in summer 2015, it was not detected in fish from Auha and in only 17% samples from Kuwait Bay.
The PAH profile in the liver of fish from each location and season was determined by expressing individual PAH concentration as the percent of ∑PAHs to compare profile difference between Auha and Kuwait Bay and summer and winter seasons of 2005–2006 and 2015 samples and the data are presented in Fig. 2. The composition percent of the group of two-, three-, four-, and five-ring PAHs is present in Fig. 3. The PAH profile showed an abundance of three- and four-ring PAHs followed by naphthalene, the only representative of two-ring compounds. Among the five-ring PAHs, only benzo(a)pyrene was detected except that in winter 2006, dibenzanthracene was also present in samples from Kuwait Bay.
PAH metabolites in fish bile
Twelve PAH metabolites were detected in the bile, and the data for averages are reported in Table 1. The profile of individual metabolites expressed as percent of the total is presented in Fig. 4, and composition % of the group of PAH metabolites in Fig. 5.
The abundance of the metabolites in the bile was of two-ring PAH naphthalene in all the fish, irrespective of the season and year of sampling. Among the two naphthalene metabolites, trans-1,2-dihydroxy-dihydro naphthalene was the major metabolite contributing more than 50% of the total metabolite concentration in most of the samples. Comparing the locations, the two-ring PAHs were higher in samples from Auha area, compared to Kuwait Bay, except in winter 2015. The percent three- and four-ring concentrations were in general, higher in Kuwait Bay; while percent five-ring PAH was higher in fish from Kuwait Bay especially in winters. In 2005–2006, percent five-ring PAHs were high in fish from Kuwait Bay in the winter months. PAH metabolites in bile were found in exceptionally higher concentration in fish from Kuwait Bay in winter 2005–2006, whereas in other samples from different locations, the levels of bile metabolites fluctuated within the same range. Collective average of 2015 when compared with 2005–2006, the difference was found statistically insignificant (P > 0.05).
Discussion
In the present study, 16-priority PAH concentration in the liver of fish was measured in samples collected in 2005–2006 and compared with recent samples collected in 2015 from Kuwait Bay and outside the Bay in the Auha area. In general, low levels of PAHs were observed in liver irrespective of the sampling location and season of collection of fish. There was apparently no difference (P < 0.05) between the liver PAH contents observed in 2005–2006 fish samples and the fish samples collected after a decade in 2015, indicating low-level PAH contamination in the marine water of Kuwait’s territorial areas. PAHs are metabolically biodegradable, and fish contain an efficient system of metabolic activation by phase 1 reactions converting PAHs to epoxide or forming hydroxylated metabolites. By phase 2 reactions, these metabolites are conjugated with glucuronic acids or sulfate and ultimately excreted in the bile as hydroxylated or conjugated metabolites. The profile of parent PAHs in liver tissues and metabolites in bile revealed that two-ring PAH metabolites were present in high concentration in bile, whereas three- and four-ring compounds were high in the liver; but their metabolites were less in bile samples, indicating the faster metabolic disposition of two-ring compounds from liver tissue. In general, five-ring compounds were present in low levels in liver tissue, and detection of their metabolites in bile indicated the ability of the fish to metabolize high molecular weight PAHs also efficiently. High molecular weight compounds were detected mainly in winter months, especially from Kuwait Bay. The ratio of phenanthrene/anthracene (Phen/Ant) and fluoranthene/pyrene (FA/Py) have been used to determine that PAHs in the fish liver were from petroleum or combustion sources. It has been reported if Phen/Ant ratio is less than 10, the PAHs are from pyrolytic sources (Baumard et al. 1998; Yang et al. 2003), and the ratio of FA/Py less than 1 indicates petrogenic sources (Zhu et al. 2005). These ratios indicated that the source of PAHs detected in fish liver was both petrogenic and pyrogenic.
There were some differences in the PAH profile in the liver tissue in Auha and Kuwait Bay, but any consistent pattern did not emerge except that five-ring compounds were mostly detected in winter samples. In bile compared to 2005–2006 fish samples, the concentration of three- and four-ring compounds was higher in 2015 samples. The metabolites in bile have been correlated with the exposure concentrations (de Silva et al. 2006); therefore, a difference in the profile of metabolites indicates differential exposure of fish to these groups of hydrocarbon.
Repeated studies conducted in Kuwait suggested a low level of petroleum hydrocarbons in almost the entire marine area. In a baseline survey, sediment from 29 locations in Kuwait’s marine area was examined and ΣPAHs ranged 40.7–97.7 ng g−1 dry weight (Lyons et al. 2015). In an earlier study, sediment from the area of present sampling was analyzed earlier, and ΣPAHs ranged from 0.79 to 17.08 ng g−1 dry weight. The concentration observed in offshore sediment was less than that reported from coastal areas of Kuwait, the United Arab Emirates, and Kharg Island in the region (Beg et al. 2015). Arabian Gulf is surrounded by the oil-producing countries and receives consistent input of petroleum hydrocarbons because of oil production, refining, and transportation activities (ROPME 1999). In spite of that, PAH contamination in the sediment from most of the areas was found to be < 100 ng g−1 that is categorized as background non-polluted, whereas 250–500 ng g−1 as low- to medium-pollution, and > 5000 ng g−1 as high-pollution areas (Long et al. 1995; Notar et al. 2001).
Selection of a control site was not feasible for the collection of fish because of the persistent low levels of PAHs in the marine area of Kuwait. Therefore, EROD activity reported for hatchery-bred sea bream 12.93 ± 3.03 pmol min−1 mg−1 protein (Beg et al. 2001) was used as the intrinsic activity for comparison with the levels of EROD in wild sea bream captured from the field. In general, EROD activity in wild fish was higher than in the hatchery-bred fish. The EROD activity in 2005–2006 samples was within the same range in fish from the two sampling areas collected in winter and summer seasons, but in 2015, EROD was significantly higher in winter season from both sampling areas. Increased EROD activity showed exposure to the compounds capable of inducing cytochrome P450 1A since EROD is used as a catalytic measure of cytochrome P4501A1 (Whyte et al. 2000). However, no episodic increase in PAHs and also EROD was observed in any of the samples analyzed. The results also demonstrated insignificant differences in EROD between Auha and Kuwait Bay sites, which run parallel to PAHs in liver and metabolites in bile that showed a similar profile in both sites. The study provided information on PAH exposure in fish from Kuwait marine area and its variation in the last 10 years and impact on biotransformation mechanism. Apparently, no difference between PAH contents and profile, and EROD activity in the liver was observed in 2005–2006 fish samples and the fish samples collected after a decade in 2015, indicating low levels of PAH contamination in the marine environment of Kuwait’s territorial areas.
A pertinent question may be raised about the stability of samples stored at − 80 °C for 10 years or so. It is to be mentioned that the fish immediately after collection were brought to the laboratory in liquid nitrogen cylinder and transferred in deep freezer for storage. It is not expected any metabolic activity during storage; however, some decay during storage cannot be ascertained. The stored fish and the fish collected in 2015 were analyzed together to avoid procedural variations. In 2005–2006, from the same batch, whole fish were analyzed and the levels of ΣPAHs obtained were 5.77 (range 0.28–18.97) and 2.55 (0.82–7.46) μg g−1 lipid from Auha area and 5.51 (0.42–22.21) and 3.77 (0.58–15.27) μg g−1 lipid from Kuwait Bay area in winter and summer respectively (Beg et al. 2009). The PAHs in whole fish cannot be compared with that in PAHs in liver tissue determined in the present study but earlier data support that the accumulation of PAHs in yellowfin seabream was not of any serious nature. More so, some of the samples contained higher content of PAHs in liver of fish samples of 2005 compared to 2015 supporting that possibly the process of decay was not operative during storage. The same is true for EROD that is fluctuated within the same range in all the samples analyzed in the present study and reported earlier (Beg et al. 2015).
Thus, environmental quality of the Kuwait marine area concerning PAH contamination and PAH accumulation in fish tissue has not been changed to any serious extent in the last decade. The level of PAHs in the marine environment of Kuwait is low but its uptake and accumulation in liver and its disposition in bile are not free from its biological effects in the exposed organism. Al-Zaidan et al. (2015) reported that two fish varieties collected from five locations in Kuwait’s marine area contained low levels of PAH metabolites in the range observed in the present study. However, histological examination of liver showed certain lesions, though of less significance, but comparable with other studies as an effect of environmental toxicants (Al-Zaidan et al. 2015), and it is well documented that metabolites of PAHs in fish have strong correlation with hepatic lesions and neoplasm (Krahn et al. 1986; Jhonson-Restrepo et al. 2008).
References
Aas, E., Beyer, J., & Goksøyer, A. (1998). PAH in fish bile detected by fixed wavelength fluorescence. Marine Environmental Research 48, 225–228.
Aas, E., Beyer, J., & Goksøyer, A. (2000). Fixed wavelength fluorescence (FF) of bile as a monitoring tool for polyaromatic hydrocarbon exposure in fish: evaluation of compound specificity, inner filters effect, and signal interpretation. Biomarkers, 5, 9–23.
Al-Sarawi, H. A., Jha, A. N., Al-Sarawi, M. A., & Lyons, B. P. (2015). Historic and contemporary contamination in the marine environment of Kuwait: an overview. Marine Pollution Bulletin, 100, 621–628.
Al-Yakoob, S., Saeed, T., & Al-Hashash, H. (1993). Polycyclic aromatic hydrocarbons in edible tissue of fish from the Gulf after the 1991 oil spill. Marine Pollution Bulletin, 27, 297–301.
Al-Yamani, F. Y., Bishop, J., Ramadhan, E., Al-Husaini, M., & Al-Ghadban, A. N. (2004). Oceanographic atlas of Kuwait’s waters. Kuwait: EPA and KISR Publications.
Al-Zaidan, A. S., Al-Sarawi, H. A., Massoud, M. S., Al-Enezi, M., Smith, A. J., Bignell, J. P., Green, M. J., Askem, C., Bolamb, T. P. C., Barber, J. L., Bersuder, P., & Lyons, B. P. (2015). Histopathology and contaminant concentrations in fish from Kuwait’s marine environment. Marine Pollution Bulletin, 100, 637–645.
Baumard, P., Budzinski, H., Michon, Q., Garrigues, P., Burgeot, T., & Bellocq, J. (1998). Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuarine, Coastal and Shelf Science, 47, 77–90.
Beg, M. U., Al-Bahloul, M., Jacob, P. G., Beg, K. R., Al-Matrouk, K., & Abdel-Elah, K. (2001). Biomarker response in sheim (Acanthopagrus latus) exposed to polycyclic aromatic hydrocarbons. Bulletin of Environmental Contamination & Toxicity, 67, 210–216.
Beg, M. U., Gevao, B., Al-Jandal, N., Beg, K. R., Butt, S. A., Ali, L. N., & Al-Hussaini, M. (2009). Polycyclic aromatic hydrocarbons in three varieties of fish from Kuwait Bay. Polycyclic Aromatic Compounds, 29, 75–89.
Beg, M. U., Al-Subiai, S. N., Al-Jandal, N., Butt, S. A., Beg, K. R., & Al-Hussaini, M. (2015). Seasonal effect on biomarkers of exposure to petroleum hydrocarbons in fish from Kuwait’s marine area. Marine Pollution Bulletin, 100, 673–680.
De Silva, D., Buzitis, J., Krahn, M. M., Bicego, M. C., & Pires-Vanin, A. M. S. (2006). Metabolites in bile of fish from Sao Sebastiano Channel, Sao Paulo, Brazil as biomarker of petrogenic polycyclic aromatic compounds. Marine Pollution Bulletin, 52, 175–180.
Fowler, S. W., Readman, J. W., Oregion, B., Villeneuve, J. P., & McCay, K. (1993). Petroleum hydrocarbon, trace metals in nearshore gulf sediments, biota, before and after the 1991 war: an assessment or temporal and spatial trends. Marine Pollution Bulletin, 27, 171–182.
ICES. (2001). The International Council for the Exploration of the Sea: Report of the ICES Advisory Committee on the Marine Environment, No. 248, Helsingør, 5.9 June 2001, Palægade 2.4 DK-1261 Copenhagen K Denmark, pp. 257.
Jhonson-Restrepo, B., Olivero-Verbel, J., Lu, S., Guette-Fernandez, J., Baldiris-Avila, R., O’Byrne-Hoyos, I., Aldous, K. M., Addink, R., & Kannan, K. (2008). Polycyclic aromatic hydrocarbons and their hydroxylated metabolites in fish bile and sediments from coastal waters of Columbia. Environmental Pollution, 151, 452–459.
Kannan, K., & Perrotta, E. (2008). Polycyclic aromatic hydrocarbons in livers of California sea otters. Chemosphere, 71, 649–655.
Krahn, M. M., Rhodes, L. D., Myers, M. S., Moore, L. K., MacLeod Jr., W. D., & Malins, D. C. (1986). Associations between metabolites of aromatic compounds in bile and the occurrence of hepatic lesions in English sole (Parophrys vetutus) from Puget Sound, Washington. Archives of Environmental Contamination and Toxicology, 15, 61–67.
Krahn, M. M., Ylitalo, G. M., Buzitis, J., Botton, J. N., Wigren, C. A., Chan, S. L., & Varanasi, U. (1993). Analyses for petroleum-related contaminants in marine fish and sediments following the Gulf oil spill. Marine Pollution Bulletin, 27, 285–292.
Lin, E., Cormier, M., & Racine, R. N. (1996). Fish biliary polycyclic aromatic hydrocarbon metabolites estimated by fixed-wavelength fluorescence comparison with HPLC-fluorescent detection. Ecotoxicology and Environmental Safety, 35, 16–23.
Literathy, P., Morel, G., Zarba, M., Samhan, M., Al-Bloushi, A., Al-Hashash, H., Al-Matrouk, K., Jacob, P. G., & Saeed, T. (1992). Petroleum compounds in the marine environment of Kuwait. Kuwait Institute for Scientific Research, Report No. KISR 4054, Kuwait.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 18–97.
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Lyons, B. P., Barber, J. L., Rumney, H. S., Bolam, T. P. C., Bersuder, P., Law, R. J., Mason, C., Smith, A. J., Morris, S., Devlin, M. J., Al-Enezi, M., Massoud, M. S., Al-Zaidan, A. S., & Al-Sarawi, H. A. (2015). Baseline survey of marine sediments collected from the State of Kuwait: PAHs, PCBs, brominated flame retardants and metal contamination. Marine Pollution Bulletin, 100, 629–636.
Notar, M., Leskov-Sek, H., & Faganel, J. (2001). Composition, distribution and sources of polycyclic aromatic hydrocarbons in sediments of the Gulf of Trieste, northern Adriatic Sea. Marine Pollution Bulletin, 42, 36–44.
OSPAR Commission. (1999). The joint assessment and monitoring program, Guidelines for Monitoring Contaminants in Biota, no. 1999-2. Oslo and Paris Commision.
ROPME. (1999). Regional report of the state of the marine environment. The regional Organization for the Protection of marine environment, Kuwait, ROPME/GC-9/002.
Ruddock, P. J., Bird, D. J., McEvoy, J., & Peters, L. D. (2003). Bile metabolites of polycyclic aromatic hydrocarbons in European eels Anguilla Anguilla from United Kingdom estuaries. The Science of the Total Environment, 301, 105–117.
Sadiq, M., Saeed, T., & Fowler, S. W. (2002). Seafood contamination. In N. Y. Khan, M. Munawar, & A. R. G. Price (Eds.), The Gulf ecosystem: health and sustainability (pp. 327–351). Leiden: Backhuys Publisher.
Saeed, T., Al-Yakoob, S., Al-Hashash, H., & Al-Bahloul, M. (1995). Preliminary exposure assessment for Kuwaiti consumers to polycyclic aromatic hydrocarbons in sea food. Environment International, 21, 255–263.
Salvo, L. M., Severino, D., deAssis, H. C. S., & DaSilva, J. R. M. C. (2016). Photochemical degradation increases polycyclic aromatic hydrocarbon (PAH) toxicity to the grouper Epinephelus marginatus as assessed by multiple biomarkers. Chemosphere, 144, 540–547.
Sole, M., Anto, M., Baena, M., Carrasson, M., & Cartes, J. E. (2010). Hepatic biomarkers of xenobiotic metabolism in eighteen marine fish from NW Mediterranean shelf and slope waters in relation to some of their biological and ecological variables. Marine Environmental Research, 70, 181–188.
Stagg, R. M., McIntosh, A., & Mackie, P. (1995). Elevation of hepatic monooxygenase activity in the das Limanda limanda L. in relation to environmental contamination with petroleum hydrocarbons in the northern North Sea. Aquatic Toxicology, 33, 245–264.
Stegeman, J. J., & Lech, J. J. (1991). Cytochrome P-450 monooxygenase systems in aquatic species: carcinogens metabolism and biomarkers for carcinogen and pollutant exposure. Environmental Health Perspectives, 90, 101–109.
Stroomberg, G. J., de Knecht, J. A., Ariese, F., van Gestel, C. A. M., & Velthorst, N. H. (1999). Pyrene metabolites in the hepatopancreas and gut of the isopod Porcellio scaber, a new biomarker for polycyclic aromatic hydrocarbon exposure in terrestrial ecosystems. Environmental Toxicology and Chemistry, 18, 2217–2224.
Torre, C. D., Tornambe, A., Cappello, S., Mariottini, M., Perra, G., Giuliani, S., Amato, E., Falugi, C., Crisari, A., Yakimov, M. M., & Magaletti, E. (2012). Modulation of CYP1A and genotoxic effects in European seabass (Dicentrarchus labrax) exposed to weathered oil: a mesocosm study. Marine Environmental Research, 76, 48–55.
Van Schanke, A., Holtz, F., van der Meer, J., Boon, J. P., Ariese, F., Stroomberg, G., van der Berg, M., & Everaarts, J. M. (2001). Dose- and time-dependent formation of biliary benzo (a) pyrene metabolites in in the marine flatfish dab (Limanda limanda). Environmental Toxicology and Chemistry, 20, 1641–1647.
Varanasi, U., Chan, S. L., MacLeod, W. D., Brown, D. W., Burrows, D. G., Tilbury, K. L., Landahl, J. T., Wigren, C. A., Hom, T., & Pierce, S. M. (1990). Survey of subsistence fish and shellfish for exposure to oil spilled from Exxon Valdez. First year: 1989. National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Seattle, WA NOAA Tech. Mem.NMFS F/NWC-191.
Whyte, J. J., Jung, R. E., Schmitt, C. J., & Tillitt, D. E. (2000). Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Critical Reviews in Toxicology, 30, 347–570.
Yang, X. A., Peterson, D. S., Baumann, P. C., & Lin, E. L. C. (2003). Fish biliary PAH metabolites estimated by fixed-wavelength fluorescence as an indicator of environmental exposure and effects. Journal of Great Lakes Research, 29, 116–123.
Zhu, I. Z., Cai, X. F., & Wang, J. (2005). PAHs in aquatic sediment in Hangzhou, China: analytical method, pollution pattern, risk assessment and sources. Journal of Environmental Sciences (China), 17, 748–755.
Acknowledgements
The authors are grateful to Dr. Samira A. S. Omar, Director General, Kuwait Institute for Scientific Research, Kuwait, for the interest and financial support to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beg, M.U., Butt, S.A., Al-Dufaileej, S. et al. Biomarkers in fish as a measure of the state of marine environment of Kuwait. Environ Monit Assess 190, 325 (2018). https://doi.org/10.1007/s10661-018-6704-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6704-5