Abstract
Carbaryl (a carbamate insecticide) and cypermethrin (a synthetic pyrethroid insecticide) are extensively used in agriculture. However, the presence of these insecticides in the environment constitutes a significant source of ecological contamination. This study determined the levels of these insecticides in Lisungwi, Neno District, a cotton-growing area in Southern Malawi. A total of 81 soil samples (500 g each) were collected from six sampling points (33 in dry season and 48 rainy season). A total of 86 water samples (500 ml each) were collected seasonally (20 from shallow wells, 18 from streams, and 48 from boreholes). High-performance liquid chromatography (HPLC) was used to determine carbaryl while UV/Vis spectrophotometer detected cypermethrin. Paired t test showed a significant (p < 0.05) difference between rainy season carbaryl (in water and soil) and maximum recommended levels (MRL) (Canadian guidelines and Australian Guidelines). Similarly, a significant (p < 0.05) difference between carbaryl and WHO limits and US MRL was reported in water and soil during the rainy season. A similar observation was made for cypermethrin. Specifically, carbaryl (0.083–0.254 mg/L in surface water and 0.165–0.492 mg/L in groundwater) and cypermethrin (8.115–15.460 mg/L in surface water and 4.48–12.18 mg/L in groundwater) concentrations during the rainy season were above the recommended limits. On the other hand, cypermethrin (0.01–0.048 mg/L) in the soil samples during the rainy season was below the recommended limits while carbaryl (1.67–1.305 mg/L) was above the recommended limits. Higher carbaryl and cypermethrin values were recorded during the rainy season than the dry season. Nitrate, pH, and EC and the insecticides (carbaryl and cypermethrin) depicted strong correlation and significance (p < 0.05), suggesting that these factors influenced their persistence in both soil and water. The study provides evidence that Lisungwi has cypermethrin and carbaryl concentrations above the recommended limits and their presence varies with seasons—a situation considered to be a serious threat to both aquatic biota and humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides (which include insecticides, herbicides, rodenticides, and nematicides, among others) are detrimental to human health and the environment (Tariq et al. 2007). Evidence has shown that approximately 2.5 million tons of pesticides are used globally in agriculture (Pimentel 1995) and the trend is exponentially increasing annually (United Nations 2017). Approximately 200,000 acute poisoning deaths are caused by pesticides annually and about 99% occur in the developing countries (such as Malawi), where health, safety, and environmental regulations are weaker and less strictly applied (United Nations 2017). A World Health Organization (WHO) report revealed that approximately 87,000 cancer cases occur annually in the developing countries and are associated with pesticide use (WHO 1990). The developing countries are in quandary especially due to the use of banned chemicals (Tariq et al. 2007). In Malawi, it is believed that the cases of pesticide poisoning may be even greater than imagined due to under-reporting as a result of data scarcity (Soko 2018). In Malawi, some of the most commonly used insecticides are cypermethrin and carbaryl. Cypermethrin (Cyano-(3-phenoxyphenyl)methyl]3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate) is a highly active synthetic pyrethroid insecticide, effective against a wide range of pests in agriculture, public health, and animal industry. The structure of cypermethrin is shown in Fig. 1.
On the other hand, carbaryl (1-naphthyl methylcarbamate), a carbamate insecticide, was introduced in 1956 by the Union Carbide Corporation to control a broad spectrum of insects on more than 120 different crops. These crops include cereals, cotton, and vegetables, among others. The structure of carbaryl is shown in Fig. 2.
Currently, these insecticides (cypermethrin and carbaryl) are the most commonly applied in agriculture (Zhao et al. 2014, 2016; Tallur et al. 2015; Chen et al. 2012) globally. In Malawi, cypermethrin and carbaryl are commonly applied in cotton-growing areas such as Neno, Salima, and other districts to control a wide range of pests (Kishimba and Mihale 2004). The effects of carbaryl and cypermethrin exposure have been reported by Azmi et al. (2006) in 14 different fruit and vegetable farm station workers existing in Gadap, Karachi, Pakistan. Tariq et al. (2007) reported a massive fish killing in Rawal Lake in Pakistan as a result of carbaryl and cypermethrin contamination. Human health risks such as nausea, vomiting, blurred vision, coma, and difficulty in breathing have been reported by Zhao et al. (2014). A review of the biological monitoring of insecticides has also made it evident that incidents of insecticide exposure are common among field workers especially women as a result of inadequate awareness (Tariq et al. 2007). Several studies have reported the detection of carbaryl and cypermethrin in soils and water (Bano and Siddique 1991; Tariq et al. 2006) in cotton-growing areas (Ahad et al. 2001; Tariq 2005). Studies have shown that over 80% of the rural population in Malawi depends on unprotected water sources from rivers, hand-dug wells, streams, springs, and surface rainwater (Makwinja et al. 2019) though it has to be noted that access to safe drinking water and sanitation is currently a global concern (Meride and Ayenew 2016) . Water from these unprotected sources is heavily contaminated with agricultural chemicals such as inorganic fertilizers, insecticides, and other human contamination. In Malawi, over 80% of the rural population depends on unprotected water sources from 178 rivers, hand-dug wells, streams, springs, and surface runoff. It is very apparent that water from these sources is heavily contaminated with agricultural chemicals such as inorganic fertilizers, pesticides, and other human contamination (GoM 2006). Neno District in Southern Malawi provides the best example though scientific studies associated with the accumulation of agricultural chemicals such as insecticides in water and soil are scarcely available. Therefore, the main objective of this article was to investigate the levels of carbaryl and cypermethrin concentrations in water and soil in Lisungwi, Neno Southern Malawi. This study was designed to provide baseline data and help researchers and authorities to set up future action plans in insecticide research and control programs in Malawi and other developing countries.
Review of the analytical method for carbaryl and cypermethrin detection
Insecticides such as cypermethrin and carbaryl in soil and water samples are usually determined by chromatography. The commonly used chromatographic techniques include gas chromatography (GC), high-performance liquid chromatography (HPLC), and thin-layer chromatography (TLC). It has been observed that for carbaryl, the detection limit is < 10 ng/g using GC (Nasiri et al. 2016). On the other hand, the detection limit for carbaryl is < 0.062 μg/L when HPLC is used (Qun and Rohrer 2016). This agrees with the current study whereby the mean detection limit for carbaryl using HPLC was 0.052 μg/L. On the other hand, using GC, the detection limit of cypermethrin is around 0.007 μg/g (Hernandes et al. 2014), while as for HPLC, it is around 0.001 mg/kg (Bissacot and Vassilieff 1997). Other techniques include spectrophotometry and calorimetric methods. In this study, cypermethrin residues in soil and water samples were determined by the method of Janghel et al. (2007) with some modifications by using UV/Vis spectrophotometer whose detection limit was found to be 0.003 μg/L which is almost similar to this study which was found to be 0.0027 μg/L. Cypermethrin is hydrolyzed to cyanide ion in an acidic medium which in turn reacts with bromine water to form cyanogen bromide. The excess of bromine is removed in the solution by the addition of potassium iodide solution which reacts with p-aminoacetophenone reagent. Absorption of violet color is measured at 400 nm using a UV/Vis spectrophotometer. On the other hand, carbaryl was determined by HPLC.
Material and methods
Study area
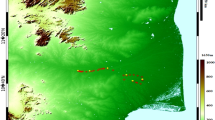
The study was conducted in Lisungwi, Neno District, Southern Malawi, geographically located at latitude 15° 33′ 35″ South and longitude 34° 30′ 16″ East. Neno District has a total area of 1469 km2 and a population of 138,291 (Government of Malawi 2018). The district has four Traditional Authorities, which are Damba, Symon, Chekucheku, and Mlauli. Approximately 38,000 ha of land is used for agriculture and maize covers 90% of arable land. Cotton is the most important cash crop grown in the dry land of Lisungwi, where rainfall is erratic (500–1200 mm) and temperature ranges from 8°C in the high-altitude areas to 35°C in the low-lying areas (Nyirenda 2011). The area is mostly dominated by Cambisol soil especially in the foot slopes and deep Lixisol and Luvisol soil on the plateau. Figure 3 shows the map of Malawi, the location of Neno District, and the sampling points.
Water sampling
Water samples were collected from streams, wells, and boreholes. These sampling points were the main sources of drinking water for the majority of rural communities in the area. The average depth of the streams was 0.5 m. Shallow wells were defined as holes drilled by the local communities to extract drinking water from the ground and had an average depth of 15 m. Borehole on the other hand was defined as those drilled using the machine and typically had an average depth of about 30 m. Water samples collected from streams represented surface water, while samples collected from wells and boreholes represented groundwater. The main sampling period was from September to October 2015 representing dry season and from February to March in 2016 representing the rainy season. Water samples were collected using an automatic ISCO sampler (Teledyne Isco, Lincoln, NE, USA). Small sub-samples were taken biweekly and pooled into a biweekly time-integrated sample that was stored in a refrigerator during the sampling period. A total of 20 samples were collected from shallow wells during both dry and rainy seasons with 10 samples collected from each season. Similarly, a total of 18 samples were collected from streams with 9 samples collected from each season. A total of 48 samples with 24 from each season were collected from boreholes. The grab samples were collected twice a month in September and March. Sampling periods and the number of samples at each sampling point are presented in Table 1. Water samples were preserved in a cooler box at 4°C (Laxen and Harrison 1981) and transported to the laboratory for analysis.
Soil sampling
Soil samples were collected from cotton fields following a systematic random sampling procedure suggested by Katalin (2011). Triplicate soil samples were collected at four corners of each rectangular cotton field and one at the central location at the depth of up to 30 cm using a Hand auger model S092 KIT: 80 mm × 1 m long, 4 kg in weight (USEPA 2014). A composite sample was made by combining the five collected soil samples (sub-samples) using a hand shovel in a bucket. About 33 representative samples each weighing 500 g were collected from the sampling points during the dry season (September to October 2015) while 48 representative samples with an average weight of 500 g were collected during the rainy season (February and March 2016) from the same sampling locations. The samples collected both during dry and rainy seasons were carefully placed in aluminum foil, fastened with masking tape, and properly labeled. The aluminum foil was then placed in a self-sealing polyethylene bag and then placed in a polyurethane container with a lid to avoid contamination. The polyurethane container was then labeled and kept in a polyurethane cooler box containing dry ice before transporting it to the laboratory for analysis. At the laboratory, the soil samples were kept in a deep freezer at − 18°C before extraction.
Determination of physicochemical parameters, phosphate, and nitrate
Water temperature and pH were measured on-site using a mobile thermometer and a portable digital pH Meter Model 213, respectively. Electrical conductivity (EC) was measured using an EC meter Model 4510 (Meride and Ayenew 2016). Before taking the measurements, the instruments were calibrated according to the manufacturer’s manual instructions. In the laboratory, phosphate concentrations were determined using the molybdenum blue method using a UV/Visible spectrophotometer (Milton Roy and electronic 20 models). In this method, phosphate in the sample is determined at 810 nm. Orthophosphate and molybdate ions were condensed in an acidic solution to form phosphomolybdic acid. Upon selective reduction (with hydrazinium sulfate), blue color was produced. The intensity of the blue color was proportional to the amount of phosphate initially incorporated into the heteropoly acid. The intensity of the color solutions was measured on the UV/Visible spectrophotometer. The calibration curve was constructed by plotting the absorbance values against the concentration of standard solutions. The straight line graph passing through the origin according to the Beer-Lambert law was used to estimate phosphate. Nitrates were determined by the sodium salicylate method. Nitrates react with sodium salicylate under strongly acidic conditions. Addition of excess sodium hydroxide solution produces the sodium salt of the organic nitro complex. The nitro compound is soluble in water and produces a strong yellow solution. The color intensity is proportional to the amount of nitrate in the sample and is measured on a UV/Visible spectrophotometer at 410 nm.
Determination of carbaryl in water samples using HPLC
A volume of 100 mL of each water sample was put into a 250-mL separatory funnel where 30 mL of methylene chloride was added and then shaken vigorously for about 2 min. Phases were allowed to separate and consequently the organic layer was transferred into a clean 100-mL volumetric flask. The extraction was repeated two more times using fresh portions of solvents. All three extracts were combined in a 100-mL volumetric flask and diluted to the mark with methylene chloride. The extract was then filtered using a disposable 0.45-μm filter into a labeled autosampler vial and then loaded into the HPLC autosampler. The HPLC (Agilent Technologies 1260 Infinity) was equipped with an auto-injector (G1329b 1260 ALS) with sample loop size of 10 μL and Diode Array Detector (G1315D 1260 DAD, Agilent) (Ahmad et al. 2001). The oven temperature was maintained at 40°C, and the system had an Acclaim 120-C18, 150 × 3 mm ID column (particle size 3 μm), and part number 063691. A guard column (Acclaim 120-C18, 5 μm, 10 × 2.1 mm ID), part number 069689, was fitted before the analytical column. The flow rate was 0.8 mL/min and has an absorbance of 280 nm. The mobile phase (60%) consisted of acetonitrile, 5 mM ammonium acetate, acetic acid (EPA method 8318a), and distilled water (40%).
Determination of carbaryl in soil samples using HPLC
Soil portions of 2.5 ± 0.1 g were weighed and put into separate 250-mL volumetric flasks with PTFE screw caps. Acetonitrile of 50 mL was then added to the portion of each sample and shaken for 2 h on a platform shaker, as described by the EPA method 8318a (USEPA 2007). The mixture was allowed to settle for about 5–10 min and then the extract was decanted into 250-mL centrifuge tubes to separate the solid particles from the liquid. The extraction was repeated two more times using 20 mL of acetonitrile and shaken for 1 h each time. All the three extracts were combined in a centrifuge tube and later centrifuged at 200 rpm for 10 min. The supernatant was carefully decanted into a 100-mL volumetric flask and diluted to the mark with acetonitrile. The extract was then filtered using a disposable 0.45-μm filter into a labeled autosampler vial and loaded into HPLC.
Calibration curve of carbaryl
Carbaryl standard was prepared by dissolving 0.050-g insecticide in 50-mL methanol; this solution was further diluted with methanol to a series of the final concentration of standards of 0.5, 1.0, 2.0, 3.0, and 5.0 mg/L. Methanol of 20 μL (solvent of blank) was analyzed before calibration of carbaryl standards starting with the lowest standard and ending with the highest standard. Standards were loaded into the autosampler vials and then loaded into the HPLC autosampler. Calibration curve was constructed by plotting a peak area against concentration.
Determination of cypermethrin in soil and water samples
Potassium iodide and p-aminoacetophenone reagents were obtained from Glassworld (South Africa). Dichloromethane (DCM), hydrochloric acid (HCl), and sodium hydroxide (NaOH) were sourced from Sigma-Aldrich (India). A cypermethrin stock solution (1000 ppm) was prepared by dissolving 0.1 g of pure reagent powder in dichloromethane (DCM) solvent to 100 mL in a volumetric flask and it was diluted to the mark using DCM. A cypermethrin intermediate solution was made by pipetting 10 mL of cypermethrin stock solution into a 100-mL volumetric flask and then diluting it to the mark using DCM.
Preparation of calibration curve
To 0.5, 1, 1.5, 2, and 2.5 mL of cypermethrin working standards, 4 mL of 20% sodium hydroxide was added in 50-mL volumetric flasks. The solution was kept for 10 min at room temperature for complete hydrolysis and neutralized with 0.1 N HCl. Then 2 mL of 0.1% potassium iodide was added followed by one drop of concentrated HCl, to liberate iodine and then followed by the addition of 2 mL of 1% p-aminoacetophenone reagent. The solutions were thoroughly shaken and kept for 15 min for color development. A violet color was observed and solutions were diluted to the mark with distilled water. Absorbance for both standards and samples were measured at 400 nm using a double-beam UV/Vis spectrophotometer (T90 model) of wavelength range 190–900 nm with spectral bandwidth of 0.1, 0.2, 0.5, 1.0, and 5.0 nm. Calibration curve was then constructed by plotting absorbance against standard concentration and the cypermethrin content in samples was established from the calibration graph: y = 0.0052× – 0.0002 and R2 = 0.9991. For water samples, 25 mL of each sample was processed for analysis using the Janghel et al. (2007) method described above.
Soil sample preparation
Soil samples were prepared by mixing 2.5 g of each soil sample portions with dichloromethane and then filtered using Whatman No. 40-cm filter paper. The filtrate of each sample portion were quantitatively transferred into separate 50-mL graduated flask and made up to the mark with DCM. Twenty-five milliliters of each aliquots were taken in a 50-mL graduated tube, and then following the Janghel et al. (2007) method described above, the samples were analyzed.
Data analysis
The experiment for each sample was performed in triplicate and results were expressed as means of the three triplicates. Data were organized using the version 2019 Microsoft office (mainly Microsoft Excel) and all statistical analyses were performed using the Paleontological Statistics Software Version 3.14.
Results and discussion
Table 2 shows the values determined from the surface and groundwater samples during the dry and rainy seasons and those recommended by the WHO for drinking water, EPA, and different authors for various aquatic biota. In this study, the water temperature during the rainy season was higher than the value recommended by the WHO (WHO 2011). Water pH on the other hand was generally within the WHO recommended limit though groundwater in both seasons showed some level of acidity for some samples. Electrical conductivity was higher in all the water samples as compared with the WHO recommended value (< 400). The results further showed that groundwater samples had the highest TDS above the recommended range while the rest had the lowest values below the WHO recommended range. The concentrations of both nitrates and phosphates were above the WHO recommended range suggesting heavy use of phosphate and nitrogenous fertilizer in the area which end up contaminating the water.
Cypermethrin and carbaryl were both detected in water (Table 2) and soil (Table 3) samples during the rainy and dry seasons. The maximum recommended (MRL) level for cypermethrin in drinking water is 0.11 mg/L while carbaryl is 0.05 mg/L. However, as seen from Table 2, samples collected from groundwater and surface water during the rainy season indicate cypermethrin and carbaryl residues above the MRL value. The concentration of cypermethrin and carbaryl in surface water during the dry season appears to be within the MRL. On the contrary, groundwater appears to have higher cypermethrin and carbaryl concentrations above MRL. Table 3 further shows that the concentration of cypermethrin in soil samples was below MRL. However, this was not the case with carbaryl which had a concentration level above MRL. Table 4 shows the results of a paired t test. As seen from Table 4, the paired t test showed a significant (p < 0.05) difference between rainy season carbaryl (in water and soil) and maximum recommended level (MRL) (WHO and USA MRL). Also, a significant (p < 0.05) difference between carbaryl and WHO limits and US MRL is noted in water and soil samples during the rainy season. A similar observation was made in cypermethrin values against Canadian guidelines and Australian guidelines. Several studies have also detected insecticide residues in both surface and groundwater samples (Károly et al. 2001; Maloschik et al. 2007). Pfeuffer (1991) and reported high carbaryl and cypermethrin values in streams of the USA during the spring season. Goswami et al. (2013) also detected cypermethrin residues in soil samples in India. In Pakistan, India, and Bangladesh, researchers such as Bhattacharjee et al. (2012) also reported high carbaryl and cypermethrin values in river ecosystems during the rainy season. In France, Bedos et al. (2002) also detected cypermethrin in groundwater while in the United Kingdom, cypermethrin concentration was reported in river systems and sediments. In Bangladesh, the level of various harmful insecticides such as cypermethrin and carbaryl exceeded the standard limit (Rahman and Alam 1997) in various river systems.
Cypermethrin and carbaryl are generally toxic to freshwater aquatic biota above MRL (de Bon et al 2014; US EPA 2003). The laboratory tests on fish have indicated the LC50 values of 0.4–2.8 μg/L (WHO 1989). The U.S. Environmental Protection Agency report also indicates the acute and chronic level of carbaryl of 2.1 μg/L (US EPA 2010, 2012) on various aquatic biota. Wernersson and Dave (1997) reported the LC50 values ranging from 0.66 to 21 μg/L for exposure lasting 24 h. Other studies have also reported LC50 levels in fish within the range of 4 to 13 ppm (Beyers et al. 1994; Sinha et al. 1991; WHO 1989). As seen from Table 2, various aquatic biota in the study area are under serious threat. Carlson et al. (1998) explained that the efficient reaction of carbaryl and water can lead to the formation of 1-naphthol as a primary product in natural waters within a couple of days and this suggests that consuming raw water from both ground and surface sources in this study area could pose a serious health risk. Researchers such as Downing et al. (2008) noted that zooplankton richness, diversity, abundance, and oxygen concentration decreased while phytoplankton and microbial abundance increase shortly after carbaryl concentration. These authors concluded that despite the complete degradation of carbaryl over time, the ecological effects of the insecticides could persist even after degradation. Researchers such as Relyea (2006) and Hanazato (1991) also noted that zooplankton richness, diversity, and abundance all declined and composition changed due to the known effects of carbaryl on zooplankton.
Figure 4 shows that cypermethrin and carbaryl residues in surface water and groundwater vary, with the highest recorded during the rainy season and lowest during the dry season. The high concentration of cypermethrin (which was also the case with carbaryl) in the rainy season was attributed to the application time. Both of these insecticides are applied during the cotton-growing season which coincides with the rainy season. Karthigayani et al. (2014) also reported the same in India. Székács et al. (2015) also reported high pesticide residues in both surface and groundwater in Hungary during the rainy season and attributed it to heavy application rates. Vig et al. (2008) also noted that the degradation of carbaryl and cypermethrin is facilitated by microorganisms and slow degradation has been observed in anaerobic and waterlogged conditions and increased persistence is observed in soils with high organic matter, high clay content, reduced microbial activity, and anaerobic conditions (Goswami et al. 2013). It should be noted that the excessive amount of these insecticides is drained into the river systems by drainage during the rainy season and hence increase their concentration in the surface water. Similar findings were also reported elsewhere by Phong et al. (2010), Canadian Council of Ministers of the Environment (2009), Gunasekara (2007), and Xu (2002). Kreuger (1988) and Tanabe et al. (2000) also noted that rainfall associated with runoff increases the movement of pesticides into nearby water bodies.
The photodegradation half-life of cypermethrin in river water has been shown to be 0.6 to 0.7 days while in soils the duration has been shown to be 0.6 to 1.9 days (Takahashi et al. 1985). What this shows is that the half-life of cypermethrin in water is shorter than in soils. However, cypermethrin is hydrophobic as such in rivers tends to attach itself to sediments or soil particles in suspension, making it more persistent. Due to high siltation rate in most Malawian rivers (including those of the study area), the availability and persistence of cypermethrin in water are very likely which explains the high concentrations recorded in this study. The carbaryl photodegradation half-life in river water has been found to be 2.1 days (Derbalah et al. 2020) while as in soils it is more persistent with half-life of up to 41 days being recorded (Das 1990). This indicates that carbaryl is more persistent than cypermethrin; hence, it will easily be found in both water and soils after its application. On the other hand, the half-lives indicate that the concentration of both pesticides will decrease with duration after application in addition to the dose rate as explained below.
In a study done by Caro et al. (1974), the results showed that carbaryl content in the soil decreased with an increase in duration after application. Johnson and Stansbury (1965) on the other hand noted that the carbaryl dose rate could significantly influence the rate of degradation in the soil. Figure 5 shows that as the initial dose decreases, the rate of degradation also exponentially decreases, suggesting that heavy application of these insecticides could lead to high persistence in the soil and water. On the other hand, degradation of cypermethrin is affected linearly with increasing soil metal concentrations (Rafique and Tariq 2015). Lisungwi area is mostly dominated by Cambisol soils especially in the foot slopes and deep Lixisol and Luvisol soils on the plateau. This type of soil has a high concentration of heavy metals. The enhanced concentration of heavy metals and their binding with soil organic matter and clay minerals could probably be the reason for the persistence of cypermethrin during the dry season (Fig. 5).
Degradation of carbaryl with time per initial dose (mg/kg) (Johnson and Stansbury 1965)
Table 5 shows the results of the correlation coefficient of both cypermethrin and carbaryl and nitrate, phosphate, pH, and electrical conductivity. The correlation coefficient of cypermethrin and pH was − 0.735 and significant (p < 0.01), demonstrating the strongest relationship between the two variables. The correlation coefficient of cypermethrin and electrical conductivity was 0.538 and significant (p < 0.05), suggesting the existence of a relationship between the two variables. On the other hand, a significant (p < 0.05) correlation coefficient (0.6851) was noted in the paired relationship between cypermethrin and nitrates while phosphate demonstrated weaker relationship with cypermethrin and non-significant (p > 0.05). On the other hand, the correlation coefficient of carbaryl and pH was − 0.792 and significant (p < 0.01) demonstrating the strongest relationship (Table 5), while electrical conductivity was 0.622 and significant (p < 0.01). However, nitrate also showed the existence of a relationship (− 0.574) with carbaryl (p < 0.05). Phosphates on the other hand showed a weaker relationship (− 0.0976) and not significant (p > 0.05).
Previous researchers agree with the present findings. For example, Fojut et al. (2001) reported a negative correlation between cypermethrin concentration in water samples and pH. The study further noted that the reduced pH increased the toxicity of cypermethrin which consequently affected the physiological responses of the organisms and causes severe chemical stress (Parithabhanu and Deepac 2014). Al-Mughrabi et al. (1992) noted that at high pH above 6, the stability of cypermethrin was reduced. On the other hand, Fisher and Lohner (1986) reported a greater amount of carbaryl in natural water at pH 4 than at pH 6 and 8. Fisher and Lohner (1986) further explained that the hazard associated with aquatic contamination by carbaryl is affected by pH and is more significant when contamination of water exceeds the capacity of aquatic biota to metabolize the chemical. Tomlin (2003) also reported a strong negative correlation between pH and carbaryl in the soil samples. Burauel and Bassmann (2005) also related the pH of the soil with the persistence of insecticides. Moral et al. (2000) on the other hand explained that pH can alter the rate at which carbamates are decomposed by hydrolysis. Hence, the persistence of carbaryl in water under low pH could be longer due to slow degradation (Fisher and Lohner 1986). Stephenson and Solomon (1993) also noted that acidic soil has the capacity of increasing the persistence of insecticides in the soil. Soil and groundwater contamination studies conducted by Tariq et al. (2007) also demonstrated the potential effect buffering capacity of the soil against the persistence of insecticides in the soil. Abrahams (2002) noted that soil in respect to its chemistry has a potential of insecticide transport in contaminating water, air, plants, food, and ultimately in human via runoff and sub-surface drainage, interflow and leaching, and the transfer of mineral nutrients and pesticides from the soil into plants and animals that constitute food chain. Fisher and Lohner (1986) also noted that the sensitivity of aquatic organisms to toxic agents in water depends on the chemical characteristics of the medium. In other words, pH, EC, and dissolved oxygen influence carbaryl and cypermethrin toxicities in both water and soil.
Conclusion
In this study, carbaryl and cypermethrin residues in both water and soil samples were detected in a cotton-growing area of Lisungwi, Neno District, Malawi. Their concentration in water samples was above the WHO MRL (0.11 and 0.05 mg/L) during the rainy season as compared with the dry season. Cypermethrin concentration in the soil samples was below MRL while carbaryl was above MRL. The study further shows that both insecticides undergo degradation. It was noted that high concentrations in both water and soil samples were recorded during the rainy season which coincides with heavy fertilizer and insecticide application as opposed to the dry season. The results further showed that the persistence of these insecticides in the soil and water samples during the dry and rainy seasons depends on pH, EC, and nitrate concentration in the water and soil. These factors depicted a strong correlation and significance. Therefore, the use of these insecticides should be done with all precautionary measures especially in rural areas.
Limitations
This study only focused on Lisungwi, Neno, Southern part of Malawi. Therefore, future research must take into consideration other cotton-growing areas in the Central and Northern Malawi.
References
Abrahams, P. (2002). Soils: their implications to human health. The Science of the Total Environment, 291, 1–32.
Ahad, K., Hayat, Y., Ahmad, I., & Soomro, M. (2001). Capillary chromatographic determination of pesticides residues in groundwater of Multan Division. Nucleus, 38(2001), 145–149.
Ahmad, R., Kookana, R. S., Alston, A. M., & Bromilow, R. H. (2001). Differences in sorption behavior of carbaryl and phosalone in soils from Australia, Pakistan, and the United Kingdom. Australian Journal of Soil Research, 39, 893–908.
Al-Mughrabi, K., Nazer, I., & Al-Shuraiqia, Y. (1992). Effect of pH of water from the King Abdallah Canal in Jordan on the stability of Cypermethrin. Crop Protection, 11(4), 341–344.
Azmi, M., Naqvi, S., Azmi, M., & Aslam, M. (2006). Effect of pesticide residues on health and different enzyme levels in the blood of farm workers from Gadap (rural area) Karachi—Pakistan. Chemosphere, 64(2006), 1739–1744.
Bano, A., & Siddique, S. (1991). Chlorinated hydrocarbons in the sediments from the coastal waters of Karachi (Pakistan). Pakistan Journal of Scientific and Industrial Research, 34(1991), 70–74.
Bedos, C., Cellier, P., Calvelet, R., & Barruiso, E. (2002). Occurrence of pesticides in the atmosphere in France. Agronomie, 22(2002), 35–49.
Beuter, L.K., Dören, L., Hommen, U., Kotthof, M., Schäfers, C., & Ebke, K.P. (2019). Testing efects of pesticides on macroinvertebrate communities in outdoor stream mesocosms using carbaryl as example test item. Environmental Sciences Europe, 31(5), 1–17.
Beyers, D., Keefe, T., & Carson, C. (1994). Toxicity of carbaryl and malathion to two federally endangered fishes, as estimated by regression and ANOVA. Environmental Toxicology and Chemistry, 9(1994), 30–39.
Bhattacharjee, S., Fakhruddin, A., Chowdhury, M., Rahman, M., & Alam, M. (2012). Monitoring of selected pesticides residue levels in water samples of paddy fields and removal of cypermethrin and chlorpyrifos residues from water using rice bran. Bulletin of Environmental Contamination and Toxicology, 89(2012), 348–353.
Bissacot, D. Z., & Vassilieff, I. (1997). HPLC determination of flumethrin, deltamethrin, cypermethrin, and cyhalothrin residues in the milk and blood of lactating dairy cows. Journal of Analytical Toxicology, 21, 397–402.
Burauel, P., & Bassmann, F. (2005). Soils as filter and buffer for pesticides—experimental concepts to understand soil functions. Environmental Pollution, 133(2005), 11–16.
Canadian Council of Ministers of the Environment. (2009). Canadian water quality guidelines for the protection of aquatic life: Carbaryl. Winnipeg: Canadian Council of Ministers of the Environment.
Carlson, R., Bradbury, S., Drummond, R., & Hammermeister, E. (1998). Neurological effects on startle response and escape from predation by medaka exposed to organic chemicals. Aquatic Toxicology, 43(1998), 51–68.
Caro, H., Freeman, H., & Turner, B. (1974). Persistence in soil and losses in run off of soil incorporated carbaryl in a small watershed. Journal of Agricultural and Food Chemistry, 22(5), 860–863.
Chen, S., Luo, J., Hu, M., Lai, K., Geng, P., & Huang, H. (2012). Enhancement of Cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Bioresource Technology, 10(2012), 97–104.
Das, Y.T. (1990). Photodegradation of [1-naphthyl-14C]carbaryl on soil under artificial sunlight, Vol. 169-208 #87095. Department of Pesticide Regulation, Sacramento, CA.
de Bon, H., Huat, J., Parrot, L., Sinzogan, A., Martin, T., Malézieux, E. & Vayssières, J.F. (2014). Pesticide risks from fruit and vegetable pest management by small farmers in sub-Saharan Africa. A review. Agronomy for Sustainable Development, 34, 723–736.
Derbalah, A., Chidya, R., Kaonga, C., Iwamoto, Y., Takeda, K., & Sakugawa, H. (2020). Carbaryl residue concentrations, degradation, and major sinks in the Seto Inland Sea, Japan. Environmental Science and Pollution Research, 27, 14668–14678.
Downing, A., DeVanna, K., Rubeck-Schurt, C., Tuhela, L., & Grunkemeyer, H. (2008). Community and ecosystem responses to a pulsed pesticide disturbance in freshwater ecosystems. Ecotoxicology, 17(2008), 539–548.
EPA, U. (2012). Aquatic life ambient water quality criteria for carbaryl-2012. Washington DC: USA Environmental protection agency.
Fisher, S., & Lohner, T. (1986). Studies on the environmental fate of carbaryl as a function of pH. Archives of Environmental Contamination and Toxicology, 15(1986), 661–667.
Fojut, T., Mulligan, R., & Tjeerdema, R. (2001). Water quality criteria report for cypermethrin: phase III: application of pesticide water quality criteria methodology. Davis: Department of Environmental Toxicology: University of California.
GoM. (2006). The National Sanitational Policy. Lilongwe: Malawi Government.
Goswami, M., Pati, U., Chowdhury, A., & Mukhopadhyay, A. (2013). Studies on the effect of cypermethrin on soil microbial biomass and its activity in an alluvial soil. International Journal of Agriculture and Food Science, 3(1), 1–9.
Government of Malawi. (2018). 2018 Malawi population and housing. Zomba: National Statistical Office.
Gunasekara, A. (2007). Environmental fate of carbaryl. Environmental monitoring, Branch. CA, USA: Branch Department of pesticide regulation. California Environmental Protection agency, Sacramento.
Hanazato, T. (1991). Effects of repeated application of carbaryl on zooplankton communities in experimental ponds with or without the predator Chaoborus. Environmental Pollution, 74(1991), 309–332.
Hernandes, T., Dores, E. F. G. C., Ribeiro, M. L., Rossignoli, P. A., & Malm, O. (2014). Simple method to determine residual cypermethrin and deltamethrin in bovine milk. Journal of the Brazilian Chemical Society, 25(9), 1656–1661.
Janghel, E. K., Rai, J. K., Rai, M. K., & Gupta, V. K. (2007). A new sensitive spectrophotometric determination of cypermethrin insecticide in environmental and biological samples. Journal of the Brazilian Chemical Society, 18(3), 590–594.
Johnson, D., & Stansbury, H. (1965). Adaptation of Sevin insecticide (carbaryl) residue method in various crops. Journal of Agricultural and Food Chemistry, 13(1965), 235–238.
Károly, G., Györfi, L., & Ocskó, Z. (2001). Surveys of Hungarian surface waters for pesticide contamination. Növényvédelem, 37(2001), 539–545.
Karthigayani, T., Denis, M., Remy, A., & Shettu, N. (2014). Effect of cypermethrin toxicity in the gills of the fish Oreochromis mossambicus. Journal of Modern Biotechnology, 3(3), 35–41.
Katalin, S. (2011). Nutrient management. Soil testing and plant analysis. https://regi.tankonyvtar.hu/hu/tartalom/tamop425/0010_1A_Book_angol_02_tapanyaggazdalkodas/ch06s03.html. Accessed 11 Nov 2019.
Kishimba, M., & Mihale, M. (2004). Levels of pesticide residues and metabolites in soil at Vikuge Farm, Kibaha District, Tanzania – a classic case of soil contamination by obsolete pesticides. Tanzania Journal of Science, 30(2), 77–87.
Kreuger, J. (1988). Pesticides in stream water within an agricultural catchment in southern Sweden, 1990–1996. Sci Total Environ, 216(1988), 227–251.
Laxen, D., & Harrison, R. (1981). Cleaning methods for polythene containers prior to the determination of trace metals in fresh water samples. Analytical Chemistry, 53(1981), 345–350.
Makwinja, R., Kosamu, I. B. M., & Kaonga, C. C. (2019). Determinants and values of willingness to pay for water quality improvement: insights from Chia Lagoon, Malawi. Sustainability, 11, 4690. https://doi.org/10.3390/su1117460.
Maloschik, E., Ernst, A., Hegedűs, G., Darvas, B., & Székács, A. (2007). Monitoring water-polluting pesticides in Hungary. Microchemical Journal, 85(1), 88–97.
Meride, Y., & Ayenew, B. (2016). Drinking water quality assessment and its effects on residents’ health in Wondo genet campus, Ethiopia. Environmental Systems Research, 5(1), 1–7.
Moral, B., Martnez-Tabche, L., Sahnchez-Hidalgo, E., Hernandez, G., Ruiz, M., & Murrieta, F. (2000). Relationship between toxicokinetics of carbaryl and effect on acetylcholinesterase activity in Pomacea patula snail. Ecotoxicology and Environmental Safety, 46(2000), 234–239.
Nasiri, A., Amirahmadi, M., Mousavi, Z., Shoeibi, S., Khajeamiri, A., & Kobarfard, F. (2016). A multi residue GC-MS method for determination of 12 pesticides in cucumber. Iranian Journal of Pharmaceutical Research., 15(4), 809–816.
Nyirenda, C. (2011). Project design document for the Trees of Hope Plan Vivo Project. Lilongwe: Clinton Development Initiative.
Parithabhanu, A., & Deepac, M. (2014). Toxicity of cypermethrin influenced pH and temperature on the fresh fish Oreochromis mossambicus. International Journal of Scientific and Research Publications, 4(1), 1–4.
Pfeuffer, R. J. (1991). Pesticide residue monitoring in sediment and surface water within the South Florida Water Management District: Volume 2. technical publication 91-01, DRE 293. West Palm Beach, FL: South Florida Water Management District.
Phong, T., Yoshino, K., Hiramatsu, K., Harada, M., & Inoue, T. (2010). Pesticide discharge and water management in a paddy catchment in Japan. Paddy and Water Environment, 8(2010), 361–369.
Pimentel, D. (1995). Amounts of pesticides reaching target pests: environmental impacts and ethics. Journal of Agricultural and Environmental Ethics, 8, 17–29.
Qun, X. & Rohrer, J. (2016). Rapid HPLC Determination of carbofuran and carbaryl in tap and environmental waters using on-line SPE. Thermo Scientific Application Update 186. Accessed on 04/08/2020 from https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AU-186-LC-Carbofuran-Carbaryl-Waters-LPN3045-EN.pdf.
Rafique, N., & Tariq, S. (2015). Photodegradation of a-cypermethrin in soil in the presence of trace metals (Cu2+, Cd2+, Fe2+ and Zn2+). Environmental Science: Processes & Impacts, 17(2015), 166–176.
Rahman, M., & Alam, M. (1997). Risk assessment of pesticide used in Bangladesh. Journal of Civil Engineering, 25(1997), 97–106.
Relyea, R. (2006). The effects of pesticides, pH, and predatory stress on amphibians under mesocosm conditions. Ecotoxicology, 1(2006), 503–551.
Sinha, N., Lal, B., & Singh, T. (1991). Carbaryl induced thyroid dysfunction in the freshwater catfish Clarias batrachus. Ecotoxicological and Environmental safety, 21(1991), 240–247.
Soko, J. (2018). Agricultural pesticide use in Malawi. Journal of Health and Pollution, 20(2018), 181–201.
Stephenson, G., & Solomon, K. (1993). Pesticides and the environment. Guelph: Department of Environmental Biology, University of Guelph.
Székács, A., Mörtl, M., & Darvas, B. (2015). Monitoring pesticide residues in surface and ground water in Hungary: Surveys in 1990–2015. Journal of Chemistry, 2015, 1–15. https://doi.org/10.1155/2015/717948.
Takahashi, N., Mikami, N., Matsuda, T., & Miyamoto, J. (1985). Photodegradation of the pyrethroid insecticide cypermethrin in water and on soil surface. Journal of Pesticide Science, 10, 629–642.
Tallur, P., Mulla, S., Megadi, V., & Talwar, M. (2015). Biodegradation of cypermethrin by immobilized cells of Micrococcus sp. strain CPN 1. Brazilian Journal of Microbiology, 46(3), 667–672.
Tanabe, S., Prudente, M., Kan-atireklap, S., & Subramania, A. (2000). Mussel watch: marine pollution monitoring of butyltins and organochlorines in coastal waters of Thailand, Philippines and India. Ocean and Coastal Management, 43(200), 819–839.
Tariq, M. (2005). Leaching and degradation of cotton pesticides on different soil series of cotton growing areas of Punjab, Pakistan in lysimeters. Ph. D. Thesis,. Lahore, Pakistan: University of the Punjab.
Tariq, M., Afzal, S., & Hussain, I. (2006). Degradation and persistence of cotton pesticides in sandy loam soils from Punjab, Pakistan. Environmental Research, 100(2006), 184–196.
Tariq, M., Afzal, S., Hussain, I., & Sultana, N. (2007). Pesticides exposure in Pakistan: a review. Environment International, 33(2007), 1107–1122.
Tomlin, C. (2003). The Pesticide Manual (13th ed.). Surrey: British Crop Protection Council.
United Nations. (2017). Report of the Special Rapporteur on the right to food. Geneva: United Nations.
US EPA. (2010). Registration review – preliminary problem formulation for ecological risk and environmental fate, endangered species, and drinking water assessments for carbaryl. Washington DC: US Environmental Protection Agency.
US EPA (2012). Aquatic life ambient water quality criteria for carbaryl. EPA-820-R-12-007. Washigton DC: US Enviromental Protection Agency.
USEPA. (2003). Environmental fate and ecological risk assessment for re-registration of carbaryl: Washington DC: U.S Protection Agency, Prevention, Pesticides and Toxic substances PC code 056801.
USEPA. (2007). SW-846 Method 8318a: N-methylcarbamates by high performance liquid chromatography (HPLC). Cincinnati: Environmental Monitoring and Support Laboratory.
USEPA. (2014). Environmental fate and ecological risk assessment for re-registration of carbaryl. Washington DC: U.S Protection Agency, Prevention, Pesticides and Toxic substances PC code 056801.
Vig, K., Singh, D., Agarwal, H., Dhawan, A., & Dureja, P. (2008). Soil microorganisms in cotton fields sequentially treated with insecticides. Ecotoxicology and Environmental Safety, 69(2), 263–276.
Wernersson, A., & Dave, G. (1997). Phototoxicity identification by solid phase extraction and photo-induced toxicity to Daphnia magna. Archives of Environmental Contamination and Toxicology, 32(3), 268–273.
WHO. (1989). Cypermethrin. Genever: United Nations Environment Programme, the International Labour Organization, and the World Health Organization.
WHO. (1990). Public health impact of pesticides used in agriculture. Geneva: World Health Organization.
WHO. (2004). Guidelines for drinking-water quality. Geneva: World Health Organization.
WHO. (2006). In water, sanitation and health. Geneva: World Health Organization.
WHO. (2011). Guidelines for drinking-water quality (4th ed.). Geneva: World Health Organization.
Xu, S. (2002). Environmental fate of carbaryl. California: California environmental protection agency, Department of Pesticide Regulation.
Zhao, L., Zhao, F., & Baizhao, Z. (2014). Electrochemical determination of carbaryl by using a molecularly imprinted polymer/graphene-ionic liquid-nano Au/chitosan-AuPt alloy nanoparticles composite film modified electrode. International Journal of Electrochemical Science, 9(2014), 1366–1377.
Zhao, J., Chi, Y., Xu, Y., Jia, D., & Yao, K. (2016). Co-metabolic degradation of β-cypermethrin and 3-phenoxybenzoic acid by co-culture of Bacillus licheniformis B-1 and Aspergillus oryzae M-4. PLoS One, 11, e0166796. https://doi.org/10.1371/journal.pone.0166796.
Acknowledgment
The authors wish to acknowledge Mr. Albert Maliro and Mr. Frank Kunkundi for the technical support. The authors are also grateful to the Soroptimist International Great Britain and Ireland (SIGBI) for the research grant provided to Charity Kanyika-Mbewe. The authors further acknowledge the University of Malawi, The Polytechnic, for making this study possible. Many thanks should also go to the communities for the lively participation during the data collection.
Author information
Authors and Affiliations
Contributions
Charity Kanyika-Mbewe, MSc student in Water Resources and Supply Management at the University of Malawi, The Polytechnic, designed the research, collected data, and analyzed and developed the manuscript. Benard Thole and Chikumbusko Chiziwa Kaonga from the University of Malawi, The Polytechnic, Rodgers Makwinja, currently a PhD candidate at Addis Ababa University, were involved significantly at each stage of the manuscript writing, field scoping, and reviewing the study tools.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kanyika-Mbewe, C., Thole, B., Makwinja, R. et al. Monitoring of carbaryl and cypermethrin concentrations in water and soil in Southern Malawi. Environ Monit Assess 192, 595 (2020). https://doi.org/10.1007/s10661-020-08557-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08557-y