Abstract
Pesticides are applied throughout the world often with unintended consequences on ecological communities. In some regions, pesticides are associated with declining amphibians, but we have a poor understanding of the underlying mechanisms. Pesticides break down more slowly under low pH conditions and become more lethal to amphibians when combined with predatory stress, but these phenomena have not been tested outside of the laboratory. I examined how pH, predatory stress, and a single application of an insecticide (carbaryl) affected the survival and growth of larval bullfrogs (Rana catesbeiana) and green frogs (R. clamitans) in outdoor mesocosms. Decreased pH had no effect on survival, but caused greater tadpole growth. Low concentrations of carbaryl had no effect on either species, but high concentrations caused lower survival and greater growth in bullfrogs. Predatory stress and reduced pH did not make carbaryl more lethal likely due to the rapid breakdown rate of carbaryl in outdoor mesocosms. Thus, whereas the stress of pH and predators can make carbaryl (and other pesticides) more lethal under laboratory conditions using repeated applications of carbaryl, these stressors did not interact under mesocosm conditions using a single application of carbaryl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While anthropogenic chemicals occur in a variety of ecological communities, ecologists have a poor understanding of their impacts on species within these communities. Aquatic communities in particular are commonly contaminated with chemicals including a wide variety of pesticides used to improve both agriculture and human health (McConnell 1998; LeNoir et al. 1999; Sparling et al. 2001; Kolpin et al. 2002, Fellers et al. 2004). Laboratory experiments have provided basic data about the effects of pesticides on model organisms, but ultimately we would like to know how pesticides affect a diversity of organisms under more natural conditions.
As we have begun to investigate the lethal and nonlethal impacts of pesticides, we have made a number of surprising discoveries about the lethality of pesticides. For example, pesticides can become more lethal in laboratory settings when we include more natural abiotic conditions including variation in pH, temperature, and UV-B light (Lohner and Fisher 1990; Zaga et al. 1998; Boone and Bridges 1999; Broomhall 2002; Chen et al. 2004). Moreover, pesticides can become more lethal in the laboratory when combined with the biotic stress of predator cues (Relyea and Mills 2001; Relyea 2003, 2004b, 2005b). These studies have advanced our understanding of the interplay between pesticides and natural variation in biotic and abiotic stressors. However, because these studies are conducted in the laboratory, we do not know whether (or under what conditions) these synergistic interactions occur when species are embedded into more natural ecological communities.
While synergistic interactions between pesticides and natural biotic or abiotic stressors occur in a variety of taxonomic groups, amphibians are one group in which pesticide effects are of utmost concern. Amphibians are experiencing declines around the globe and in some locations there appears to be a connection with pesticides (Wake 1998; Alford and Richards 1999; Davidson et al. 2001; Houlihan et al. 2001; Kiesecker et al. 2001). For example, in the western United States, some species of amphibians are declining and the declining populations are often those that are downwind from agricultural areas (Davidson et al. 2001, 2002). Carbamates and organophosphates, two classes of insecticides that inhibit acetylcholine esterase, are most closely correlated with these population declines (Davidson 2004). Moreover, amphibians living in ponds with declining populations exhibit decreased activity of the acetylcholine esterase enzyme, a potential signal of exposure to carbamates and organophosphates (Sparling et al. 2001). While pesticide concentrations in these wetlands tend to be low, laboratory experiments demonstrate that low concentrations of carbamates and organophosphates can be lethal if combined with chemical cues emitted by the aquatic predators that frequently coexist with amphibians (Relyea and Mills 2001; Relyea 2003, 2004b).
In this study, I examined how two species of larval anurans are affected by a carbamate pesticide (i.e., carbaryl) when combined with different levels of pH and predatory stress under outdoor mesocosm conditions. I selected larval bullfrogs and green frogs as the target species because both have shown synergistic responses with predator cues under laboratory conditions (Relyea 2003). I tested the following hypotheses: (1) carbaryl alone will have no effect on tadpole survival or growth, (2) carbaryl combined with low pH will cause reduced tadpole survival and growth, and (3) carbaryl combined with predatory stress will cause reduced tadpole survival and growth.
Pesticide background
Carbaryl is a carbamate insecticide that operates as a nerve agent by inhibiting acetylcholine esterase. Often sold under the commercial name Sevin®, carbaryl is one of the top ten pesticides used in the United States with 1–2 million kg applied annually to forests, rangeland, homes, and 1.3 million ha of cropland (Donaldson et al. 2002; National Pesticide Use Database www.ncfap.org ). It is a broad-spectrum insecticide whose half-life depends upon site conditions including sunlight and pH; for example, the half-life of carbaryl ranges from 0.1 d at pH = 8 to 1500 d at pH < 6 (Aly and El-Dib 1971; Wauchope and Haque 1973). Carbaryl concentrations in wetlands can be up to 4.8 mg/l, but typical concentrations are substantially lower (≤1 mg/l; Norris et al. 1983; Peterson et al. 1994). Fortunately, we know a great deal about the impacts of carbaryl from a large number of recent experiments on amphibians both in the laboratory and in outdoor mesocosms (Marian et al. 1983; Bridges 1997, 1999, 2000; Bridges and Semlitsch 2000, 2001; Semlitsch et al. 2000; Boone and Semlitsch 2001, 2002; Relyea and Mills 2001; Relyea 2003, 2005b). This wealth of knowledge allows in-depth investigations using carbaryl as a model pesticide.
Methods
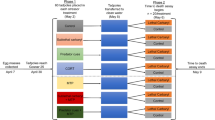
I conducted the experiment using a completely randomized design with a factorial combination of predator cues (absent or present), pH (6 or 8), and three carbaryl concentrations. The 12 treatment combinations were replicated four times for a total of 48 experimental units. The experimental units were 800-l cattle watering tanks filled with 580 l of well water on 19–20 June 2003. On 20 June, I added 15 g of rabbit chow to serve as an initial nutrient source. On 24 June, the tanks were made into pond mesocosms by adding 300 g of dried leaf litter (primarily Quercus spp.) and a 150-ml aliquot of pond water that served as an initial source of algae and zooplankton.
Each mesocosm was equipped with four predator cages constructed of 10 × 10 cm drain pipe with a screen on each end to permit the diffusion of chemical cues from the predators. In mesocosms assigned the predator treatment, each cage held an adult newt (Notophthalmus viridescens). Importantly, this predator coexists with both bullfrogs and green frogs in nature. In mesocosms assigned the no-predator treatment, the cages remained empty. Each newt was fed approximately 200 mg of mixed bullfrogs and green frogs and the feeding occurred three times per week. To equalize disturbance among tanks, I briefly lifted the no-predator cages out of the water during each feeding.
I manipulated the pH of the mesocosms by adding sulfuric acid. While acid additions are effective at lowering pH, one cannot hold pH at a constant level because photosynthesis will continually raise the pH during the daylight hours and throughout the duration of the experiment. For mesocosms assigned the high pH (i.e., pH = 8), I simply used the standard well water which has a natural pH of ∼8. For the pH = 6 treatment, I added 50 ml of sulfuric acid on 11 July (an amount that was determined based on titrations using two of the mesocosms). Quantification of pH on 15 July demonstrated that the desired pH treatments were achieved (see Results). I subsequently quantified pH on 25 and 28 July and found that the difference in pH between the low and high treatments began to converge. To maintain a lower pH in the low pH tanks, I added a second aliquot of sulfuric acid (10 ml) on 29 July and this was effective at reestablishing the desired pH (see Results). For comparison, the pH of natural ponds typically ranges from 5 to 8 (Mitsch and Gosselink 1986).
After altering the pH and allowing the mesocosms to establish algal communities, I added 20 tadpoles of each species on 12 July. The tadpoles came from eggs that were collected as ≥10 egg masses from two nearby ponds and hatched in 200-l wading pools containing aged well water. This ensured that the tadpoles were not exposed to either pesticides or predator cues prior the experiment. Once the tadpoles achieved the free-swimming stage (Gosner stage 25; Gosner 1960), I haphazardly selected individuals from a mixture of the egg masses (initial tadpole mean mass ± 1 SE: bullfrogs = 33 ± 3 mg and green frogs = 16 ± 2 mg).
After adding the tadpoles to the mesocosms, I applied carbaryl using a commercial formulation of carbaryl (Sevin®) whose stock concentration (22%) was confirmed using high-pressure liquid chromatography (Mississippi State Chemical Laboratory, Mississippi State, MS). Previous work has demonstrated no difference between technical grade carbaryl and commercial forms of carbaryl on amphibian growth and survival (C. M. Bridges, personal communication, USGS Toxicology Laboratory, Columbia, MO). The nominal concentrations desired were 0, 1, and 5 mg/l. Thus, given 580 l of water in each tank, I added 0, 2.57 ml and 12.85 ml of Sevin, respectively. It is important to note that the actual concentrations achieved in the mesocosms were not measured. As a result, I will refer to the three carbaryl treatments as no-carbaryl, low-carbaryl, and high-carbaryl, respectively.
After being exposed to the treatments for one month, I terminated the experiment on 12 August. I drained the tanks and collected all surviving tadpoles. I quantified the survival of both bullfrog and green frog larvae and then weighed all tadpoles to quantify the mean individual growth rate of both species ((final mass-initial mass)/31 d). The survival and mean growth rate for each tank served as the response variables.
Statistical analysis
I conducted a multivariate analysis of variance on the four response variables (the survival and growth of larval bullfrogs and green frogs). For all significant multivariate effects, I then conducted univariate tests. For significant univariate effects for treatments with more than two levels (i.e., the pesticide treatment), I conducted mean comparisons using Fisher’s LSD test. The pH manipulations were done incorrectly for two tanks and the data from these two tanks were excluded from the analysis (both coincidentally happened to be assigned the pH = 6, no-predator, no-carbaryl treatment). While the normality and homoscedasticity assumptions of the analysis were largely met by the growth data, the assumptions were not met by the survival data. Thus, I ranked the survival data prior to analysis. For the water chemistry data (pH and temperature), I conducted a repeated-measures analyses of variance.
Results
There were significant multivariate effects of pH and carbaryl on the tadpoles, but no multivariate effect of caged predators (Table 1, Figs. 1–2). In addition, there were no significant two- or three-way interactions.
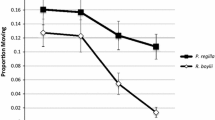
The survival of larval bullfrogs and green frogs when raised under a factorial combination of predators (NP = no predator, P = caged predator), pH (pH = 6 or 8), and three additions of carbaryl (0, 2.57 ml and 12.85 ml of Sevin containing 22.5% carbaryl) to 580-l mesocosms for nominal concentrations of 0, 1, and 5 mg/l. Data are means ± SE
The growth of larval bullfrogs and green frogs when raised under a factorial combination of predators (NP = no predator, P = caged predator), pH (pH = 6 or 8), and three additions of carbaryl (0, 2.57 ml and 12.85 ml of Sevin containing 22.5% carbaryl) to 580-l mesocosms for nominal concentrations of 0, 1, and 5 mg/L. Data are means ± SE
Univariate analyses indicated that the pH manipulations had no effect on tadpole survival but did affect tadpole growth (Table 1B). Both bullfrog and the green frog tadpoles grew more at low pH than high pH. Across all carbaryl and predator treatments, growth at low pH was 45% higher for bullfrogs and 65% higher for green frogs.
Univariate tests also indicated that carbaryl affected bullfrog survival and growth but had no effect on green frog survival and growth (Table 1B). Across all predator and pH treatments, bullfrog survival was nearly identical between the no-carbaryl and low-carbaryl treatments (P = 0.998). However, at high-carbaryl treatments, bullfrog survival declined by 10% (P = 0.039). Across all predator and pH treatments, bullfrog growth was similar between the no-carbaryl and low-carbaryl treatments (P = 0.417), but improved by 33% in the high-carbaryl treatment (P = 0.001).
To determine if the increase in bullfrog growth in the high-carbaryl treatment was simply a reflection of the 10% lower survival of bullfrogs, I conducted a subsequent ANCOVA on bullfrog growth using survival as a covariate. Whereas the relationship between density and growth is typically nonlinear, across this small change in density an assumption of linearity is reasonable. As in the previous analysis, the ANCOVA indicated no effects of predators or any interactions (P > 0.4). However, the survival covariate also was not significant (F 1,33 = 1.1, P = 0.300) while carbaryl and pH continued to have significant effects on bullfrog growth (F 2,33 = 4.0, P = 0.027 and F 1,33 = 26.6, P < 0.001, respectively). Thus, the increase in bullfrog growth associated with increased concentrations of carbaryl was not related to the 10% decline in bullfrog density.
Mesocosm pH
The repeated-measures ANOVA on mesocosm pH indicated significant effects of pH treatment (F 1,33 = 191.0, P < 0.001), time (F 3,99 = 69.3, P < 0.001) and pH treatment-by-time interaction (F 1,33 = 40.0, P < 0.001). There were no effects of carbaryl, predators, or any other interactions on pH (P ≥ 0.1). Shortly after the initial addition of sulfuric acid (15 July), the difference between pH treatments was significant (F 1,34 = 517, P < 0.001); the mean pH (±SE) was 8.19 ± 0.05 in high-pH mesocosms and 6.48 ± 0.06 in low-pH mesocosms. As time passed, the difference in pH began to converge (8.33 ± 0.03 versus 7.62 ± 0.03 on 25 July; 8.17 ± 0.04 versus 7.44 ± 0.04 on 28 July) but remained significantly different (ANOVA F 1,34 = 313, P < 0.001 for July 25; ANOVA F 1,34 = 161, P < 0.001 for July 25). Following the addition of a second aliquot of sulfuric acid (10 ml) on 29 July, the desired difference in pH was reestablished (8.01 ± 0.16 versus 6.02 ± 0.18; ANOVA F 1,33 = 69, P < 0.001).
Discussion
The results of the experiment demonstrated that the addition of carbaryl and the manipulations of pH affected amphibian performance but the addition of caged predators did not. While carbaryl caused some death (i.e., 10%) in one of the two species, this lethal effect happened only in the high-carbaryl treatment. Importantly, the mesocosm water was not tested, preventing me from confirming the nominal concentrations and assessing the rate of pesticide breakdown in the mesocosms. In static-renewal laboratory experiments, a considerable amount of mortality occurs at 2–3 mg/l (LC5016-d for green frogs = 2.6 mg/l; LC5016-d for bullfrogs = 2.3 mg/l; Relyea 2003). However, the current experiment only used a single application of carbaryl that could break down over time rather than re-applications of carbaryl as in static-renewal experiments. In other mesocosm experiments using single applications of carbaryl, low mortality rates are a common observation (Boone et al. 2001; Boone and Semlitsch 2001; Boone and James 2003).
The 10% bullfrog mortality caused by the high-carbaryl treatment was accompanied by a significant 33% increase in bullfrog growth and a nonsignificant 19% increase in green frog growth. The subsequent ANCOVA suggested that the increased growth was not caused by the reduction in bullfrog density (i.e., reduced competition). In laboratory experiments, we have not observed increased tadpole growth with the addition of carbaryl (Relyea and Mills 2001; Relyea 2003), suggesting that carbaryl does not affect tadpole physiology in a way that increases growth. Observing carbaryl-associated growth increases in the mesocosm experiment but not in laboratory experiments implies that the growth change might be mediated through indirect changes in the community that caused greater food availability and, as a result, increased tadpole growth.
Increased tadpole growth with carbaryl has been observed in past mesocosm experiments, although this outcome has been inconsistent. For example, as in the current experiment, Boone and Semlitsch (2003) found that adding carbaryl caused a significant increase in bullfrog growth. In general, however, mesocosm experiments using 2–7 mg/l of carbaryl have shown that carbaryl can cause a wide variety of effects on tadpole growth. If we consider the 29 cases that have been observed in past mesocosm experiments (29 combinations of species × carbaryl concentrations), carbaryl can cause increased growth (five cases), decreased growth (two cases), both increased and decreased growth (depending upon density; three cases) or no change in growth (19 cases; Boone et al. 2001; Boone and Semlitsch 2001, 2002, 2003; Bridges and Boone 2003; Boone and James 2003; Boone and Bridges 2003; Mills and Semlitsch 2004; Boone et al. 2004). The variation in outcomes does not appear to be a reflection of species-specific responses; a given species can show any of the growth responses. Instead, the diversity of observed growth outcomes suggests that we need to understand the mechanisms underlying community responses to carbaryl and other pesticides that share carbaryl’s mode of action (i.e., other carbamates and organophosphates).
When the addition of carbaryl is associated with increased tadpole growth, a number of studies have hypothesized that the underlying mechanism is an indirect effect of carbaryl reducing zooplankton abundance (due to direct toxicity) which would result in an algal bloom that could be consumed by the tadpoles (Boone and Semlitsch 2002, 2003; Bridges and Boone 2003). Indeed, there is abundant evidence that carbaryl can reduce zooplankton abundance (primarily cladocerans) and cause cascading positive effects on phytoplankton (Hanazato and Yasuno 1987, 1989; Bridges and Boone 2003; Mills and Semlitsch 2004; Relyea 2005a). However, North American tadpoles are not known to consume phytoplankton (i.e., suspended algae); instead, they consume periphyton (i.e., attached algae). As detailed by Leibold and Wilbur (1992), because of competition between phytoplankton (the resource of zooplankton) and periphyton (the resource of tadpoles), the addition of carbaryl should lead to a decline in periphyton and, therefore, a reduction in tadpole growth. Mills and Semlitsch (2004) confirmed this mechanism by quantifying all members of the community and finding that carbaryl caused a large reduction in zooplankton, a three-fold increase in phytoplankton, a three-fold decrease in periphyton, and an 18% reduction in metamorph mass. This underscores the need for future experiments to identify the mechanisms of the indirect interactions when identifying pesticide effects in communities.
Given that the expected indirect effect of adding carbaryl is a decrease in tadpole growth and given that only small a fraction of mesocosm experiments have observed an increase in tadpole growth with carbaryl, we are in need of alternative mechanisms. One alternative mechanism for instances of increased tadpole growth is that the addition of carbaryl, because it is a neurotoxin that reduces tadpole foraging activity (Bridges 1997, 1999), allows periphyton to experience an exponential increase in biomass. After the degradation of carbaryl, this increased biomass of periphyton can be cropped down by the tadpoles, resulting in greater final growth. Interestingly, this mechanism has been observed in herbivores living with predators. When predators induce herbivores to reduce their foraging activity, it allows resources to increase and the animals end up being more massive than animals living in predator-free environments (Relyea and Werner 2000, Peacor 2002). Importantly, this mechanism would predict that growth effects of carbaryl would be determined by species-specific behavioral responses to carbaryl and by the timing of the pesticide addition; both factors have been cited as being important in past studies (Boone and Bridges 2003; Mills and Semlitsch 2004). In summary, there are multiple possible mechanisms that could affect amphibian growth. While the behavioral mechanism seems more likely to be correct, future experiments should work to definitively test both mechanisms.
When I reduced the pH in the mesocosms, there was no effect on tadpole survival, but it did cause a 45–65% increase in growth. Given that a major food resource for tadpoles is periphyton, these results suggest that the reduction in pH caused an increase in tadpole resources (i.e., periphyton). Previous research has documented that reductions in pH from 8 to 6 commonly cause increases in periphyton biomass (reviewed in Planas 1996). Thus, the mechanism underlying the pH effect appears to be a straightforward increase in tadpole resources.
Based on previous laboratory experiments, I expected to find interactive effects among carbaryl, pH, and predator cues; however, I found no interactive effects. For example, I observed no interaction between carbaryl and pH, despite the fact that laboratory experiments have shown that the half-life of carbaryl is considerably longer at lower pH. In the laboratory, the half-life of carbaryl is ∼1 day at pH = 8 but ∼400 days at pH ≤ 6 (Aly and El-Dib 1971; Wauchope and Haque 1973; Sikka et al. 1975; Sharom et al. 1980). However, carbaryl can also be broken down by sunlight (Wolfe et al. 1978). Under mesocosm conditions with water at a pH of 7.8–8.4, the half-life of carbaryl has been estimated at 4 h to 4 d (Boone and Bridges 2003; Boone et al. 2004). The breakdown by sunlight therefore likely plays a very important role in determining the effect on amphibians and the rest of the aquatic community. The weak effects of carbaryl on bullfrog survival and the lack of any effect on green frog survival under either pH condition in the mesocosm experiment suggests that carbaryl broke down quite rapidly in the experiment under both pH conditions, although I did not have the data to confirm this.
I also found no evidence of interactions between carbaryl and predatory stress, despite the fact that such interactions have been repeatedly observed in laboratory experiments using several species of amphibians (including green frogs and bullfrogs) and several types of pesticides (Relyea and Mills 2001; Relyea 2003, 2004b, 2005b). In trying to reconcile the different outcomes between laboratory and mesocosm experiments, we must consider the commonalities and differences in the two experimental venues and their associated protocols. The current mesocosm experiment shared a number of commonalities with the past laboratory experiments: (1) the mesocosm experiment used a mixture of two populations of bullfrogs and green frogs that included the population originally used in the laboratory experiments, (2) both experiments used the same species of predator (adult newts), (3) both experiments used the same water source (a nearby well), and (4) the high pH treatment of the mesocosm experiment was similar to the pH in the laboratory experiments (pH = 7.8–8.0; Relyea 2003). However, there were four major differences between the mesocosm experiment and past laboratory experiments. First, the mesocosms had four predators fed 300 mg of tadpole every 2 d in 580-l tanks whereas the laboratory tubs contained one predator fed 100 mg of tadpoles every 2 d in an 8-l tub. Thus, on a per-liter basis, the concentration of the predator cue was nearly 6-fold higher in the laboratory experiment and this higher concentration of predator cues might make a synergistic interaction more likely. This possibility seems unlikely because other experiments have shown that behavioral and morphological responses to caged predators in a cattle tank plateau at four predators, suggesting that higher concentrations of predator cues do not cause additional stress (Relyea 2004a). Second, the laboratory experiments were static-renewal experiments in which the water was changed every 4 d (to prevent fouling) and the pesticide was re-applied after each water change. In contrast, mesocosm experiments typically do not foul (because zooplankton help crop down bacteria) and the typical protocol is to apply the desired pesticide concentration as a single pulse at the beginning of the experiment (e.g., Boone and Semlitsch 2002; Mills and Semlitsch 2004; Relyea 2005a). Third, as noted above, mesocosm experiments are conducted outside where they are exposed to sunlight that breaks down carbaryl more rapidly than under laboratory conditions (within a few days; Boone and Bridges 2003; Boone et al. 2004). Finally, mesocosms contain a community of organisms that may permit a variety of potential direct and indirect effects that are not possible in single-species laboratory experiments. It is likely that the latter three factors all contributed to the lack of any synergistic effects in the mesocosm experiment.
These results highlight the critical importance of experimental protocols when extrapolating our results to natural habitats. For example, when we examine pesticides that rapidly break down and are applied only once per year (e.g., a one-time application of a pesticide in the spring), static-renewal tests that maintain a constant pesticide concentration may bias us toward finding larger effects than one would observe in nature. In contrast, when we examine pesticides that rapidly break down and are applied repeatedly throughout the spring and summer, single-pulse mesocosm experiments may bias us toward finding smaller effects than one would observe in nature. In the case of pesticides that inhibit acetylcholine esterase such as carbaryl, applications occur throughout the agricultural growing season and, therefore, are likely entering aquatic habitats repeatedly during the larval period of amphibians. Thus, to definitively test whether predatory stress interacts with pesticides under more natural conditions, we need to conduct experiments that mimic the pesticide application protocols that occur in nature.
Conclusions
Global use of pesticides is important to human health and agriculture, but the impacts on ecological communities are often unknown. Our understanding of pesticide effects often comes from single-species laboratory experiments, but we can arrive at very different conclusions under more natural conditions. In the case of pesticide impacts on amphibians, static renewal laboratory experiments have discovered that changes in pH and predatory stress can have substantial impacts on the lethality of pesticides, but the current study demonstrates that these synergistic effects do not occur with single applications to pond mesocosms. Such disparate results suggest that we need to carefully consider how experimental conditions relate to the conditions experienced by organisms in nature and that we need to track changes in the food web to understand the plethora of potential indirect effects that pesticides can produce when species are embedded back into their natural community context. Although it can require more time, energy, and financial resources, this approach will undoubtedly lead us to a better understanding of the impacts of pesticides on aquatic communities and how pesticides might lead to global declines in amphibians.
References
Alford RA, Richards SJ (1999) Global amphibian declines: a problem in applied ecology. Ann Rev Ecol Syst 30:133–165
Aly OM, El-Dib MA (1971). Studies on the persistence of some carbamate insecticides in the aquatic environment—I: hydrolysis of sevin, baygon, pyrolan and dimetilan in waters. Water Research 5:1191–1205
Boone MD, Bridges CM (1999) The effect of temperature on the potency of carbaryl for survival of tadpoles of the green frog (Rana clamitans). Environ Toxicol Chem 18:1482–1484
Boone MD, James SM (2003) Interactions of an insecticide, herbicide, and natural stressors in amphibian community mesocosms. Ecol Appl 13:829–841
Boone MD, Bridges CM (2003) Effects of carbaryl on green frog (Rana clamitans) tadpoles: timing of exposure versus multiple exposures. Environ Toxicol Chem 22:2695–2702
Boone MD, Bridges CM, Rothermel BB (2001) Growth and development of larval green frogs (Rana clamitans) exposed to multiple doses of an insecticide. Oecologia 129:518–524
Boone MD, Semlitsch RD (2001) Interactions of an insecticide with larval density and predation in experimental amphibian communities. Conserv Biol 15:228–238
Boone MD, Semlitsch RD (2002) Interactions of an insecticide with competition and pond drying in amphibian communities. Ecol Appl 12:307–316
Boone MD, Semlitsch RD (2003) Interactions of bullfrog tadpole predators and an insecticide: predation release and facilitation. Oecologia 137:610–616
Boone MD, Semlitsch RD, Fairchild JF, Rothermel BB (2004) Effects of an insecticide on amphibians in large-scale experimental ponds. Ecol Appl 14:685–691
Bridges CM (1997) Tadpole swimming performance and activity affected by acute exposure to sublethal levels of carbaryl. Environ Toxicol Chem 16:1935–1939
Bridges CM (1999) Effect of a pesticide on tadpole activity and predator avoidance behavior. J Herpetol 33:303–306
Bridges CM (2000) Long-term effects of pesticide exposure at various life stages of southern leopard frog (Rana sphenocephala). Arch Environ Con Toxicol 39:91–96
Bridges CM, Boone MD (2003) The interactive effects of UV-B and insecticide exposure on tadpole survival, growth and development. Biol Conserv 113:49–54
Bridges CM, Semlitsch RD (2000) Variation in pesticide tolerance of tadpoles among and within species of Ranidae and patterns of amphibian decline. Conserv Biol 14:1490–1499
Bridges CM, Semlitsch RD (2001) Genetic variation in insecticide tolerance in a population of southern leopard frogs (Rana sphenocephala): implications for amphibian conservation. Copeia 2001:7–13
Broomhall S (2002) The effects of endosulfan and variable water temperature on survivorship and subsequent vulnerability in predation in Litoria citropa tadpoles. Aqua Toxicol 61:243–250
Chen CY, Hathaway KM, Folt CL (2004) Multiple stress effects of Vision® herbicide, pH, and food on zooplankton and larval amphibian species from forest wetlands. Environ Toxicol Chem 23:823–831
Davidson C (2004) Declining downwind: amphibian population declines in California and historical pesticide use. Ecol Appl 14:1892–1902
Davidson C, Shafer HB, Jennings MR (2001) Declines of the California red-legged frog: climate, UV-B, habitat, and pesticides hypotheses. Ecol Appl 11:464–479
Davidson C., Shafer HB, Jennings MR (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601
Donaldson D, Kiely T, Grube A (2002) Pesticides Industry Sales and Usage: 1998 and 1999 Market Estimates. USEPA Report No. 733-R-02-001
Fellers GM, McConnell LL, Pratt D, Datta S (2004) Pesticides in mountain yellow-legged frogs (Rana muscosa) from the Sierra Nevada Mountains of California, USA. Environ Toxicol Chem 23:2170–2177
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hanazato T, Yasuno M (1987) Effects of a carbamate insecticide, carbaryl, on summer phyto- and zooplankton communities in ponds. Environ Pollut 48:145–159
Hanazato T, Yasuno M (1989) Effects of carbaryl on spring zooplankton communities in ponds. Environmental Pollut 56:1–10
Houlihan JE, Findlay CS, Schmidt BR, Meyers AH, Kuzmin SL (2001) Quantitative evidence for global amphibian population declines. Nature 404:752–755
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian population declines. Nature 410:681–684
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Leibold MA, Wilbur HM (1992) Interactions between food-web structure and nutrients on pond organisms. Nature 360:341–343
LeNoir JS, McConnell LL, Fellers GM, Cahill TM, Seiber JN (1999) Summertime transport of current-use pesticides from California’s central valley to the Sierra Nevada Mountain range, USA. Environ Toxicol Chem 18:2715–2722
Lohner TW, Fisher SW (1990) Effects of pH and temperature on the acute toxicity and uptake of carbaryl in the midge, Chironomus riparius. Aqua Toxicol 16:335–354
Marian MP, Arul V, Pandian TJ (1983) Acute and chronic effects of carbaryl on survival, growth, and metamorphosis in the bullfrog (Rana tigrina). Arch Environ Con Toxicol 12:271–275
McConnell LL, LeNoir JS, Datta S, Seiber JN (1998) Wet deposition of current-use pesticides in the Sierra Nevada Mountain range, California, USA. Environ Toxicol Chem 17:1908–1916
Mills NE, Semlitsch RD (2004) Competition and predation mediate the indirect effects of an insecticide on southern leopard frogs. Ecol Appl 14:1041–1054
Mitsch WJ, Gosselink JG (1986) Wetlands. Van Nostrand Reinhold, New York, p 539
Norris LA, Lorz HW, Gregory SZ (1983) Influence of forest and range land management on anadramous fish habitat in western North America: forest chemicals. PNW-149. General Technical Report (U. S. D. A. Forest Service, Portland, OR)
Peacor SD (2002) Positive effect of predators on prey growth rate through induced modifications of prey behaviour. Ecol Lett 5:77–85
Peterson HG, Boutin C, Martin PA, Freemark KE, Ruecker NJ, Moody MJ (1994) Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aqua Toxicol 28:275–292
Planas D (1996) Acidification effects. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology. Academic Press, London, pp 497–530
Relyea RA (2003) Predator cues and pesticides: a double dose of danger for amphibians. Ecol Appl 13:1515–1521
Relyea RA (2004a) Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecology 85:172–179
Relyea RA (2004b) Synergistic impacts of malathion and predatory stress on six species of North American tadpoles. Environ Toxicol Chem 23:1080–1084
Relyea RA (2005a) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl 15:618–627
Relyea RA (2005b) The lethal impacts of Roundup and predatory stress on six species of North American tadpoles. Arch Environ Con Toxicol 48:351–357
Relyea RA, Werner EE (2000) Morphological plasticity in four larval anurans distributed along an environmental gradient. Copeia 2000:178–190
Relyea RA, Mills N (2001) Predator-induced stress makes the pesticide carbaryl more deadly to grey treefrog tadpoles (Hyla versicolor). PNAS 98:2491–2496
Semlitsch RD, Bridges CM, Welch AM (2000) Genetic variation and a fitness tradeoff in the tolerance of gray treefrog (Hyla versicolor) tadpoles to the insecticide carbaryl. Oecologia 125:179–185
Sharom MS, Miles JRW, Harris CR, McEwen FL (1980) Persistence of 12 insecticides in water. Water Res 14:1089–1093
Sikka HC, Miyazaki S, Lynch RS (1975) Degradation of carbaryl and 1-naphthol by marine microorganisms. Bull Environ Con Toxicol 13:666–672
Sparling DW, Fellers GM, McConnell LS (2001) Pesticides and amphibian population declines in California, USA. Environ Toxicol Chem 20:1591–1595
Wake DB (1998) Action on amphibians. TREE 13:379–380
Wauchope RD, Haque R (1973) Effects of pH, light and temperature on carbaryl in aqueous media. Bull Environ Con Toxicol 9:257–260
Wolfe NL, Zepp RG, Paris DF (1978) Carbaryl, propham and chlorpropham: a comparison of the rates of hydrolysis and photolysis with the rate of biolysis. Water Res 12:565–571
Zaga A, Little EE, Raben CF, Ellersieck MR (1998) Photoenhanced toxicity of a carbamate insecticide to early life stage anuran amphibians. Environ Toxicol Chem 17:2543–2553
Acknowledgments
My thanks to Josh Auld, Adam Marko, Jason Hoverman, Elizabeth Kennedy, Stacy Phillips, and Nancy Schoeppner for assisting with the experiments. Josh Auld, Jason Hoverman, and Nancy Schoeppner provided insightful reviews of the manuscript. This research was funded by the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Relyea, R.A. The effects of pesticides, pH, and predatory stress on amphibians under mesocosm conditions. Ecotoxicology 15, 503–511 (2006). https://doi.org/10.1007/s10646-006-0086-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-006-0086-0