Abstract
Solvent-terminated dispersive liquid-liquid microextraction (ST-DLLME) as a simple, fast, and low-cost technique was developed for simultaneous extraction of Cd2+ and Cu2+ ions in aqueous solutions. Multiobjective evolutionary algorithm based on decomposition with the aid of artificial neural networks (ANN–MOEA/D) was used for the first time in chemistry, environment, and food sciences to optimize several independent variables affecting the extraction efficiency, including disperser volume and extraction solvent volume, pH, and salt addition. To perform the ST-DLLME operations, xylene, methanol, and dithizone were utilized as an extraction solvent, disperser solvent, and chelating agent, respectively. Non-dominated sorting genetic algorithm versions II and III (NSGA II and NSGA III) as multiobjective metaheuristic algorithms and in addition central composite design (CCD) were studied as comparable optimization methods. A comparison of results from these techniques revealed that ANN-MOEA/D model was the best optimization technique owing to its highest efficiency (97.6% for Cd2+ and 98.3% for Cu2+). Under optimal conditions obtained by ANN-MOEAD, the detection limit (S/N = 3), the quantitation limit(S/N = 10), and the linear range for Cu2+ were 0.05, 0.15, and 0.15–1000 μg L−1, respectively, and for Cd2+ were 0.07, 0.21, and 0.21–750 μg L−1, respectively. The real sample recoveries at a spiking level of 0.05, 0.1, and 0.3 mg L−1 of Cu2+ and Cd2+ ions under the optimal conditions obtained by ANN–MOEA/D ranged from 94.8 to 105%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are numerous sources of heavy metal contaminations such as metal processing factories, mines, and sewage sludge. They enter into the human body via air, water, and food. Some of these metals such as copper ions are essential micronutrient at low concentrations but can be toxic at higher concentrations and others; for instance, cadmium ions are toxic even at very low concentrations (Furini 2012). In water and food samples, the concentration of heavy metals in trace levels can cause serious health problems to humans, and therefore, preconcentration of analyte was carried out before final assay. Preconcentration of heavy metal ions was carried out by using liquid–liquid extraction (Karadaş and Kara 2014) and solid-phase extraction (Mohammadi et al. 2016). The employments of these methods were limited due to high consumption of organic solvent and high-cost procedure (Shirkhanloo et al. 2010). To eliminate these problems, dispersive liquid–liquid microextraction (DLLME) as an efficient miniaturized approach was introduced in 2006 (Rezaee et al. 2006). The extraction in this technique was restricted to the solvents with higher density and also poisonous property which should be isolated in solution using a centrifuge apparatus. Chen and coworkers developed a solvent-terminated dispersive liquid–liquid microextraction (ST-DLLME) with a lower density solvent (H. Chen et al. 2010). The ST-DLLME process can be completed by the injection of demulsifier solvent like acetonitrile into the final turbid solution of the aqueous sample, and as the extraction solvent eliminates the emulsion and consequently, the centrifugation step is not necessary (Mansour and Danielson 2018). One of the issues that has always been of interest to scientists is to optimize the experimental conditions and obtaining the most appropriate response. Recently, scientists have focused on the development of high efficiency multiobjective metaheuristic optimization algorithms such as non-dominated sorting genetic algorithm versions II and III (NSGA-II and NSGA-III) (Deb et al. 2002; Deb and Jain 2014) and multiobjective evolutionary algorithm based on decomposition (MOEA/D) (Zhang and Li 2007). In comparison to NSGA versions II and III, the MOEA/D method uses a different approach for optimization and lower computational complications. MOEA/D decomposes a multiobjective problem (MOP) into a number of single objective subproblems and solving them simultaneously with respect to pareto optimal solutions (Neri and Tirronen 2010). This technique has been applied in limited fields, and so, few reports have been given in the literature in this regard (C.M. Chen et al. 2010; Zhou et al. 2011).
In our previous works, DLLME technique was employed for extraction of several analytes in various samples (Moradi et al. 2017; Maham et al. 2014; Maham et al. 2013c; Maham et al. 2013a; Maham et al. 2013b; Kiarostami et al. 2014; Farajvand et al. 2018). So as far as we are aware, practically no research has been carried out on the simultaneous microextraction of Cd2+ and Cu2+ ions using ST-DLLME, and furthermore, a MOEA/D approach has not been used in the field of chemistry, environment, and food sciences. Therefore, this study describes the employment of multiobjective evolutionary algorithm based on decomposition with the aid of artificial neural networks(ANN–MOEA/D) to optimize the ST-DLLME of Cu2+ and Cd2+ ions with dithizone as a complexing agent in water, wastewater, milk, tea, and apple juice samples. In addition, the central composite design (CCD), NSGA-II, and NSGA-III have also been employed as comparable techniques for microextraction optimization.
Materials and methods
Chemical and reagents
All laboratory reagents were analytical grade and purchased from Merck and Sigma-Aldrich. Cadmium and copper ion stock solutions with concentration of 1000 mg L−1 were made by dissolution of CdCl2 and CuCl2 (Titrasol, Merck) in deionized water. Dilutions of heavy metal stock solutions in deionized water were carried out to prepare calibration solutions. Adjustment of pH solution was controlled by adding HCl (0.1 M) and NaOH (0.1 M). The natural water (river and well water) and wastewater were collected from agricultural areas and a factory wastewater, respectively (Islamshahr, Iran). The river samples were obtained directly from three separate three sites separated from each other by 1 km. They were then mixed to prepare a bulk sample. The bulk well water samples were prepared by collecting samples of well water at an interval of 3 days from only one site. The bulk wastewater samples were collected from a factory wastewater over a 3-day period. One liter of the laboratory sample was collected directly from the homogenized bulk sample. Additionally, all the other samples such as apple juice (Sunich Co, Iran), low-fat bottled milk (Damdaran Co, Iran), black tea (Ahmad Co, England), and bottled mineral water (Vata Co, Iran) were purchased from a local market and applied to study the proposed ST-DLLME percentage recoveries.
Instruments
Atomic absorption spectrometer equipped with a deuterium background correction and an air-acetylene burner (Varian spectra 240 fs (USA)) was employed for determination of copper and cadmium ions. pH meter Jenway 3510 (United States of America) was used to determine the solution pH.
Real sample preparation
A sample of tea drink was conventionally made by putting 5 g of the tea leaves in 250 mL of boiling water. The samples of wastewater, water, milk, and apple juice were stored individually in a sterile food container and kept at 4 °C. Water and wastewater samples were acidified with nitric acid to a pH 2. The aqueous samples were centrifuged for 20 min at 4000 rpm, and after, the supernatants were passed through a 0.45-μm membrane filter (Millipore Co, MA, USA). After filtration step, the cleaned apple juice and milk were mixed with deionized water for dilution in the ratio of 1:4. Lastly, the prepared aqueous samples were used for ST-DLLME process.
ST-DLLME procedure
To perform ST-DLLME procedure, a 10 mL of deionized water spiked with 0.5 mg L−1 Cd2+ and Cu2+ ions with adjusted pH of 6 was transferred into a 10-mL glass volumetric flask. Triple mix containing 250 μL xylene as an extraction solvent, 100 μL dithizone (1 × 10−4 mol L−1) as a complexing agent, and 550 μL methanol as a disperser solvent was injected by a Hamilton syringe (1 mL) into the above solution, in which a turbid solution was formed. Then, with injection of a 500 μL acetonitrile as a demulsifier, the phase separation was resulted. A Hamilton syringe (100 μL) was used to collect the upper phase which was evaporated using a N2 gas gentle stream. After evaporation, 1 mL of nitric acid (0.1 M) was added to the obtained residual and finally nebulized into the flame atomic absorption spectrometer for ion determination.
Optimization methods
For optimization of three independent factors such as extraction, disperser solvent types, and extraction time, one variable at a time (OVAT) technique was applied. Other four variables were optimized by multiobjective evolutionary algorithm based on decomposition with the aid of artificial neural networks (ANN–MOEA/D), non-dominated sorting genetic algorithm versions II and III, and response surface methodology (RSM) with regard to the microextraction efficiency.
Central composite design
In this project, a CCD technique with four variables and three levels as a response surface methodology with α = 2 including 30 treatment combinations with 16 factorial points, eight axial points, and six replicates at center point was tested using Design Expert (version 7.00, Stat-Ease Inc., Minnea polis, MN). The low and high levels of four independent variables in CCD such as the volumes of extraction (V1) and disperser (V2) solvents, pH (V3), and salt addition (V4) were 50 and 450 μL, 300 and 800 μL, 2 and 10, and 0 and 7.5%, respectively.
Artificial neural networks-multiobjective evolutionary algorithm
The nonlinear relation between the independent factors (input data) and the dependent factors (output data) was obtained by multilayer preceptrons (MLP) artificial neural network using MATLAB R2010a software. Training of the network was performed by the levenberg marquardt back propagation method. Tansig and pureline were employed as activation functions in the hidden and output layers, respectively.
A problem with multiobjective state (MOP) is introduced as Eq. 1 (Ying et al. 2017).
where v∈ π is the feasible search region and v = (v1, v2, …, vn)T is the vector of decision factors.
- F::
Ω → Rm, m is the objective function number and Rm is the multi-dimensional space for objective.
By developing the multiobjective genetic algorithm (MOGA), Srinvas and coworkers proposed the non-dominated sorting genetic algorithm (NSGA) (Ying et al. 2017). The basis of this algorithm is based on the genetic algorithm, but the process way of the selection operator is different. The operators of mutation and cross-over remain unchanged. Later, Deb and coworkers developed the NSGA-II (Deb et al. 2002) as a fast non-dominated sorting method for ranking results in selection procedure. The NSGA-II was not very efficient for problems with many objectives, and consequently, a new version of NSGA called NSGA-III was developed (Deb and Jain 2014). Zhang and Li introduced multiobjective evolutionary algorithm based on decomposition (MOEA/D) in 2007 (Zhang and Li 2007). This technique degrades the multiobjective problem into several sub-problems which are optimized simultaneously through neighborhood scalar methods. In all the generations, the population for individual subproblem is composed of the best solution obtained relative to the starting point of the algorithm. The neighborhood relationships between the subproblems are established upon the basis of the intervals among the vectors of their collection coefficients. For two subproblems that are neighbors, the optimal solutions are similar. In MOEA/D method, the information obtained from all neighbor subproblems is used to optimize them. The process begins with a population comprising N generated random solutions and each particular solution is assigned to an individual. vi is the solution of subproblem i. A weight vector range λi is stated as the T nearest weight vectors with λ1, λ2…, λN. Bi (T) is used to define the collection for the T indices of neighboring subproblems of item i. In the operation of recreation, two solutions are selected from the range of the subproblem i for reproducing an offspring using the operators of genetic algorithm. Next, the range of the subproblem i is renovated using the previous generated offspring. Finally, the optimal solutions for all subproblems are equal to the solutions in the population. The framework of MOEA/D is shown directly below (Ying et al. 2017).

Results and discussions
Optimization methods
The extraction solvent type
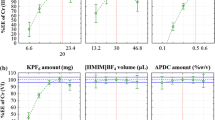
For studying the effect of extraction solvent type, several low density solvents such as cyclohexane, toluene, n-hexane, cyclohexanone, and xylene were investigated. Triplicate experiments of 10-mL sample were carried out under conditions of 300 μL ethanol, 100 μL extraction solvent, no salt addition, and pH 5.45. Figure 1a reveals that the maximum efficiency was acquired with xylene along with significant difference (p < 0.05, single factor ANOVA). Thus, xylene was chosen as the extraction solvent for further experiments.
The disperser solvent type
The disperser solvent type influences the viscosity of the solvent, generation of the droplet, and efficiency of the extraction. Methanol, ethanol, and acetone were studied as disperser solvents. As indicated in Fig. 1b, methanol gave the maximum efficiency with significant difference (p < 0.05, single factor ANOVA). Moreover, methanol had the minimum toxicity and price compared to others and consequently, was selected as the disperser solvent for the next experiments.
The time of extraction
The influence of time of extraction was investigated at the time intervals between 2 and 10 min. Figure 1c illustrates the percentage relative recovery of the extraction for Cu2+ and Cd2+ ions against the time of extraction. As can be seen in Fig. 1c, the extraction time of 6 min was chosen as the maximum extraction efficiency with significant difference (p < 0.05, one-way ANOVA).
Central composite design optimization
The CCD was utilized to optimize the extraction and disperser solvent volumes, solution pH, and salt addition of ST-DLLME for Cu2+ and Cd2+ ions from aqueous solutions. The quadratic response surface models were confirmed for parameters of regression by the analysis of variance (ANOVA) as shown in Table 1. Table 1 indicates the high F value and a low probability (p < 0.001) for models, and consequently, the models were significant. According to the values of p, the V1, V12, V32, and V42 for Cu2+ and V1, V12, V22, and V42 for Cd2+ were significant. The rest of the variables and interactions were insignificant. The lack of fit for both metal ions was insignificant (p > 0.05). The coded quadratic equations based on the CCD analysis for Cu2+ and Cd2+ ions are given by Eqs. 2 and 3, respectively.
Based on the optimal conditions acquired from the CCD for multivariate optimization along with desirability of 0.96 (550 μL methanol, 250 μL xylene, 3.75% salt addition, and pH 6), the predicted response, experimental response, and absolute error were 96.31%, 95.11%, and 1.2 for Cu2+ and 94%, 93.5%, and 0.5 for Cd2+, respectively.
ANN–MOEA/D optimization
MOEAD with the aid of artificial neural networks (ANN–MOEA/D) was utilized for optimizing the ST-DLLME of Cu2+ and Cd2+ ions according to the nonlinear equations which were acquired from artificial neural networks. Figure 2 shows the structure of multilayer artificial neural networks with a population of four variables such as the volumes of disperser and extraction solvents, salt addition, and pH that outlined by the CCD. The values of bias and weight for each layer of ANN model were computed, and corresponding equations for Cu2+ and Cd2+ ions are obtained individually as Eq. 4.
where β1 and K1 are the bias and weight of middle layer with 10 neurons, respectively, and β2 and K2 are the bias and weight of outer layer with one neuron, respectively. The initial factors like the volumes of disperser and extraction solvents, salt addition, and pH were indicated by V (1), V (2), V (3), and V (4), respectively. The weights and biases for Cu2+ and Cd2+ ions are presented in Table 2. These data were used to obtain an objective function for ANN–MOEA/D optimization technique with 100 maximum iteration number of iteration, subproblem number of 50, and neighbor number of 8. The pareto fronts of two objectives for NSGA (II), NSGA (III), and MOEA/D optimization methods of ST-DLLME for Cu2+ and Cd2+ ions are illustrated in Fig. 3. MOEA/D showed the best homogeneity and the highest extraction efficiency as stated in Fig. 3 and Table 3, respectively, and consequently, it was proved to be the best technique for optimization. Under the optimal conditions obtained by ANN-MOEA/D (extraction solvent volume 260 μL, disperser solvent volume 500 μL, pH 6, and salt addition 3%), the percentage relative recoveries of ST-DLLME method for Cu2+ and Cd2+ ions were 98.30 and 97.6%, respectively.
Figures of merit
In accordance with ANN-MOEAD optimal conditions, some important figures of merit in the ST-DLLME method such as determination (S/N = 10) and detection (S/N = 3) limits, regression line, linear range, and coefficient of determination (R2) in spiked water samples were acquired and the results are indicated in Table 4.
Study of real samples
Several real samples such as apple juice, milk, tea drink, mineral water, river water, well water, and wastewater were studied to get microextraction efficiency and accuracy. To accomplish this purpose, the percentage relative recovery (R%) was calculated by the following equation:
where Cfounded, Creal, and Cadded are the concentrations of analyte after addition of a known amount of standard in the real sample, the concentration of analyte in the real sample, and the concentration of known amount of standard which was spiked to the real sample, respectively (Rezaee et al. 2006). Real samples were tested individually with spiking levels of 50, 100, and 200 μg L−1 of Cu2+ and Cd2+ ions. As presented in Table 5, the mean percentage relative recoveries with three replicate experiments for each sample ranged from 94.6 to 105.1% for Cu2+ and 94.8 to 106.1% for Cd2+ which were in the acceptable range with standard deviations of 0.12 to 0.43 for Cu2+ and 0.10 to 0.61 for Cd2+ ions. Therefore, the suggested ST-DLLME method can be applied successfully for determination of the above heavy metal ions in real samples.
Comparison with other methods
As presented in Table 6, several pretreatment methods for Cd2+ and Cu2+ ions that included in the literatures were compared to the proposed ST-DLLME based on the figures of merit and type of optimization techniques. Among the data that are shown in Table 6, the proposed ST-DLLME method, in relation to other methods such as solid-phase extraction, flow injection analysis, and cloud point extraction (rows 1–5 of the Table 6), presents lower solvent and sample consumption, shorter extraction time, more suitable determination, and detection limits and linear range. Unlike DLLME method such as in situ DLLME using ionic liquid and conventional DLLME (rows 6 and 7 of the Table 6), in the ST-DLLME method, there is no need for centrifugation step for phase separation, and consequently, the extraction time is much shorter. In addition, there are other advantages using ST-DLLME method; namely rapidity, simple processing, and lower cost of operation. One factor at a time (OFAT) method was employed in the most comparable methods presented in Table 6 (rows 1–5 and 7). In the case of interaction between some variables, OFAT method cannot provide the correct optimal conditions. Chemometric optimization methods such as metaheuristic algorithm and experimental design can be used successfully for solving these difficulties. As compared to other methods except the method in row 6 of Table 6, the proposed method showed the suitable potential of ANN–MOEA/D and CCD as multivariate methods to find the optimal conditions.
Conclusions
The presented ST-DLLME method for simultaneous extraction of Cu2+ and Cd2+ ions can be regarded as a safe method due to a very low consumption of hazardous solvents (260 μL) and low volume of sample (10 mL) with suitable figures of merit and acceptable percentage relative recoveries. In addition, the proposed method was performed with uncomplicated equipment and lower solvent volume in a shorter time along with a powerful multiobjective optimization algorithm. Thus, the introduced ST-DLLME method can be recommended as an inexpensive, highly accurate, and fast method for simultaneous analysis of Cu2+ and Cd2+ ions in various laboratories.
References
Arslan O, Karadaş C, Kara DJJoAI (2016) Simultaneous Preconcentration of copper and cadmium by dispersive liquid–liquid microextraction using N, N′-Bis (2-Hydroxy-5-Bromo-Benzyl) 1, 2 diaminopropane and their determination by flame atomic absorption spectrometry 99:1356–1362.
Chen, H., Chen, R., & Li, S. (2010). Low-density extraction solvent-based solvent terminated dispersive liquid–liquid microextraction combined with gas chromatography-tandem mass spectrometry for the determination of carbamate pesticides in water samples. Journal of Chromatography A, 1217(8), 1244–1248.
Chen, C.M., Chen, Y.p., Shen, T.C., & Zao, J.K. (2010) Optimizing degree distributions in LT codes by using the multiobjective evolutionary algorithm based on decomposition. In 2010 IEEE Congress on Evolutionary Computation (CEC), (pp. 1–8). Barcelona: IEEE.
Deb, K., & Jain, H. (2014). An evolutionary many-objective optimization algorithm using reference-point-based nondominated sorting approach, part I: Solving problems with box constraints. IEEE Transactions on Evolutionary Computation, 18(4), 577–601.
Deb, K., Pratap, A., Agarwal, S., & Meyarivan, T. (2002). A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Transactions on Evolutionary Computation, 6(2), 182–197.
Farajvand, M., Kiarostami, V., Davallo, M., & Ghaedi, A. (2018). Optimization of Solvent Terminated Dispersive Liquid–Liquid Microextraction of Copper Ions in Water and Food Samples Using Artificial Neural Networks Coupled Bees Algorithm. Bulletin of Environmental Contamination and Toxicology, 100(3), 402–408.
Furini, A. (2012). Plants and heavy metals: Springer Science & Business Media.
Karadaş, C., & Kara, D. (2013). On-line preconcentration and determination of trace elements in waters and reference cereal materials by flow injection–FAAS using newly synthesized 8-hydroxy-2-quinoline carboxaldehyde functionalized Amberlite XAD-4. Journal of Food Composition and Analysis, 32(1), 90–98.
Karadaş, C., & Kara, D. (2014). Determination of copper (II) in natural waters by extraction using No-vanillidine-2-amino-p-cresol and flame atomic absorption spectrometry. Instrumentation Science & Technology, 42(5), 548–561.
Khiat M, Pacheco-Fernández I, Pino V, Benabdallah T, Ayala JH, Afonso AMJAM (2018) A guanidinium ionic liquid-based surfactant as an adequate solvent to separate and preconcentrate cadmium and copper in water using in situ dispersive liquid–liquid microextraction 10:1529–1537.
Kiarostami, V., Rouini, M.-R., Mohammadian, R., Lavasani, H., & Ghazaghi, M. (2014). Binary solvents dispersive liquid—liquid microextraction (BS-DLLME) method for determination of tramadol in urine using high-performance liquid chromatography. DARU Journal of Pharmaceutical Sciences, 22(1), 25.
Maham, M., Karami-Osboo, R., Kiarostami, V., & Waqif-Husain, S. (2013a). Novel Binary Solvents-Dispersive Liquid—Liquid Microextraction (BS-DLLME) method for determination of patulin in apple juice using high-performance liquid chromatography. Food Analytical Methods, 6(3), 761–766.
Maham, M., Kiarostami, V., Waqif-Husain, S., Abroomand-Azar, P., Tehrani, M. S., Sharifabadi, M. K., et al. (2013b). Extraction and determination of cyproheptadine in human urine by DLLME-HPLC method. Iranian journal of pharmaceutical research: IJPR, 12(2), 311–318.
Maham, M., Kiarostami, V., WAQIF-HUSAIN, S., Karami-Osboo, R., & Mirabolfathy, M. (2013c). Analysis of ochratoxin A in malt beverage samples using dispersive liquid-liquid microextraction coupled with liquid chromatography-fluorescence detection. Czech Journal of Food Sciences, 31, 520–525.
Maham, M., Kiarostami, V., Waqif-Husain, S., & Sharifabadi, M. K. (2014). Analysis of chlorpheniramine in human urine samples using dispersive liquid-liquid microextraction combined with high-performance liquid chromatography. Brazilian Journal of Pharmaceutical Sciences, 50(3), 551–557.
Mansour, F. R., & Danielson, N. D. (2018). Solvent-terminated dispersive liquid-liquid microextraction; a tutorial. Analytica Chimica Acta, 1016, 1–11.
Mohammadi, S., Hamidian, H., Karimzadeh, L., & Moeinadini, Z. (2016). Tween 80 coated alumina: An alternative support for solid phase extraction of copper, nickel, cobalt and cadmium prior to flame atomic absorption spectrometric determination. Arabian Journal of Chemistry, 9, S1290–S1296.
Moradi, Z., Kiarostami, V., & Amini, M. (2017). Rapid Analysis of Styrene in Drinking Water and Tea Samples Using Dispersive Liquid-Liquid Microextraction Combined with Liquid Chromatography-Ultraviolet Detection. Food Analytical Methods, 10(1), 41–48.
Neri, F., & Tirronen, V. (2010). Recent advances in differential evolution: a survey and experimental analysis. Artificial Intelligence Review, 33(1–2), 61–106.
Pourreza, N., Rastegarzadeh, S., & Larki, A. (2014). Simultaneous preconcentration of Cd (II), Cu (II) and Pb (II) on Nano-TiO2 modified with 2-mercaptobenzothiazole prior to flame atomic absorption spectrometric determination. Journal of Industrial and Engineering Chemistry, 20(5), 2680–2686.
Rezaee, M., Assadi, Y., Hosseini, M.R.M., Aghaee, E., Ahmadi, F., & Berijani, S. (2006). Determination of organic compounds in water using dispersive liquid–liquid microextraction. Journal of Chromatography A, 1116(1–2), 1–9.
Shirkhanloo, H., Rouhollahi, A., & Mousavi, H. Z. (2010). Preconcentration and Determination of Trace Amount of Nickel in Water and Biological Samples by Dispersive Liquid-Liquid Microextraction. Journal of the Chinese Chemical Society, 57(5A), 1035–1041.
Silva, E. L., dos Santos Roldan, P., & Giné, M. F. (2009). Simultaneous preconcentration of copper, zinc, cadmium, and nickel in water samples by cloud point extraction using 4-(2-pyridylazo)-resorcinol and their determination by inductively coupled plasma optic emission spectrometry. Journal of Hazardous Materials, 171(1–3), 1133–1138.
Yalçınkaya, Ö., Kalfa, O. M., & Türker, A. R. (2011). Chelating agent free-solid phase extraction (CAF-SPE) of Co (II), Cu (II) and Cd (II) by new nano hybrid material (ZrO2/B2O3). Journal of Hazardous Materials, 195, 332–339.
Ying, S., Li, L., Wang, Z., Li, W., & Wang, W. (2017). An improved decomposition-based multiobjective evolutionary algorithm with a better balance of convergence and diversity. Applied Soft Computing, 57, 627–641.
Zhang, Q., & Li, H. (2007). MOEA/D: A multiobjective evolutionary algorithm based on decomposition. IEEE Transactions on Evolutionary Computation, 11(6), 712–731.
Zhou, A., Qu, B.Y., Li, H., Zhao, S.Z., Suganthan, P. N., & Zhang, Q. (2011). Multiobjective evolutionary algorithms: A survey of the state of the art. Swarm and Evolutionary Computation, 1(1), 32–49.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farajvand, M., Kiarostami, V., Davallo, M. et al. Simultaneous extraction of Cu2+ and Cd2+ ions in water, wastewater, and food samples using solvent-terminated dispersive liquid–liquid microextraction: optimization by multiobjective evolutionary algorithm based on decomposition. Environ Monit Assess 191, 287 (2019). https://doi.org/10.1007/s10661-019-7383-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7383-6