Abstract
In this project, a simple, low-cost and rapid procedure based on dispersive liquid–liquid microextraction (DLLME) technique coupled with high performance liquid chromatography-ultraviolet detector (HPLC-UV) has been used for the extraction and determination of styrene in aqueous solutions. Several factors, such as type of extraction and dispersive solvents and their volumes, salt addition, and pH were optimized. Under optimal conditions, the recoveries of styrene for tea and water samples spiked with 10 and 15 ng mL−1 were in the range of 91.4–97.8 %, whereas the temperature was set at 0, 4, 20, 70 and 91 °C for 15, 30, 60, 1440, and 14,400 min. The linear range was obtained in the interval of 1.86–50 ng mL−1. The limits of detection (S/N = 3) and quantitation (S/N = 10) were 0.6 and 1.86 ng mL−1, respectively. The relative standard deviations (RSDs) for three replicated analysis of styrene in aqueous samples ranged from 0.01 to 0.3 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics are defined as processable material based on a wide range of organic polymer which can be reshaped while soft by heating and then set into rigid or slightly elastic form and have many applications including packaging of foods, pharmaceutics, etc. Polystyrene (PS) occupies the fourth place behind polyethylene (PE), polypropylene (PP), and polyvinylchloride (PVC) on the bulk polymer with an annual production of over 12 × 106 tons. Products formed from PS are hard and transparent, with high brilliance and resistance to many chemicals. Its disadvantages are its brittleness, sensitivity to stress cracking, and migration of its additives and monomers into food contact with polymer (Piringer and Baner 2008). Styrene, a monomer used in the manufacture of numerous types of plastics such as polystyrene and acrylenenitrile–butadiene–styrene, can be in direct contact with food when polymers such as polystyrene are used as packaging materials (Arab-Tehrany and Gonzalez 2015; Silva et al. 2000). The migration of undesirable substances such as additive and styrene as a residual unreactive monomer which may be present in the polystyrene matrix can cause various problems for food quality. Evaluation of potential health risks of exposure to styrene indicating that it can adversely affect humans in some ways raises public health and safety (Cohen et al. 2002). Long-term exposure to small amounts of styrene can cause neurotoxic (fatigue, nervousness, sleeplessness), hematological (low platelet and hemoglobin values), cytogenetic (chromosomal and lymphatic abnormalities), and carcinogenic effects (Dowty et al. 1976). The international agency for research on cancer (IRAC) in 1978 reclassified styrene from a non-carcinogenic group to possibly carcinogenic to humans (ASTDR, 1992). Sensitive and reliable analytical methods are therefore necessary for determination of styrene residues in water, milk, juice, and other food products which may be in contact with polystyrene. The most widely used methods for the determination of styrene in the polymer matrix and foodstuff include gas chromatography (Gennari et al. 2012; Lau et al. 1995; Paraskevopoulou et al. 2012) and high performance liquid chromatography (Gawell and Larsson 1980; Khaksar and Ghazi-Khansari 2009). Various preconcentration and pre-treatment techniques have been studied before final analysis. Liquid–Liquid extraction (Carrillo-Carrión et al. 2007; Garrigós et al. 2004) has been widely used for extraction of styrene and gave satisfactory efficiency but can be replaced by greener and low-cost method. Solid-phase extraction (Szűcs et al. 2002)and solid-phase microextraction (Chiesa et al. 2010; Kusch and Knupp 2002; Verzera et al. 2010)have been used to the preconcentration of styrene in food stuff and drinking water. Rezaee and coworkers (Rezaee et al. 2006) have reported a new technique in 2006 based on liquid-phase microextraction which was named dispersive liquid–liquid microextraction (DLLME) for determination of polycyclic aromatic hydrocarbons in water samples. The technique is based on the formation of tiny droplets of the extraction solvent (water-immiscible organic solvent) in the sample solution using dispersive solvent (water-miscible organic solvent). This microextraction technique has been applied for analysis of different organic (Farajzadeh et al. 2009; Pena et al. 2009; Xiong et al. 2009) and inorganic (Moghadam et al. 2011; Taher et al. 2014) compounds in various materials. In our previous works (Kiarostami et al. 2014; Maham et al. 2013a; Maham et al. 2013b; Maham et al. 2013c; Maham et al. 2014), DLLME has been developed for preconcentration of some organic compounds in several samples. In this work, the technique was applied as a low-cost, rapid, and safe extraction method for extraction of styrene in drinking water and tea which was in direct contact with polystyrene cups in several temperatures using high performance liquid chromatography with UV–Vis detection.
Material and Methods
Reagent and Materials
Standard of styrene with 99.7 % purity was purchased from Merck (Darmstadt, Germany). Methanol and acetonitrile with HPLC grade and all other chemicals such as acetone, dichloromethane, chloroform, carbon tetrachloride, and dichloroethane used in this study were of analytical grade from Merck (Darmstadt, Germany). Food-grade, rigid, and open polystyrene cups (100 mL), which are commonly used for drinking of water and tea, and Cylon black tea (Golestan Co., Iran) were purchased from a local market. A stock standard solution of styrene was prepared in methanol with a concentration level of 1000 µg mL−1 and stored at 4 °C in a refrigerator. Working standard solutions were prepared daily by appropriate dilution of the stock solution.
Instrumentation
A high-performance liquid chromatography system (Agilent 1200) equipped with a rotary injection valve with 20 μl loop, quaternary HPLC pump (G1311 A), degasser (G1322 A) and absorbance UV–Vis detector (G1314B) were used. Azorbax eclips XDB C18 column (150 × 4.6 mm, 5 μm, Agilent) was used for separation at ambient temperature. A Metrohm digital pH meter model 827 was employed for pH measurements. For sedimentation of tiny droplets of the extractant, a Hettich centrifuge model Universal 320R was used.
DLLME Procedure
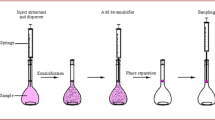
An aliquot of 5 mL of an aqueous solution containing 10 ng mL−1 of styrene was placed in a 10-mL screw cap glass tube test tube with conical bottom. 0.5 mL of acetone (as a dispersive solvent) and 70 μl of chloroform (as an extraction solvent) were mixed and injected rapidly into the aqueous sample. After injection, the cloudy solution was formed in the test tube and styrene was extracted to tiny droplets of extraction solvent. The solution was then centrifuged for 3 min at 8000 rpm and the extraction solvent sedimented in the bottom of the tube. The sedimented phase was completely transferred to another test tube with conical bottom using 100 μl HPLC syringe (Hamilton) and desolvated with mild stream of nitrogen gas. Afterwards, the residue was dissolved in mobile phase solvent system (acetonitrile–water, 75/25) and 20 μl was injected into the HPLC system.
Chromatographic Separation
An isocratic mode was performed for mobile phase binary solvent system made of acetonitrile–water (75/25, v/v) for chromatographic separation at flow rate of 1 ml min−1 on Agilent C18 column. UV–Vis detection was performed at a wavelength of 245 nm.
Results and Discussion
Optimization of DLLME Procedure
In order to obtain high extraction efficiency of styrene in aqueous solutions, several factors that influence the efficiency of DLLME such as the type and volume of extraction solvent, type and volume of dispersive solvent, salt addition, and pH were evaluated and optimized. Analysis was carried out on water samples spiked with styrene at a concentration of 10 ng mL−1.
Selection of Extraction Solvent
The type of extraction solvent is one of the most important factors affecting the extraction efficiency with conical test tube and should be heavier than water and immiscible in it. Several chlorinated solvents including carbon tetrachloride (CCl4), dichloromethane (CH2Cl2), 1, 2-dichloroethane (C2H4Cl2), and chloroform (CHCl3) with higher density than water, low solubility in water, and various polarities were chosen as extraction solvents. The experiments were performed by using 70 μl of each extraction solvent and 500 μl of methanol as a dispersive solvent. No droplet was formed by dichloromethane. As shown in Fig.1, the maximal value of extraction efficiency and minimal error bar was obtained by chloroform for styrene. Therefore, chloroform was chosen as a solvent extraction for subsequent experiments.
Selection of Dispersive Solvent
High solubility of a dispersive solvent in the organic and aqueous phases is the important factor for the selection of a dispersive solvent. Hence, acetone, methanol, isopropyl alcohol, and acetonitrile were tested. A series of sample solutions were studied using 70 μl chloroform (as an extraction solvent) and 500 μl of each dispersive solvent. As shown in Fig. 2, acetone as a dispersive solvent offered the best extraction efficiency. Acetone also has the lowest price and toxicity than other dispersive solvents.
Effect of Extraction Solvent Volume
Variation of extraction and dispersive solvent volumes can change the extraction efficiency because of variation in volume of the sedimented phase. To study the effect of the extraction solvent volume, different volumes of chloroform (as an extraction solvent) in the range of 50–200 μl were mixed with 500 μl acetone (as a dispersive solvent). As indicated in Fig.3, with increasing the extraction solvent volume to 100 μl, the extraction efficiency increased, then decreased by increasing the solvent volume since the sedimented droplet volume also decreased. Thus, 100 μl of extraction solvent volume was selected as an optimal value in the subsequent experiments.
Effect of Dispersive Solvent Volume
For obtaining optimal volume of acetone, different experiments were performed by using various volumes of dispersive solvent in the range of 300–1000 μl. Figure 4 shows that by increasing the volume of dispersive solvent from 300 to 750 μl the extraction efficiency of styrene increased, then decreased by increasing of its volume from 750 to 1000 μl. According to the results, a volume of 750 μl was chosen as the optimal volume value for the dispersive solvent.
Effect of Salt Addition
The effect of ionic strength on the extraction efficiency is important since it can be effected on the mass transfer of target analytes. The results (Fig. 5) show that by addition of salt concentration from 0 to 5 %, the extraction efficiency of styrene decreased due to increasing the ionic strength and decreasing the mass transfer of styrene with high hydrophobicity to the extraction solvent. Thus, the all subsequent experiments performed without adding salt.
Effect of Sample pH
The influence of pH was studied by addition of strong acid and base in the sample solution. Various DLLME experiments were performed by different pH in the range of 3 to13. As indicated in Fig. 6, by increasing of pH from 3 to 7, the extraction efficiency increased, but with increasing pH from 7 to 13, the efficiency decreased. The reason for this variation may be related to increase of ionic strength in the acidic and basic solutions due to increase of hydronium or hydroxide ion concentrations. Therefore, pH 7 was selected as the optimal pH in the subsequent experiments.
Figures of Merit
The analytical figures of merit of the DLLME method including the calibration curve, limits of detection (S/N = 3) and quantitation (S/N = 10) and repeatability were studied under the optimized condition (Table 1.). Quantitative analysis was carried out by the external standard method. External standard calibration curve was prepared for the styrene after the extraction of a standard series of spiked water samples in the range of 5–50 ng mL−1. Repeatability was expressed as relative standard deviation (RSD) and calculated on three replicate experiments at a concentration of 0.01–0.3 %.
Validity of the Method
To assess the applicability and evaluation of accuracy of the proposed method, water and tea samples which were in direct contact with rigid polystyrene cups were investigated and analyzed in different temperatures and storage times by proposed DLLME method with UV–Vis detection. All samples were spiked with 10 and 15 ng mL−1 of styrene. The mean recoveries of styrene from water and tea samples at spiking levels of 10 and 15 ng mL−1 were in the range of 91.8–97.8 % (Table 2.). Thus, the proposed method can be applied for the determination of the styrene in the aqueous solutions with good accuracy. For analyzing the samples at 0 and 4 °C, first the samples were placed in a special laboratory refrigerator at pre-set temperature. After a specific period of time, the samples were taken out and allowed to reach the ambient temperature. Then the DLLME method was performed. Keeping the samples at higher temperatures (20, 70 and 91 °C) for various periods of time were carried out using benmary bath with an adjustable temperature system. The rigid polystyrene cups were covered with parafilm and after a period of time, samples were then taken out and let to reach the ambient temperature, and after which, the DLLME procedure was carried out. The experiments were replicated three times for the mentioned low and high temperatures. The results for initial concentrations due to migration of styrene from the rigid polystyrene cup to liquid has shown some interesting facts. After maintaining the drinking water (as frozen liquid) at 0 °C for a period of 10 days (14,400 min), the concentration of styrene reached to 4.3 ng mL−1, whereas no styrene was detected for the water sample at 4 °C after a period of 60 min and tea sample at 20 °C after period of 15 min, and also, low concentrations of styrene were detected for drinking water and tea samples at various temperature higher than 0 °C. This can be due to maintaining the frozen drinking water at 0 °C after a long period of time(14,400 min or 10 days) and also a phase change to liquid in ambient temperature which caused internal stress in the rigid polystyrene cups, consequently accelerated the migration of styrene. The initial concentrations for the drinking water samples in comparison to tea samples indicated that the lower migration occurred in tea samples which is due to the presence of some constituents which can act as attenuator for the styrene migration. Representative chromatograms with good resolution obtained for styrene from a blank water sample and spiked water sample (10 ng mL−1) which were extracted by DLLME method under the optimal conditions (Fig.7.).
Typical HPLC after employing DLLLME chromatograms of (a) blank water sample and (b) water sample spiked with styrene at concentration level of 10 ng mL−1 after performing DLLME under optimum conditions (Solvent extraction and its volume, 100 μl chloroform; dispersive solvent and its volume, 750 μl acetone; pH value, 7, no salt addition)
Comparison of DLLME with Other Methods
The important figures of merits of the proposed DLLME method for analysis of styrene in drinking water and tea samples have been compared with earlier reported methods. As shown in Table 3, the proposed method has the lowest LOQs and LODs, good linear range, and acceptable relative recoveries in comparison with other methods. Since solvents are generally hazardous materials, the use of lesser solvent in any experimental work is much safer in comparison to the larger amounts in other experiments. In over study, only a small drop (100 μL) of the extraction solvent and a small volume of the sample (10 mL) was used; therefore, it was considered as the safe method to be applied. In addition, this experiment was carried out with a cheap and simple apparatus and with a small amount of expensive solvent in a very short period of time; consequently, it can be accounted as a fast and low-cost method and does not involve any labor-intensive and time-consuming steps.
Conclusions
The present study has proposed a simple, low-cost and rapid method with easy operation for determination of styrene in drinking water and tea samples followed by HPLC with UV–Vis detection as a low-cost detector which is available for most research laboratories. Compared with the previously published methods, the proposed DLLME method shows adequately low limits of detection and quantitation, good repeatability, and low consumption of solvent and sample volumes. Therefore, the presented method can be considered as a laboratory routine method for analysis of styrene in aqueous samples.
References
Arab-Tehrany E, Gonzalez LS (2015) Transfer phenomena in food/packaging system. In: Functional polymers in food science. John Wiley & Sons, Inc., pp. 67–94. doi:10.1002/9781119109785.ch3
Carrillo-Carrión C, Lucena R, Cárdenas S, Valcárcel M (2007) Liquid–liquid extraction/headspace/gas chromatographic/mass spectrometric determination of benzene, toluene, ethylbenzene, (o-, m- and p-)xylene and styrene in olive oil using surfactant-coated carbon nanotubes as extractant. J Chromatogr A 1171:1–7. doi:10.1016/j.chroma.2007.09.039
Cecair TEFPK (2012) A study on the migration of styrene from polystyrene cups to drinks using online solid-phase extraction liquid chromatography (SPE-LC). Malaysian J Anal Sci 16:49–55
Chiesa L, Panseri S, Soncin S, Vallone L, Dragoni I (2010) Determination of styrene content in gorgonzola PDO cheese by headspace solid phase micro-extraction (HS-SPME) and gas-chromatography mass-spectrometry (GC-MS). Vet Res Commun 34:167–170
Cohen JT et al. (2002) A comprehensive evaluation of the potential health risks associated with occupational and environmental exposure to styrene. J Toxicol Environ Health Part B, Crit Rev 5:1–265. doi:10.1080/10937400252972162
Dowty BJ, Laseter JL, Storer J (1976) The transplacental migration and accumulation in blood of volatile organic constituents. Pediatr Res 10:696–701. doi:10.1203/00006450-197607000-00013
Farajzadeh MA, Seyedi SE, Shalamzari MS, Bamorowat M (2009) Dispersive liquid-liquid microextraction using extraction solvent lighter than water. J Sep Sci 32:3191–3200. doi:10.1002/jssc.200900109
Flanjak J, Sharrad J (1984) Quantitative analysis of styrene monomer in foods. A limited East Australian survey. J Sci Food Agric 35:457–462. doi:10.1002/jsfa.2740350416
Garrigós MC, Marín ML, Cantó A, Sánchez A (2004) Determination of residual styrene monomer in polystyrene granules by gas chromatography–mass spectrometry. J Chromatogr A 1061:211–216. doi:10.1016/j.chroma.2004.10.102
Gawell B-M, Larsson B (1980) Determination of styrene in foods by reversed-phase high-performance liquid chromatography. J Chromatogr A 198:198–202. doi:10.1016/S0021-9673(00)80110-7
Gennari O, Albrizio S, Monteiro M (2012) A GC–FID method to determine styrene in polystyrene glasses. Food Anal Methods 5:1411–1418. doi:10.1007/s12161-012-9395-5
Hansson E, Hakkarainen M (2006) Multiple headspace single-drop microextraction—a new technique for quantitative determination of styrene in polystyrene. J Chromatogr A 1102:91–95. doi:10.1016/j.chroma.2005.10.060
Khaksar M-R, Ghazi-Khansari M (2009) Determination of migration monomer styrene from GPPS (general purpose polystyrene) and HIPS (high impact polystyrene) cups to hot drinks. Toxicol Mech Methods 19:257–261. doi:10.1080/15376510802510299
Kiarostami V, Rouini M-R, Mohammadian R, Lavasani H, Ghazaghi M (2014) Binary solvents dispersive liquid–liquid microextraction (BS-DLLME) method for determination of tramadol in urine using high-performance liquid chromatography DARU. J Pharm Sci 22:25–25. doi:10.1186/2008-2231-22-25
Kusch P, Knupp G (2002) Analysis of residual styrene monomer and other volatile organic compounds in expanded polystyrene by headspace solid-phase microextraction followed by gas chromatography and gas chromatography/mass spectrometry. J Sep Sci 25:539
Lau O-W, Lung M-T, Mok CS (1995) Distillation-extraction of styrene migrating from polystyrene containers to foods by gas chromatography. Int J Food Sci Technol 30:397–404. doi:10.1111/j.1365-2621.1995.tb01387.x
Maham M, Karami-Osboo R, Kiarostami V, Waqif-Husain S (2013a) Novel binary solvents-dispersive liquid–liquid microextraction (BS-DLLME) method for determination of Patulin in apple juice using high-performance liquid chromatography. Food Anal Methods 6:761–766. doi:10.1007/s12161-012-9483-6
Maham M et al. (2013b) Extraction and determination of cyproheptadine in human urine by DLLME-HPLC method. Iranian J Pharm Res: IJPR 12:311–318
Maham M, Kiarostami V, WAQIF-HUSAIN S, Karami-Osboo R, Mirabolfathy M (2013c) Analysis of ochratoxin a in malt beverage samples using dispersive liquid-liquid microextraction coupled with liquid chromatography-fluorescence detection. Czech J Food Sci 31:520–525
Maham M, Kiarostami V, Waqif-Husain S, Sharifabadi MK (2014) Analysis of chlorpheniramine in human urine samples using dispersive liquid-liquid microextraction combined with high-performance liquid chromatography. Brazilian J Pharm Sci 50:551–557
Moghadam MR, Shabani AMH, Dadfarnia S (2011) Spectrophotometric determination of iron species using a combination of artificial neural networks and dispersive liquid–liquid microextraction based on solidification of floating organic drop. J Hazard Mater 197:176–182. doi:10.1016/j.jhazmat.2011.09.073
Paraskevopoulou D, Achilias DS, Paraskevopoulou A (2012) Migration of styrene from plastic packaging based on polystyrene into food simulants. Polym Int 61:141–148. doi:10.1002/pi.3161
Pena MT, Casais MC, Mejuto MC, Cela R (2009) Development of an ionic liquid based dispersive liquid–liquid microextraction method for the analysis of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A 1216:6356–6364. doi:10.1016/j.chroma.2009.07.032
Piringer OG, Baner AL (2008) Plastic packaging materials for food: barrier function, mass transport, quality assurance, and legislation. Wiley,
Rezaee M, Assadi Y, Hosseini M-RM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Silva FC, de Carvalho CR, de L Cardeal Z (2000) Solid-phase microextraction method for the quantitative analysis of styrene in water. J Chromatogr Sci 38:315–318
Szűcs S, Tóth L, Legoza J, Sárváry A, Ádány R (2002) Simultaneous determination of styrene, toluene, and xylene metabolites in urine by gas chromatography/mass spectrometry. Arch Toxicol 76:560–569. doi:10.1007/s00204-002-0384-0
Taher MA, Daliri Z, Fazelirad H (2014) Simultaneous extraction and preconcentration of copper, silver and palladium with modified alumina and their determination by electrothermal atomic absorption spectrometry. Chin Chem Lett 25:649–654. doi:10.1016/j.cclet.2013.12.025
Verzera A, Condurso C, Romeo V, Tripodi G, Ziino M (2010) Solid-phase microextraction coupled to fast gas chromatography for the determination of migrants from polystyrene-packaging materials into yoghurt. Food Anal Methods 3:80–84
Xiong C, Ruan J, Cai Y, Tang Y (2009) Extraction and determination of some psychotropic drugs in urine samples using dispersive liquid-liquid microextraction followed by high-performance liquid chromatography. J Pharm Biomed Anal 49:572–578. doi:10.1016/j.jpba.2008.11.036
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Zeinab Moradi declares that she has no conflict of interest. Vahid kiarostami declares that he has no conflict of interest. Mohsen Amini declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Moradi, Z., Kiarostami, V. & Amini, M. Rapid Analysis of Styrene in Drinking Water and Tea Samples Using Dispersive Liquid-Liquid Microextraction Combined with Liquid Chromatography-Ultraviolet Detection. Food Anal. Methods 10, 41–48 (2017). https://doi.org/10.1007/s12161-016-0547-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0547-x