Abstract
Urbanization, agriculture, and other land transformations can affect water quality, decrease species biodiversity, and increase metal and nutrient concentrations in aquatic systems. Metal pollution, in particular, is a reported consequence of elevated anthropogenic inputs, especially from urbanized areas. The objectives of this study were to quantify metal (Cu, Al, Cd, Ni, and Pb) concentrations in the waters and biota of four streams in South Georgia, USA, and relate metal concentrations to land use and abiotic and biotic stream processes. Additionally, macrophytes, invertebrates, and fish were identified to assess biodiversity at each site. Metal concentrations in the three trophic levels differed among sites and species, correlating to differences in land use surrounding the rivers. The highest metal concentrations (except Al) were found in the streams most impacted by urbanization and development. Al concentrations were highest in streams surrounded by land dominated by forested areas. Metal content in macrophytes reflected metal concentrations in the water and was at least three orders of magnitude higher than any other trophic level. Despite metal concentration differences, all four streams contained similar water quality and were healthy based on macroinvertebrate community structure. This study provides insight into the impact of urbanization and the fate and effects of metals in river ecosystems with varying degrees of anthropogenic impact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human alterations to landscapes can substantially influence stream and river chemistry and the input of contaminants (Correll 1983; Peierls et al. 1991; Correll et al. 1992; Jordan et al. 1997a, b; Pyati et al. 2012; Gong et al., 2013). Water quality changes in streams and rivers may persist for decades, thus altering habitat and affecting biotic communities (Behnke 1990; Vinson and Hawkins, 1998). Metal pollution, in particular, is problematic in many aquatic environments, especially in densely populated areas (Klein 1979).
Metals may be suspended in the water column for various time periods, depending on a variety of abiotic and biotic factors. In the water column, metals can form inorganic complexes, reversibly bind to organic and particulate matter, and be passed through the food chain (Di Toro, et al. 2001). Eventually, metals partition in the sediment over time; however, metals may be remobilized into the interstitial water by both physical and chemical disturbances. Many metals (e.g., aluminum, iron) occur naturally in sediment, and, as sediment composition changes with local geography and environment, so do the concentrations of these elements in water bodies. Anthropogenic contributions of metals disrupt the natural cycles of these elements, which can result in the unidirectional movement of dissolved metals into aquatic systems (Eisler 1988a, 1988b, 1996; Klee and Graedel 2004).
Anthropogenic land-based sources of metal contamination include industrial discharges into streams or rivers, agricultural runoff, domestic stormwater runoff, and sewage treatment discharge (Pratt et al. 1981; Guzman and Jimenez 1992; Gonzalez et al. 1999; Echols et al. 2009). Naturally occurring trace metals such as copper and nickel are essential micronutrients required by all organisms; however, in excess, these metals, as well as nonessential metals, such as cadmium and lead, may accumulate in organisms and cause adverse biological effects (Bielmyer et al. 2005; Bielmyer et al. 2006; Bury et al. 2003; Rainbow and Dallinger 1993).
Most waterborne metals exert toxicity by binding to and inhibiting enzymes on the gills or gill-like structures of aquatic organisms (Bielmyer et al. 2006; Bury et al. 2003). This leads to a disruption in ion and water balance in the organism and possibly death, depending on the metal concentration and exposure time. Ingestion of metal-contaminated diets can also result in intestinal metal accumulation and potential toxicity to the consumer (Bielmyer et al. 2005; Bielmyer and Grosell 2011). Decreased respiration, decreased reproductive capacity, kidney failure, neurological effects, bone fragility, mutagenesis (genetic mutation), and other effects have been observed in aquatic biota after metal exposure. Several water quality parameters can modify the toxicity of metals including dissolved oxygen (DO), dissolved organic carbon concentration, sulfide and chloride concentrations, pH, water hardness, and alkalinity, as well as other variables (Campbell 1995). Metal toxicity may therefore vary in different water bodies, reflecting the changes in water chemistry (Campbell 1995) as well as the organisms that reside there.
Agriculture and urban land usage can impact the metal concentrations found in aquatic environments. Excess metals may accumulate in biota to varying degrees and potentially cause devastating effects in aquatic ecosystems, such as reduced species diversity and modified community composition (Mangun 1989; Grubaugh and Wallace 1995; Lenat and Crawford 1994; Roth et al. 1996; Roy et al. 2003; Dallinger et al. 1987; Cardwell et al. 2002; Vardanyan and Ingole 2006; Mortimer 1985). Therefore, the objectives of this study were to (1) quantify metal concentrations in the waters and biota of four streams with varying order and levels of anthropogenic disturbance and (2) relate metal concentrations at multiple trophic levels (water-abiotic, macrophytes, macroinvertebrates, small fish, large fish) to land use differences and governing abiotic and biotic processes.
Methods
Field sites
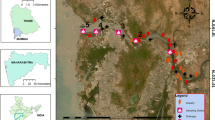
The population of Lowndes County, Georgia, is 114,552 spread throughout 496.07 mile2 (U.S. Census Bureau: State and County QuickFacts, 2012). In this project, four streams in Lowndes County, each with varying levels of anthropogenic impact, were studied including One Mile Branch, OMB; Sugar Creek, SC; Withlacoochee River, WIT; and Little River, LR.
OMB and SC are both predominantly surrounded by urban landscapes and have experienced substantial human alterations. OMB is a first-order stream and is susceptible to metal inputs from impervious surfaces and other urban inputs. Over 50 % of the land use surrounding OMB is classified as medium density residential, followed by 22 % as light industrial, commercial, and institutional, 18 % as heavy industrial and roadways, and only 8 % as forest, open, and park (City of Valdosta, 2010). OMB is surrounded with high levels of vegetation and organic debris. This river runs through the main parking area of Valdosta State University, and the sampling site is located at 30° 50′ 35.9″ N, 83° 17′ 52.7″ W. A beaver dam was also noted 100 ft upstream from sampling site. While the dam was not a focus of our study, it did reduce flows and water levels for part of our study. OMB and some other streams confluence into SC. SC is a second-order stream that is surrounded by impervious surfaces as well as urban landscape. Approximately 37 % of the total land use surrounding SC is classified as medium density residential, 24 % as light industrial, commercial, and institutional, 17 % as forest, open, and park, and 16 % as heavy industrial and roadways (City of Valdosta 2010). The SC sampling site is located near a restaurant and parking lot with coordinates of 30° 51′ 40.6″ N, 83° 19′ 05.2″ W. This stream runs through residential areas as well as shopping centers in downtown Valdosta, GA. It has a large amount of foliage surrounding the stream and many large stones positioned on the banks as a form of bank restoration.
LR, a third order stream, is surrounded mainly by forest (40 %) interspersed with cropland (36 %) and pasture (18 %), with only slight urban (6 %) influence, as determined by the United States Department of Agriculture. SC and LR both merge into WIT (a fourth-order stream) at separate locations. LR, sampled at 30° 51′ 08.5″ N, 83° 20′ 49.2″ W, and WIT, sampled at 30° 42′ 43.0″ N, 83° 27′ 20.6″ W, are both surrounded by dense vegetation and trees line the banks. WIT is predominantly surrounded by wetlands (36 %), agriculture (27 %), and upland forests (14 %) and only to a small degree by urban and built-up (13 %) and rangeland (6 %) (Southwest Florida Water Management District 2009). All four streams are considered black water and limestone base streams. LR and WIT both contain a large number of limestone boulders as part of the bedload in both streams. For the purpose of this study, OMB and SC were considered metal-impacted sites and LR and WIT were considered reference sites (Fig. 1).
Field sampling
During two large sampling periods (October 2013 and December 2013), fish were collected from all four stream sites using a dip net to capture small fish and a seine net to capture larger fish. Multiple habitats were sampled so that each stream habitat was represented. Fish were transferred to the Valdosta State University laboratory and then euthanized using tricaine methanesulfonate (MS-222, Argent Chemical Laboratories, Redmond, WA). During the same sampling periods, macrophytes were collected by hand from OMB and SC only, because macrophyte presence was low in LR and WIT. Given the clumped and sometimes sparse distribution of the macrophyte communities in our systems, macrophyte collection did not follow a standardized technique. Collections gathered each species represented in the system forming a representative sample. Bryophytes were collected from boulders of limestone located throughout each stream basin. Given the karst topography, boulders greater than 20 cm are numerous and common in these systems providing some of the primary habitat. It should be noted that macrophytes are a minor component to each system with the exception of OMB. However, in the areas where they did exist, they were able to alter flows and cause sedimentation. Macrophytes were refrigerated until they were processed (see below).
During these larger seasonal samplings, the pH, DO, temperature, and chloride were measured using a YSI Professional Plus meter with various probes (YSI®, Yellow Springs, OH, USA), and 4–8 L of water was collected via subsurface grab in 1 L polypropylene cubitainers at three different sites in the downstream to upstream direction of each stream. Additionally, in the other months through March 2014, the pH, DO, temperature, and chloride were measured and 2–4 L of water was collected in polyethylene cubitainers at one site at each stream (because of heavy rainfall) for further lab analysis.
Macroinvertebrate samples were collected from the OMB, SC, WIT, and LR using insect kick nets and by hand over a 200-m reach in the fall of 2013. Macroinvertebrates were collected until 100 individuals were captured.
Laboratory analyses
All fish were identified using Hoyer and Canfield (1994), and macrophytes were identified using aquatic plant identification manuals (University of Florida Center for Aquatic and Invasive Plants). Biological diversity was quantified by calculating diversity indices using plant, invertebrate, and fish data (Beck’s Index, Shannon-Weiner, Simpson’s Index of Diversity). After euthanasia, small fish, ranging in size from ∼1.5 to 7.0 cm total length, were immediately dried in an oven at 65 °C for 24 h, weighed, acidified with trace metal grade nitric acid (Fisher Scientific, Pittsburg, PA), and heated in a water bath to 60 °C until complete digestion. Large fish, ranging in size from ∼9.0 to 15.0 cm total length, were immediately dissected and the following organs removed: gills, heart, intestine, liver, gallbladder, and spleen. Each organ of the larger fish as well as all macrophyte (stem, leaves, roots) samples were dried and digested as described above. All digested tissue samples were diluted with 18 mΩ Milli-Q® water and then analyzed for Cu, Al, Cd, Ni, and Pb using atomic absorption spectrophotometry (AAS; PerkinElmer AAnalyst 800) with flame or graphite detection, as appropriate (detection limits ∼1 ppb). These metals are frequently used in modern society and are thus commonly found in areas with increased anthropogenic disturbance. Certified 1 g/mL metal standards dissolved in 2 % HCl (Fisher Chemical, Fairlawn, NJ, USA) were used for each metal, and samples were analyzed in triplicate. The blank and standards were used for re-calibration every 40 samples, and they were also analyzed as samples periodically throughout each cycle. Three replicates of a certified reference material (LUTS-1, lobster hepatopancreas) were treated as the samples to determine average extraction efficiencies. This method of digestion and metal analysis has been used in several other studies in our laboratory and has been effective (Shyn et al. 2012; Jarvis et al. 2013).
Water collected from each sampling site was filtered through pre-burned Whatman Glass Fiber Filters (0.7 μm) within an hour of returning to the laboratory using a vacuum-suction technique, and the filtrate was stored in polypropylene plastic bottles and refrigerated at 3 °C. Filtered water was measured for alkalinity, hardness, nitrite, phosphate, and carbon dioxide via a LaMotte® Freshwater Colorimetric kit. Ammonia and nitrate levels were measured using a Red Sea Marine and Freshwater Test Kit®. Two subsamples (15 mL) of the filtered water from each site at each stream were additionally filtered (0.45 μm Nylon, Fisher Scientific, Pittsburg, PA), acidified, and then analyzed for Cu, Al, Cd, Ni, and Pb using AAS with graphite furnace detection.
Bioconcentration factors (BCFs) were calculated for each metal for each species of macrophyte at the two collection sites (OMB and SC). BCFs are expressed as the ratio of milligrams of metal in the macrophyte to the milligrams of metal per liter of water (environmental metal concentration; Hemond 2000). Higher BCF values are indicative of lower metal exposure levels (McGeer et al. 2003).
Macroinvertebrate samples were taken to the laboratory at Valdosta State University and immediately preserved in ethyl alcohol solution. All invertebrates collected were identified down to genus or to species if possible. Invertebrate data was used to determine stream health using the Beck’s Biotic Index for stream health (Terrell and Perfetti 1996).
Statistical analysis
The waterborne metal data were analyzed for normality and equality of variance using a Shapiro-Wilk’s test and a Bartlett’s test, respectively. Statistical differences between sites and over time were calculated using an ANOVA followed by a post hoc Tukey’s test with Sigma-Plot 11.0. Differences over time (October and December) were not observed; therefore, the data for the metal concentrations in each the macrophytes, smaller fish, and larger fish were combined for the two larger sampling periods.
Results
Water quality parameters are presented in Table 1. The pH (ranging from 5.72 to 7.05), alkalinity (ranging from 18 to 38 mg CaCO3/L), and hardness (ranging from 22 to 58 mg CaCO3/L) values were relatively low, and CO2 (ranging from 3.9 to 11 mg/L) and DO (ranging from 5.1 to 11.7 mg/L) values were relatively high for freshwaters (Table 1). No substantial differences in these parameters were observed between sites; however, temporal changes were evident and correlated with an increase in rainfall (Fig. 2). Temperature varied by season with the coldest values observed in January. Alkalinity, CO2, and DO values were highest in the winter months, which also related to an increase in rainfall (Table 1 and Fig. 2). The lowest pH values were measured in the spring when a peak in rainfall was observed (Table 1 and Fig. 2). Valdosta had an unseasonably wet period in December and January in particular (Fig. 2). Among the four streams, DO values were lowest in OMB, with the exception of April. Nitrogen concentrations, as ammonia, nitrate, and nitrite, as well as chloride concentrations were generally low at all sites (Table 1).
Metals were detected in every stream sampled; waterborne Al concentrations were the highest, followed by Cu, Pb, Ni, and Cd (Fig. 3). Significant differences in waterborne metal concentrations were observed among streams, with the highest Cu concentrations in the two metal-impacted streams and Al concentrations highest in the two reference streams (Fig. 3). In general, concentrations of all metals except Al were most elevated in OMB, whereas Al was highest in WIT and SC. A spike in Cu, Ni, Cd, and Pb concentration was observed in WIT in November (Fig. 3).
Concentration (mean ± standard error; n = 6 in October and December; n = 4 in November, January, and February) of a copper (Cu), b aluminum (Al), c nickel (Ni), d cadmium (Cd), and e lead (Pb) in water samples from four streams: Sugar Creek, One Mile Branch, Little River, and Withlacoochee in 2013–2014. Different letters indicate significant differences in metal concentration between the streams in a given month (p ≤ 0.05)
Macrophyte metal concentrations (Table 2) largely reflected the metal concentrations in the water and were up to several orders of magnitude higher than any other trophic level for some metals. Macrophytes were only found at two of the sites, OMB and SC, with far fewer species observed at SC as compared to OMB (Table 2). Significant differences in metal accumulation in macrophytes between sites were not determined because only one species (Micranthemum sp.) was found at both sites, and the sample sizes (n = 2–4) were too small of each species.
For the two sites that macrophytes were found, Al concentrations in the macrophytes were an order of magnitude higher than any other metal measured. Cu, Ni, and Pb accumulated to a similar extent, and Cd accumulated to the lowest degree in the macrophytes (Table 2). Bryophytes accumulated the highest metal concentrations in SC, whereas Potamogeton sp., Eleocharis sp., and Micranthemum sp. generally accumulated the highest metal concentrations in OMB (Table 2). Bioconcentration factors were calculated for each metal and are presented in Table 3. Considerable variation was observed among both macrophytes and metals. Additionally, for the species (Micranthemum sp.) that was found at both sites, BCF values substantially differed, and differences were not consistent among metals (Table 3).
Metals were also observed in the tissues of higher trophic levels. Metal concentrations in the smaller fish were most elevated in OMB, with the exception of Pb in Little River (Fig. 4). Of all the metals, tissue Al concentrations were the highest in the whole body of smaller fish, similar to that observed in the water and macrophytes (Fig. 4). Of the other metals, Cu was also particularly elevated in the whole body of smaller fish. Gambusia holbrooki followed by Heterandria formosa generally had the highest metal body burdens and were found in all four streams (Fig. 4).
Concentration (mean ± standard error) of a copper (Cu), b aluminum (Al), c nickel (Ni), d cadmium (Cd), and e lead (Pb) in small fish (1.5–7.0 cm) collected from four streams: Sugar Creek (SC), One Mile Branch (OMB), Little River (LR), and Withlacoochee (WIT). The fish collected include Gambusia holbrooki (eastern mosquitofish), Labidesthes sicculus (brook silverside), Percina nigrofasciata (blackbanded darter), Noturus gyrinus (tadpole madtom), Lepomis macrochirus (bluegill), Lepomis punctatus (spotted sunfish), Heterandria formosa (least killifish), Notropis maculatus (taillight shiner), and Pomoxis nigromaculatus (black crappie)
Metals were also detected in the organs of the larger fish and varied depending on the metal and fish species (Fig. 5). Tissue metal concentrations were highest in the heart and liver of the spotted sunfish, the liver of the warmouth, the liver and gallbladder of the black crappie, and the heart and liver of the redbreast sunfish (Fig. 5). Tissue metal concentrations varied depending on the metal, in the redear sunfish (Fig. 5). For each metal, tissue metal distribution differed with fish species (Fig. 5). The redbreast sunfish, which was found in LR, had the highest tissue metal concentrations (with the exception of Al). The redear sunfish, found in SC, had the highest tissue Al concentrations and had comparable tissue Ni concentrations as those observed in the redbreast sunfish (Fig. 5).
Concentration (mean ± standard error) of a copper (Cu), b aluminum (Al), c nickel (Ni), d cadmium (Cd), and e lead (Pb) in the organs of large fish (9.0 to 15.0 cm) collected from two streams: One Mile Branch (OMB) and Sugar Creek (SC). The fish collected include Lepomis punctatus (spotted sunfish), Lepomis gulosus (warmouth), Lepomis microlophus (redear sunfish), Pomoxis nigromaculatus (black crappie), and Lepomis auritus (redbreast sunfish). n = 2–7 for each species
No significant changes in species biodiversity were observed among the four sites using Shannon-Weiner’s Index or Simpson’s Index of Diversity (Table 3). All four streams were represented as stream clean or stream clean in a monotypic habitat according to Beck’s Index Score (Table 4). Macroinvertebrate samples from SC and LR were all group one taxa for the Beck’s Index indicating good water quality (Table 5). The majority of macroinvertebrate samples collected from OMB and WIT were group 1 taxa with a few macroinvertebrate samples representing group 2 taxa indicating fair water quality.
Discussion
Metal concentrations found in the four streams sampled are comparable to those reported in other blackwater rivers (Pyati et al. 2012). In addition, a survey study in the nearby Alapahoochee River found low metal (Cu, Zn, Pb) concentrations near detection limits with the exception of sampling times following rain events (Barnett et al. 2007). However, the Alapahoochee River does not contain a direct urban influence in contrast to our study sites. OMB and SC are most impacted by urbanization and development so it follows that the metals associated with anthropogenic sources would be most elevated in these streams. OMB is also more shallow and was likely influenced by rainfall more than the other streams. The spike in Cu, Ni, Cd, and Pb concentration observed in WIT in November was likely due to excessive rainfall and thus increased inputs of nonpoint source pollution, a trend supported by other studies in the area (Barnett et al. 2007). The land use surrounding WIT and LR is dominated by forested and wetland areas mixed with agriculture (Southwest Florida Water Management District 2009). Al is a major component of clay, which is abundant in those areas. High amounts of Al have been shown to be naturally deposited in the sediments of nearby Long Pond, Lake Park, GA prior to human impacts throughout the past 4000 years (Earley 2015). Acidic rain can liberate Al from soils, and atmospheric deposition is believed to be the main cause for the acidification of freshwaters (Schuurkes et al. 1986; Van Breemen and Van Dijk 1988). Al depletion in forests can cause radical changes in soil genesis and may also lead to future reduction in acid neutralization (Mulder et al. 1989). The water chemistry observed in the streams also changed in response to the increased rainfall. The low alkalinity, hardness, and pH values in these streams could have facilitated increased bioavailability and uptake of the metals into the biota.
Macrophytes, invertebrates, and fish have been shown to accumulate metals to varying degrees and be susceptible to metal toxicity (Dallinger et al. 1987; Cardwell et al. 2002; Vardanyan and Ingole 2006; Mortimer 1985). In this study, macrophytes were only found at the two metal-impacted sites (OMB and SC), but there presence was not assumed to be a response to metal inputs. The magnitude of metal accumulation in the macrophytes was largely species dependent and similar to other reported studies (Förstner and Wittmann 1983; Cardwell et al. 2002; Kumar et al. 2008; Sanches Filho et al. 2015). Mortimer (1985) reported a higher bioconcentration of Pb than Ni or Cu in freshwater macrophytes. Venkatesha et al. (2013) reported accumulation of metals in the order of Cu > Pb > Ni > Cd. Potamogeton spp. have been shown to accumulate 242–380 μg/g dw Zn, 45–50 μg/g dw Cu, 5.8–7.5 μg/g dw Pb, and 1.2–2.2 μg/g dw Cd (Förstner and Wittmann 1983). Similar to that observed in the present study, Cardwell et al. (2002) reported a Cd concentration in Myriophyllum aquaticum of 6.5 μg/g dw.
Macrophytes have been used as biomonitors of metal pollution in freshwater environments for quite some time (Mortimer 1985; Phillips and Rainbow 1993). Additionally macrophytes are known to be more tolerant to metals than are aquatic animals (Tyler et al. 1989). Bryophytes contained some of the highest tissue metal concentrations. They have been reported in other studies to be a particularly useful bioindicator of metal pollution (Whitehead and Brooks 1969; Phillips and Rainbow 1993). The BCFs for macrophytes in this study (Table 2) were several times greater than those reported by Ndeda and Manohar (2014). However, other researchers have reported much higher (several orders of magnitude greater) BCF values (McGeer et al. 2003; Donnachie et al. 2014; Jarvis and Bielmyer-Fraser 2015), similar to those in the present study (Table 2). Even though macrophytes are generally more tolerant of metals, toxic effects have been reported. Elevated Cu has been shown to reduce photosynthesis and respiration rates in aquatic macrophytes (Vazquez et al. 2000). Jarvis and Bielmyer-Fraser (2015) reported phytotoxicity in Ulva lactuca after exposure to 100 μg/L of Cu or Cd or after exposure to a 100-μg/L mixture of Cd, Cu, Pb, Ni, and Zn.
Tissue metal concentrations in small fish were generally highest in the most urbanized (metal-impacted) site (OMB). The Cu and Ni concentrations in the small fish were similar to those reported in laboratory toxicity studies (Bielmyer et al. 2005; Bielmyer et al., unpublished). Also, the small fish species H. formosa and G. holbrooki contained some of the most elevated tissue metal concentrations, and G. holbrooki was found at every site. It is possible that G. holbrooki is a more metal-tolerant species.
The metal distribution pattern in the organs of large fish was similar to those reported in other studies (Bielmyer et al. 2005; Vinodhini and Narayanan 2008) and those we have observed in our laboratory using the fish, Fundulus heteroclitus (Bielmyer et al. 2005, 2006; Bielmyer et al., unpublished). In freshwater, the primary binding sites for most waterborne metals are on the gills (Chowdhury et al. 2003; Bielmyer et al. 2005, 2006). The liver detoxifies excess metals from both the gill and intestine of teleost fish, and metals have also been reported to accumulate in this organ (Blanchard and Grosell 2005; Bielmyer et al. 2005, 2006). Bile is secreted by the liver and stored in the gall bladder, so metal may also pass into this organ, which is consitstent with our findings. Because different larger fish species were found in each of the streams, it is difficult to compare tissue metal concentrations across sites. The redbreast sunfish, which was found in LR, had the highest tissue metal concentrations; however, this could have been a consequence of the unique physiology of the fish rather than due to site differences. Another possibility of the higher tissue metal concentrations could be an unknown source of contamination.
Metals were observed in the water and three trophic levels of the streams studied; however, concentrations differed among sites, relating to differences in land use surrounding the rivers. Despite these differences, no significant changes in species biodiversity were observed among the four sites using either index (Table 3). These rivers were also found to be healthy using Beck’s Index (Table 4). Macrophytes found and collected from the streams most impacted by human disturbance (OMB and SC) appeared to substantially accumulate and potentially remove metals from the water, as metal concentrations were most elevated in this trophic level. We did not, however, observe metal biomagnification up the food chain in this study. The observed metal removal (uptake into macrophytes) could have helped minimize effects on overall species biodiversity, thus protecting the aquatic communities in these systems. It should be noted that the unseasonable wet period could have confounded the impacts of the land use surrounding the sampling sites, and more research needs to be done in drier years to assess these endpoints. Additionally, downstream impacts of metals are not acknowledged in this paper. Lastly, the metal concentrations measured in the water column of these streams were near or below hardness-based U.S. EPA ambient water quality criterion values (USEPA 1984, 1986, 1987, 2007, 2016), yet metal accumulation was still observed in the biota. Standard stream health assessment measures, such as macroinvertebrate indices, would fail to detect biotic metal accumulation, as the presence of macroinvertebrates showed all streams to be healthy.
References
Barnett, J., Bechler, D.L., Denizman, C., Grable, J., Nienow, J., Turco, J., Tietjen, W., & Wood, G. L. (2007). Watershed restoration action strategy development in the Alapahoochee River watershed. Nonpoint Source Management Program, Section 319 Report. Environmental Protection Division, Department of Natural Resources, Georgia. 92 pp.
Behnke, A. C. (1990). A perspective on America’s vanishing streams. Journal of the North American Benthological Society, 9, 77–88.
Bielmyer, G. K., Tomasso, J., Gatlin, D., Isely, J., & Klaine, S. J. (2005). Responses of hybrid striped bass to waterborne and dietary copper in freshwater and saltwater. Comparative Biochemistry and Physiology-Part C, 140, 131–137.
Bielmyer, G. K., Tomasso, J., & Klaine, S. J. (2006). Physiological responses of hybrid striped bass to aqueous copper in freshwater and saltwater. Archives of Environmental Contamination and Toxicology, 50, 531–538.
Bielmyer, G. K., & Grosell, M. (2011). Emerging issues in marine metal toxicity. In Bury, N., & Handy, R., (Eds.), Essential Reviews in Experimental Biology vol. 2, (pp. 129–158). London: Kings College.
Blanchard, J., & Grosell, M. (2005). Effects of salinity on copperaccumulation in the common killifish (Fundulus heteroclitus). Enviromental Toxicology and Chemistry, 24, 1403–1413.
Bury N. R., Walker, P. A., & Glover, C. N. (2003). Nutritive Metal Uptake in Teleost Fish. Journal of Experimental Biology, 206, 11–23.
Campbell, P. G. C. (1995). Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In Tessier, A. & Turner, D. R. Metal Speciation and Bioavailability in Aquatic Systems.New York: John Willy & Sons.
Cardwell, A. J., Hawker, D. W., & Greenway, M. (2002). Metal accumulation in aquatic macrophytes from southeast Queensland, Australia. Chemosphere, 48, 653–663.
Chowdhury, M. J., Grosell, M., McDonald, D. G., & Wood, C. M. (2003). Plasma clearance of cadmium and zinc in non-acclimated and metal-acclimated trout. Aquatic Toxicology, 64, 259–275.
City of Valdosta (2010). Master stormwater management plan. Valdosta, GA.
Correll, D. L. (1983). N and P in soils and runoff of three coastal plain land uses. Nutrient cycling in agricultural ecosystems (pp. 207–224). Athens, Georgia: University of Georgia Press.
Correll, D. L., Jordan, T. E., & Weller, D. E. (1992). Nutrient flux in a landscape: effects of coastal land use and terrestrial community mosaic on nutrient transport to coastal waters. Estuaries, 15, 431–442.
Dallinger, R., Prosi, F., Segner, H., & Back, H. (1987). Contaminated food and uptake of heavy metals by fish: a review and a proposal for further research. Oecologia, 73, 91–98.
Di Toro, D. M., Allen, H. E., Bergman, H. L., Meyer, J. S., Paquin, P. R., & Santore, R. C. (2001). Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environmental Toxicology and Chemistry, 20(10), 2383–2396.
Donnachie, R. L., Johnson, A. C., Moeckel, C., Pereira, M. G., & Sumpter, J. P. (2014). Using risk-ranking of metals to identify which poses the greatest threat to freshwater organisms in the UK. Environmental Pollution, 194, 17–23.
Earley, S. M. (2015). The paleolimnology of Long Pond. GA, USA: Linking biogeochemical processes to historic primary producer communities p. 76.
Echols, K. R., Meadows, J. C., & Orazio, C. E. (2009). Pollution of aquatic ecosystems II: hydrocarbons, synthetic organics, radionuclides, heavy metals, acids, and thermal pollution. In G. E. Likens (Ed.), Encyclopedia of inland waters (pp. 120–128). Cambridge: Elsevier/Academic Press.
Eisler, R. (1988a). Nickel hazards to fish, wildlife, and invertebrates: a synoptic review. Laurel (MD): U.S. Fish and Wildlife Service (USFWS), Patuxent Wildlife Research Center 34. 95.
Eisler, R. (1988b). Lead hazards to fish, wildlife, and invertebrates: a synoptic review. Laurel (MD): U.S. Fish and Wildlife Service (USFWS), Patuxent Wildlife Research Center 14. 94.
Eisler, R. (1996). Silver hazards to fish, wildlife, and invertebrates: a synoptic review. Laurel (MD): U.S. Fish and Wildlife Service (USFWS), Patuxent Wildlife Research Center 32. 63.
Förstner, U., & Wittmann, G. T. W. (1983). Metal pollution in the aquatic environment. Berlin: Springer-Verlag 486.
Gong, D. C., Gao, X., Ntakirutimana, T., Guo, J. S., & Li, K. (2013). Water quality status along the Liangtan River and control planning alternatives for pollution reduction. Polish Journal of Environmental Studies, 22, 1061–1067.
Gonzalez, H., Pomares, M., Ramirez, M., & Torres, I. (1999). Heavy metals in organism and sediments from the discharge zone of the submarine sewage outfall of Havanna city, Cuba. Marine Pollution Bulletin, 38, 1048–1051.
Grubaugh, J. W., & Wallace, J. B. (1995). Functional structure and production of the benthic community in a Piedmont river: 1956-1957 and 1991-1992. Limnology and Oceanography, 40, 490–501.
Guzman, H. M., & Jimenez, C. E. (1992). Contamination of coral reefs by heavy metals along the Caribbean coast of Central America (Costa Rica and Panama). Marine Pollution Bulletin, 24, 554–561.
Hemond, H. (2000). Chemical fate and transport in the environment. Elsevier Science Publishing Company Inc., 156–157.
Hoyer, M. V., & Canfield, Jr., D. E. (1994). Handbook of common freshwater fish in Florida lakes. University of Florida, Institute of Food and Agricultural Sciences.
Jarvis, T. A., & Bielmyer-Fraser, G. K. (2015). Accumulation and effects of metals in two seaweed species. Comparative Biochemistry and Physiology-Part C, 171, 28–33.
Jarvis, T. A., Lockhart, J. M., Loughry, W. J., & Bielmyer, G. K. (2013). Metal accumulation in wild nine-banded armadillos. Ecotoxicology, 22, 1053–1062.
Jordan, T. E., Correll, D. L., & Weller, D. E. (1997a). Nonpoint source discharges of nutrients from Piedmont watersheds of Chesapeake Bay. Journal of the American Water Resource Association, 33, 631–646.
Jordan, T. E., Correll, D. L., & Weller, D. E. (1997b). Relating nutrient discharges from watersheds to land use and streamflow variability. Water Quality Research, 33, 2579–2590.
Klee, R. J., & Graedel, T. E. (2004). Elemental cycles: a status report on human or natural dominance. Annual Review of Environment and Resources, 29, 69–107.
Klein, R. D. (1979). Urbanization and stream quality impairment. Water Resource Bulletin, 15, 948–963.
Kumar, J. I. N., Soni, H., Kumar, R. N., & Bhatt, I. (2008). Macrophyte in phytoremediation of heavy metal contaminated water and sediments in Pariyej community reserve, Gujarat, India. Turkish Journal of Fisheries and Aquatic Sciences, 8, 193–200.
Lenat, D. R., & Crawford, J. K. (1994). Effects of landuse on water-quality and aquatic biota of three North Carolina Piedmont streams. Hydrobiologia, 294, 185–199.
Mangun, W. R. (1989). A comparison of five Northern Virginia (USA) watersheds in contrasting land use patterns. Journal of Environmental Systems, 18, 133–151.
McGeer, J. C., Brix, K. V., Skeaff, J. M., DeForest, D. K., Brigham, S. I., Adams, W. J., & Green, A. (2003). Inverse relationship between bioconcentration factor and exposure concentration for metals: implications for hazard assessment of metals in the aquatic environment. Environmental Toxicology and Chemistry, 22, 1017–1037.
Mortimer, D. C. (1985). Freshwater aquatic macrophytes as heavy metal monitors—the Ottawa River experience. Environmental Monitoring and Assessment, 5, 311–323.
Mulder, J., van Breemen, N., & Eijck, H. C. (1989). Depletion of soil aluminum by acid deposition and implications for acid neutralization. Nature, 337, 247–249.
Ndeda, L. A., & Manohar, S. (2014). Bio concentration factor and translocation ability of heavy metals within different habitats of hydrophytes in Nairobi Dam, Kenya. IOSR-Journal of Environmental Science, Toxicology and Food Technology, 8, 42–45.
Peierls, B. L., Caraco, N. F., Pace, M. L., & Cole, J. J. (1991). Human influence on river nitrogen. Nature, 350, 386–387.
Phillips, D. J. H., & Rainbow, P. S. (1993). The biomonitoring of trace metals and radionuclides. Biomonitoring of trace aquatic contaminants (pp. 189–192). Oxford: Elsevier Science.
Pratt, J. M., Coler, R. A., & Godfrey, P. J. (1981). Ecological effects of urban stormwater runoff on benthic invertebrates inhabiting the Green River, Massachusetts. Hydrobiologia, 83, 29–42.
Pyati, R., Bielmyer, G. K., Chalk, S., McCarthy, D., McCarthy, H., Pinto, G., Sonnenberg, L., & Welsh, P. (2012). “Case study: St. Johns River Basin, USA,” fourth United Nations world water development report, world water assessment programme 2012. Paris: UNESCO Publishing.
Rainbow P. S., & Dallinger R. (1993). Metal uptake, regulation, and excretion in freshwater invertebrates. In Dallinger, R., & Rainbow, P.S., (Eds.), Ecotoxicology of Metals in Invertebrates (pp. 119–131). Boca Raton: Lewis Publishers.
Roth, N. E., Allan, J. D., & Erickson, D. L. (1996). Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landscape Ecology, 11, 141–156.
Roy, A. H., Rosemond, A. D., Paul, M. J., Leigh, D. S., & Wallace, J. B. (2003). Stream macroinvertebrate response to catchment urbanisation (Georgia, USA). Freshwater Biology, 48, 329–346.
Sanches Filho, P. J., Nunes, L. V., Rosa, D. A., Betemps, G. R., & Pereira, R. S. (2015). Comparison among native floating aquatic macrophytes for bioconcentration of heavy metals. Ecotoxicology and Environmental Contamination, 10, 1–6.
Schuurkes, J. A. A. R., Heck, C. C., Hesen, P. L. G. M., Leuven, R. S. E. W., & Roelofs, J. G. M. (1986). Effects of sulphuric acid and acidifying ammonium desposition on water quality and vegetation of simulated soft water ecosystems. Water, Air, & Soil Pollution, 31, 267–272.
Southwest Florida Water Management District (2009). Southwest Florida Water Management District Land Use and Cover. Brooksville, FL.
Shyn, A., Chalk, S. J., Smith, K., Charnock, N., & Bielmyer, G. K. (2012). Zinc Distribution in the Organs of Adult Fundulus heteroclitus After Waterborne Zinc Exposure in Freshwater and Saltwater. Archives of Environmental Contamination and Toxicology 63, 544–553.
State and County QuickFacts, 2012. http://www.census.gov/quickfacts/table/PST045215/13185. January\15\2016
Terrell, C. R. & Perfetti, P. B. (1996). Water quality indicators guide. Surface waters. Terrene Institute. Washington, D.C. second edition.
Tyler, G., Balsberg Pahlsson, A. M., Bengtsson, G., Baath, E., & Tranvik, L. (1989). Heavy-metal ecology of terrestrial plants, microorganisms and invertebrates. Water, Air, & Soil Pollution, 47, 189–215.
USEPA (1984). Ambient water quality criteria for lead. U.S. Environmental Protection Agency. Washington, DC: Office of Water Regulations and Standards.
USEPA (1986). Ambient water quality criteria for nickel. U.S. Environmental Protection Agency. Washington, DC: Office of Water Regulations and Standards.
USEPA (1987). Ambient aquatic life water quality criteria for silver. EPA-440/5-87-011.
USEPA. (2007). Aquatic life ambient freshwater quality criteria copper—2007 revision. EPA-822-R-07-001.
USEPA. (2016). Aquatic life ambient water quality criteria cadmium—2016. EPA-820-R-16-002.
Van Breemen, N., & van Dijk, H. F. G. (1988). Ecosystem effects of atmospheric deposition of nitrogen in The Netherlands. Environmental Pollution, 54, 249–274.
Vardanyan, L. G., & Ingole, B. S. (2006). Studies on heavy metal accumulation in aquatic macrophytes from Sevan (Armenia) and Carambolim (India) lake systems. Environment International, 32, 208–218.
Vazquez, M. D., Fernandez, J. A., Lopez, J., & Carballeira, A. (2000). Effects of water acidity and metals concentration on accumulation and within-plant distribution of metals in the aquatic bryophyte Fontinalis antipyretica. Water, Air, & Soil Pollution, 120, 1–19.
Venkatesha, R., Somashekar, R. K., & Prakash, K. L. (2013). Biomonitoring of metals in freshwatermacrophytes and benthic organisms. International Journal of Innovative Research and Science, Engineering and Technology, 2, 4661–4669.
Vinodhini, R., & Narayanan, M. (2008). Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (common carp). International journal of Environmental Science and Technology, 5, 179–182.
Vinson, M. R., & Hawkins, C. P. (1998). Biodiversity of stream insects: variation at local, basin and regional scales. Annual Review of Entomology, 43, 271–293.
Whitehead, N. E., & Brooks, R. R. (1969). Aquatic bryophytes as indicators of uranium mineralization. Bryologist, 72, 501–507.
Acknowledgements
Funding of this project was provided by a Valdosta State University Quality Enhancement Program grant and the Biology Department. The authors would also like to thank Mathew Cannister from the U.S. Geological Survey for making the map in Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bielmyer-Fraser, G.K., Waters, M.N., Duckworth, C.G. et al. Assessment of metal contamination in the biota of four rivers experiencing varying degrees of human impact. Environ Monit Assess 189, 23 (2017). https://doi.org/10.1007/s10661-016-5738-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5738-9