Abstract
Copper (Cu) is an abundant trace metal, and although essential at low levels, it is also potentially toxic to aquatic organisms. Mechanisms of toxicity and consequences of exposure vary depending on ionoregulatory status (acclimated to freshwater or salt water). The goal of this research was to examine the responses of hybrid striped bass (Morone chrysops × Morone saxatilis) exposed to Cu in freshwater and 15 g/L salt water. In freshwater, a general dose- and time-dependent pattern of increasing Cu accumulation in gill tissue was evident in fish exposed to aqueous Cu (220 and 447 mg/L) for up to 96 hours. The 96-hour acute median lethal concentration for freshwater-acclimated hybrid striped bass exposed to Cu was 94 mg/L (confidence interval = 62 to 144 mg/L). Plasma osmolality and Na+ concentrations decreased in Cu-exposed fish. Freshwater-acclimated hybrid striped bass exposed to aqueous Cu (60 mg/L) for 3 weeks decreased in mass and accumulated Cu in gill, intestine, and liver. In salt water, no mortality occurred, and there were no statistical differences in growth, tissue Cu, or plasma ion concentrations in hybrid striped bass exposed to Cu compared with control fish. Freshwater-acclimated hybrid striped bass were very sensitive to Cu exposure and exhibited responses typical of commonly tested teleost fishes; however, the same sensitivity was not observed in salt water–acclimated fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Copper (Cu) is abundant in the environment and also potentially toxic to aquatic organisms (Moore and Ramamoorthy 1984). Cu bioavailability is a function of water quality, and differences in toxicity exist across the continuum from freshwater to salt water (Lauren and McDonald 1986; Wilson and Taylor 1992; Lin and Dunson 1993; Welsh et al. 1993; Hall and Anderson 1995). It is critical to characterize the bioavailability and toxicity of Cu in both marine and freshwater systems to establish feasible water-quality criteria (Hall and Anderson 1995). Although attempts have been made to address these issues, current knowledge requires the synthesis of information from different species (Lewis and Lewis 1971; Stagg and Shuttleworth 1982; Wilson and Taylor 1992; Grosell et al. 2001). Results of Cu toxicity tests with marine species are often difficult to relate to results from freshwater organisms. For instance, the gill is the primary binding site for most waterborne metals in freshwater. Cu may inhibit Na+/K+ adenosine triphosphatase (ATPase) and carbonic anhydrase at the gill, which results in net ion loss (Stagg and Shuttleworth 1982; Lauren and McDonald 1985; 1986; 1987; Li et al. 1998; Wood 2001). However, in salt water, Cu binding at the fish gill may not be as significant as in freshwater because of competition with Na+ for the gill as well as complexation of Cu by inorganic ligands, resulting in decreased bioavailability of the Cu ion (Hall and Anderson 1995). In freshwater, ionoregulation requires active uptake of Na+, whereas in salt water, organisms continuously drink water to prevent dehydration, actively excreting Na+ (Wilson and Taylor 1992). Despite the decreased Cu bioavailablity in salt water, marine biota are known to accumulate Cu in the environment (Birge et al. 2000), and some researchers suggest that Cu exposure in salt water may also disturb osmoregulation, resulting in a net gain of Na+ (Stagg and Shuttelworth 1982; Grosell et al. 2004a). This physiological difference in osmoregulation makes it difficult to compare Cu toxicity results between freshwater and marine organisms. Considering the myriad fish species used, the range of water qualities tested, and the variety of exposure scenarios examined, it is not surprising that it has been difficult to draw conclusions that allow generalized models to be derived for the prediction of metal toxicity.
The hybrid striped bass is a cross between a female white bass (Morone chrysops) and a male striped bass (M. saxatillus), which is euryhaline, and, unlike its parents, resilient to handling stress and amenable to laboratory culture (Weirich et al. 1992). Striped bass on the Atlantic coast range from St. Lawrence River in Canada to St. Johns River in Florida (Harrell 1997), and its distribution generally includes near shore waters, bays, and coastal rivers (American Fisheries Society 1990). These fish migrate into tidal freshwater to spawn and then return to the coast (Harrell 1997). Since the 1960s, striped bass have become valued in sport fishing and for commercial culture (American Fisheries Society 1990), which has resulted in the extensive characterization of the nutritional requirements of the striped bass and its hybrids. The ability of the hybrid striped bass to rapidly acclimate and grow in either freshwater or salt water (Smith et al. 1986) makes it a good candidate for toxicity testing. The objective of this research was to characterize mortality, growth, Cu accumulation, and physiological responses of hybrid striped bass to Cu exposure in freshwater and salt water.
Materials and Methods
Animal Holding
Juvenile (2-month-old) hybrid striped bass were obtained from Southland Fish Hatchery (Hampton, SC). Fish were transported to the Aquatic Animal Research Laboratory at Clemson University and held in recirculating systems. Animals were fed diets similar to those used in the experiments (46% crude protein, 18% crude fat, and 4% crude fiber). Water quality in the holding system was maintained at an average temperature of 20°C (± 1.7 mean ± SD), 13 g/L (± 1.7) salinity, 7.0 mg/L (± 0.8) dissolved oxygen, pH 7.3 (± 0.92), 0.8 mg/L (± 0.95) total ammonia-N concentration, and 0.6 mg/L (± 0.89) nitrite-N concentration. Background Cu concentration in the holding system was 1.4 μg/L (± 0.65).
Test Solutions
Moderately hard water was used for the freshwater treatments and was prepared according to standard methods (United States Environmental Protection Agency [USEPA] 1989). Salt water was prepared by adding Instant Ocean (Aquarium Systems, Mentor, OH) to 18 MΩ deionized water to attain 15 g/L salinity (approximately 43% seawater). Hybrid striped bass have been shown to survive and grow well at this salinity (Smith et al. 1986). In addition, at 15 g/L, the osmolality of the water was higher than that of the fish blood, so the hybrid striped bass were functioning as saltwater fish (Weirich et al. 1992).
Reagent-grade Cu sulfate pentahydrate (Sigma Chemical) was used to make a stock solution of 100 mg Cu/L in Milli-Q (Millipore, Bedford, MA). Aliquots from this stock were diluted in freshwater or salt water in a 200-L carboy to the desired Cu concentration for each treatment and allowed to equilibrate at least 24 hours before testing. Water-chemistry samples were taken from both carboys and from each experimental tank. Samples for Cu analysis were acidified with concentrated nitric acid and preserved in 15-mL centrifuge tubes.
Toxicity Testing
The acute median lethal concentration (LC50) for Cu to the hybrid striped bass (average individual fish mass = 9.0 ± 2.7 g) was determined in freshwater. A 96-hour static toxicity test was conducted with six concentrations of Cu (nominal Cu concentrations of 0, 50, 100, 200, 400, and 800 μg/L), each consisting of 4 replicate tanks/treatment (1 fish/tank). A 96-hour static toxicity test was also conducted in salt water with five Cu concentrations (nominal Cu concentrations of 0, 10, 100, 1000, and 10,000 μg/L), each consisting of 2 replicate tanks/treatment (1 fish/tank).
A 48-hour static test was conducted to determine the sublethal physiologic effects of aqueous Cu to hybrid striped bass in moderately hard water. The 3 treatments of 0, 220, and 447 μg/L dissolved Cu, each with 6 replicate tanks, contained 6 fish/tank (average individual fish mass = 34.0 ± 18.2 g). One fish per tank was sampled for analysis at each sampling time (0, 6, 12, 24, and 48 hours). The fish were not fed throughout the experiment. A 48-hour static test with hybrid striped bass was also conducted in 15 g/L salt water. The two treatments of 0 and 400 μg/L dissolved Cu, each with 12 replicate tanks, contained 1 fish/tank (average individual fish mass = 58 ± 16.1 g). Four fish were sampled at the start of the experiment directly from the holding tank, and 4 fish from each treatment were sampled at 0, 8, 24, and 48 hours.
A 21-day static renewal toxicity test was conducted after the 48-hour experiments. All fish were weighed, measured, and then transferred to the testing chambers (initial fish mass = 13.0 ± 4.2 g). There were 4 treatments: freshwater control, saltwater control, freshwater Cu (53 μg/L dissolved Cu), and saltwater Cu (53 μg/L dissolved Cu), each with 6 replicate tanks (1 fish/tank). Solutions were renewed 2 to 3 times weekly. The fish were fed to satiation once a day, and a qualitative assessment of feeding behavior was noted. Mortality was monitored during the experiment, and all fish were weighed, measured, and sampled at the end. Fish were not fed for 24 hours before sampling.
Experimental Design
All experiments were conducted in aerated 37-L glass aquaria filled to 30 L. During the chronic experiments, one half of the water was replaced at each renewal (2 times/wk). All salt water–acclimated fish were transferred directly from the recirculating holding tank. For freshwater treatments, fish were transferred from the recirculating holding tank to a 112-L aquarium and acclimated to fresh water conditions for at least 24 hours before testing.
Water Chemistry
Water-quality parameters were measured at the beginning and end of the 48-hour experiments and three times weekly throughout the 21-day experiment (Table 1). Hardness and alkalinity were measured by titrametric methods according to American Public Health Association (APHA) (1989) guidelines, and salinity was measured using a refractometer. Dissolved oxygen and temperature were measured using a calibrated YSI model no. 50 dissolved oxygen and temperature meter (YSI, Yellow Springs, OH), and pH was measured using a pH meter (Jenco model no. 6072). Nitrogen was quantified using a spectrophotometer according to standard methods (APHA 1989). Total Cu concentration in each freshwater treatment was determined on a Perkin Elmer (Norwalk, CT) model A Analyst 800 Atomic Absorption Spectrophotometer (AAS) with graphite furnace (detection limit of 1 μg/L) for sample concentrations <30 μg/L. All other samples in freshwater were analyzed on the same instrument with flame mode. Deionized water (Milli-Q) blanks and Cu standards (0 to 40 μg/L for graphite furnace mode and 0 to 1000 μg/L for flame mode) were used throughout the analyses. Total Cu concentration in each saltwater treatment was measured by inductively coupled plasma (ICP) analysis with a Thermo ELemental Model 61E Analyzer (Thermo Elemental, Franklin, MA). The actual Cu concentrations averaged 93% ± 12% of the nominal concentrations (range 84% to 121%).
Sampling Procedure
Sampling took 3 to 5 minutes/fish. Fish were anesthetized with 0.5 mg/L MS222 (Argent Chemical, Redmond, WA), and blood was collected from the hemal arch by severing the caudal peduncle. Gill, liver, and intestine were collected and immediately frozen (−45°C). Plasma was separated from red blood cells by centrifugation (2000 × g), and hematocrit determined. Plasma osmolality was analyzed by vapor pressure osmometer (Wescor, Logan, UT). A plasma sample was diluted (25 ×) with 18 MΩ deionized water (Milli-Q) and then frozen until later use. Plasma Na+ was analyzed by AAS using flame emission. Other cations were measured by ICP analysis, and plasma Cl– was measured by ion chromatograph (IC) analysis with a Dionex IC Model DX-500 (Dionex, Atlanta, GA). Tissue samples were later weighed, digested by way of microwave-assisted acid digestion, and analyzed for Cu by an AAS using flame mode. Plasma glucose was measured spectrophotometrically at 340 nm using Infinity Glucose Reagent and standards (Thermo Electron, St. Louis, MO).
Data Analysis
Sublethal responses were analyzed using analysis of variance followed by a Dunnett’s test (p ≤ 0.05) across treatments at each sampling time (Dunnet 1955). The LC50 was calculated using trimmed Spearman-Karber analysis (Hamilton et al. 1977).
Cu toxicity values (96-hour LC50s) for 19 fish species were obtained from the literature (Welsh et al. 1993; Hansen et al. 2002) and the USEPA Aquatic Toxicity Information Retrieval (AQUIRE) aquatic toxicology database (2005) selected based on fish age (juvenile to adult) and water type (moderately hard to hard freshwater). The 19 96-hour LC50 values, along with our hybrid striped bass value, were analyzed for normality by the Shapiro-Wilk test, ranked, assigned percentiles, and the data plotted on a log Cu concentration versus probit graph to obtain species sensitivity distribution (Posthuma et al. 2002). Data was also plotted untransformed for comparison.
Whole-body condition factors (K) were calculated to determine the effect of chronic Cu exposure to each fish at the end of the test by the formula:
Statistical differences between initial and final condition factors were determined using Student t test (p ≤ 0.05).
Results
Toxicity Tests
The 96-hour LC50 value for hybrid striped bass exposed to waterborne Cu in freshwater was 94 μg Cu/L (confidence interval [CI] = 62 to 144 μg Cu/L). The species sensitivity diagram shows that hybrid striped bass along with rainbow trout (Oncorhynchus mykiss) and bull trout (Salvelinus confluentus) were the most sensitive species in this data set (Fig. 1). A 96-hour LC50 value was not calculated in salt water because mortality did not occur at any of the concentrations tested (i.e., >10,000 μg Cu/L). The highest Cu concentrations in this experiment exceeded the solubility of Cu (<1 mg/L) in 15 g/L salt water, indicating that Cu concentrations in the water were at a maximum.
Species sensitivity distribution for 20 juvenile to adult fish species exposed to Cu for 96 hours. Data presented (A) with and (B) without probit transformation. Open circles represent values for individual fish species, and the filled diamond represents the value for hybrid striped bass. The two lowest values are for rainbow trout and bull trout. CU = copper.
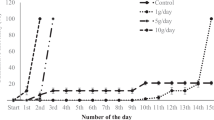
Throughout the 48-hour static test in freshwater, gill Cu concentrations increased in both a time- and concentration-dependent manner (Fig. 2), and by the end of the exposure, 50% mortality had occurred in the highest Cu-exposure group (447 μg/L). For all fish in the 48-hour saltwater experiment, the mean gill and intestinal Cu concentrations were 0.9 μg Cu/g and 2.5 μg Cu/g wet weight, respectively, and treatments did not differ statistically (data not shown). Intestinal Cu of freshwater-acclimated fish was not determined in the 48-hour experiment.
Gill Cu (mean ± SD) in hybrid striped bass exposed to aqueous Cu for 48 hours in freshwater. Data (n = 6) were analyzed by ANOVA followed by Dunnett’s test. Asterisks indicate statistical significant difference from control values at each time period (p < 0.05). ANOVA = analysis of variance; Cu = copper.
Plasma Na+ and osmolality decreased in hybrid striped bass exposed to Cu for 48 hours in freshwater (Fig. 4). The accumulation of Cu in gill (Fig. 2) corresponded to these decreases. Complete mortality occurred when osmolality decreased <240 mOsm (30% of initial values). In the 48-hour saltwater experiment, the mean values of plasma Na+ and osmolality in the Cu-exposed fish were 3,804 mg/L (165 mM) and 386 mOsm, respectively; however, treatment values were not statistically different from control values. Plasma Cl– concentrations in the Cu-exposed freshwater and saltwater fish were not statistically different from controls (data not shown). Mean Cl– values in the Cu-exposed freshwater and saltwater fish were 3,485 and 3,849 mg/L (98 and 108 mM), respectively.
Plasma Na+ (mean ± SD) in hybrid striped bass exposed to aqueous Cu for 48 hours in freshwater. Data (n = 6) were analyzed by ANOVA followed by Dunnett’s test. Asterisks indicate statistical significant difference from control values at each time period (p < 0.05). Mortality occurred at 2500 mg/L (109 mM) plasma Na+. ANOVA = analysis of variance; Cu = copper.
Plasma osmolality (mean ± SD) in hybrid striped bass exposed to aqueous Cu for 48 hours in freshwater. Data (n = 6) were analyzed by ANOVA followed by Dunnett’s test. Asterisks indicate significant differences from control values at each time period (p < 0.05). Mortality occurred at a plasma osmolality of 240 mOsm. ANOVA = analysis of variance; Cu = copper.
Plasma glucose and hematocrit were measured as additional stress responses in the 48-hour studies. Plasma glucose levels were not statistically different between treatments in either the freshwater or saltwater treatments with a mean value of 30 mg/dl (1.6 mM) for all fish (data not shown). Hematocrit increased (Fig. 5) in a time- and dose-dependent manner in freshwater but was not significantly different between saltwater treatments (data not shown).
Percent hematocrit (red blood cell volume/total serum) mean ± SD in hybrid striped bass exposed to aqueous Cu for 48 hours in freshwater. Data (n = 6) were analyzed by ANOVA followed by Dunnett’s test. Asterisks indicate statistical significant difference from control values at each time period (p < 0.05). Mortality occurred at a hematocrit of 49%. ANOVA = analysis of variance; Cu = copper.
In the 21-day experiment, growth was not significantly different between salt water–acclimated controls and salt water–acclimated Cu-exposed fish, and no mortality occurred (Fig. 6). A 30% decrease in survival was observed in the freshwater-acclimated, Cu-exposed fish compared with the controls. With respect to growth, control fish weight increased by 30%, whereas the Cu-exposed fish actually lost an average of 20% of their initial weight in freshwater. This corresponded to a statistically significant decrease from initial condition factor (K) values, reflecting that weight loss, relative to standard length, and can be interpreted as an index of toxicity (Table 2).
Percent weight gain (mean ± SD) in hybrid striped bass in either freshwater (FW) or saltwater (SW) under control conditions (CTL) or exposed to aqueous Cu (53 μg/L dissolved Cu) for 21 days. Data (n = 6) were analyzed by ANOVA followed by Dunnett’s test. Asterisks indicate statistical significant difference from respective control values (p < 0.05). Mortality occurred in 30% of the freshwater-acclimated fish exposed to Cu. ANOVA = analysis of variance; Cu = copper.
In addition to decreased growth, significant gill, liver, and intestinal Cu accumulation occurred in freshwater-acclimated hybrid striped bass exposed to 53 μg/L dissolved Cu compared with controls, but this did not occur in salt water–acclimated fish at 53 μg/L dissolved Cu (Fig. 7). Hematocrit increased as well in freshwater (Fig. 8); however, other physiological characteristics, such as water and ion balance, were not significantly different from controls in either freshwater or salt water.
Gill (A), intestine (B), and (C) liver Cu accumulation (mean ± SD) in hybrid striped bass in either freshwater (FW) or saltwater (SW) under control conditions (CTL) or exposed to aqueous Cu (53 μg/L dissolved Cu) for 21 days. Values are in given in μg/g wet weight. Data (n = 6) were analyzed by ANOVA followed by Dunnett’s test. Asterisks indicate statistical significant difference from respective control values (p < 0.05). Mortality occurred in 30% of the freshwater-acclimated fish exposed to Cu. ANOVA = analysis of variance; Cu = copper.
Plasma hematocrit (mean ± SD) in hybrid striped bass in either freshwater (FW) or saltwater (SW) under control conditions (CTL) or exposed to aqueous Cu (53 μg/L dissolved Cu) for 21 days. Data (n = 6) were analyzed by ANOVA followed by Dunnett’s test. Asterisks indicate statistical significant difference from respective control values (p < 0.05). Mortality occurred in 30% of the FW-acclimated fish exposed to Cu. ANOVA = analysis of variance; Cu = copper.
Discussion
The 96-hour LC50 value of 94 μg Cu/L (CI = 62 to 144 μg Cu/L) in freshwater was within the range of other 96-hour LC50 values reported in the literature for fishes commonly used in metal-toxicity testing (Welsh et al. 1993; Hansen et al. 2002). The gill has previously been identified as the primary binding site for most waterborne metals in freshwater (Lauren and McDonald 1985; 1986; Playle et al. 1992), resulting in a disturbance of branchial ionoregulation because of inhibition of Na+ and Cl– active transport and increased ionic permeability of the gills (Lauren and McDonald 1987; Li et al. 1998). The resulting plasma Na+ depletion has been observed in several studies characterizing acute Cu toxicity in freshwater (Stagg and Shuttleworth 1982; Lauren and McDonald 1985; Grosell et al. 1998), and a time- and dose-dependent depletion was observed in this freshwater study, and a 45% plasma Na+ loss caused mortality (Fig. 3). Previously published studies indicated that a 30% decrease in exchangeable Na+ pool led to rainbow trout mortality in 50% of the adult population (Lauren and McDonald 1986; Wood et al. 1996). We attribute the initial loss in plasma ions and osmolality in freshwater (Figs. 3 and 4), at the first sampling time (6-hours) to handling stress. By 48 hours, the control fish values had stabilized or started moving toward initial concentrations, whereas Cu-exposed fish continued to lose plasma ions. In addition to ionoregulatory responses, hematocrit, a secondary stress response, increased (Fig. 5) in a time- and dose-dependent manner in freshwater, which is consistent with the findings of other researchers (Dethloff et al. 1999; De Boeck et al. 2001; Cerqueira and Fernandes 2002).
The Cu concentration and/or exposure duration in this experiment may have been too low to cause an ionoregulatory effect in salt water. In a previous study, plasma Na+ levels of salt water–adapted flounder (Platichthys flesus L) exposed to 170 μg/L Cu for 28 days were not significantly different from control fish; however, when these fish were exposed for 35 and 42 days to 170 μg/L Cu, a significant increase in plasma Na+ was observed as was an increase in gill Cu accumulation (Stagg and Shuttleworth 1982). Lewis and Lewis (1971) found that adult golden shiners (Notemigonus crysoleucas Mitchill) exposed to 5 mg/L Cu in freshwater had an 18% decrease in osmolality causing complete mortality in 46 hours; however, when 235 mOsm NaCl was added to the 5 mg/L Cu, no mortality occurred in golden shiners, and plasma osmolality of the blood serum increased more than that of fish held in salt water alone. Channel catfish (Ictalurus punctatus) fingerlings exposed to 2.5 mg/L Cu in freshwater experienced a 12% decrease in osmolality and then recovered with no mortality (Lewis and Lewis 1971). Similar to the response of the golden shiners, channel catfish exposed to 5 mg/L Cu with 235 mOsm NaCl had an increase in blood serum osmolality compared with control fish in salt water.
Effects of waterborne Cu exposure in salt water are not as well documented as in freshwater, but in addition to Cu uptake by the gill, intestinal Cu accumulation may also be a factor related to drinking. Blanchard and Grosell (2005) reported that Fundulus heteroclitus acclimated to either 11 or 20 g/L salt water had no significant increase in gill Cu accumulation after exposure to 30 or 150 μg/L Cu for 12 days; however, significant accumulation did occur in fish exposed to 150 μg/L Cu for 30 days at both salinities. F. heteroclitus acclimated to 20 g/L salt water and exposed to 30 or 150 μg/L Cu for 30 days had significant accumulation of intestinal Cu; however, at 4- and 12-day Cu exposures, significant intestinal Cu accumulation was not observed (Blanchard and Grolsell 2005). Similarly, F. heteroclitus acclimated to 11 g/L salt water accumulated significant intestinal Cu when fish were exposed to 30 or 150 μg/L Cu for 4, 12, or 30 days (Blanchard and Grolsell 2005). Recently, the intestine was indicated as an important site for Cu uptake in the marine gulf toadfish (Opsanus beta); however, more rapid Cu accumulation occurred in the gill (Grosell et al. 2004b). Furthermore, Grosell et al. (2004) reported an initial inhibition of drinking rate in O. beta after exposure to 3.5 μg/L Cu (55 mM) followed by an increased drinking rate from day 3 onward.
We expected intestinal Cu accumulation to occur in salt water–acclimated fish exposed to Cu for 21 days because these fish were presumably drinking to compensate for diffusive ion losses. If Cu uptake had been occurring at the intestine and then being regulated, then Cu accumulation would have also occurred in the liver. This pattern of intestinal Cu uptake in hybrid striped bass has been previously shown after dietary exposure to 500 to 900 mg/kg Cu (Bielmyer et al. 2005). Neither the intestine nor the liver had increased Cu levels, suggesting that the fish were not drinking excessively and/or were able to rapidly metabolize and excrete Cu at the tested exposure level.
Cu accumulated in the gill, intestine, and liver of freshwater-acclimated fish exposed to Cu for 21 days, which subsequently led to a decrease in growth and condition factor. Growth effects and liver Cu accumulation have been reported in several fish species after chronic Cu exposure (Drummond et al. 1973; Benoit 1975; Lett et al. 1976; Buckley et al. 1982; Lanno et al. 1985; Dethloff et al. 1999). During the first 2 weeks of our experiment, fish in the freshwater Cu-exposed treatment appeared to eat less than fish in other treatments. This observation is consistent with De Boeck et al. (1997) who reported a 36% decrease in the food intake of carp exposed to 51 μg Cu /L. In another study, there was little or no effect on the survival, growth, or swimming performance of rainbow trout exposed to 60 μg Cu/L for 30 days (Taylor et al. 2000). These investigators suggested that the availability of food (3% fish wet body weight/d and distributed as three 1% meals) prevented growth inhibition and initial ion losses, which usually result from Cu exposure. This was not the case in our study. The fish were offered food to satiation, and those exposed to Cu in freshwater decreased their intake regardless of the availability.
Marr et al. (1996) developed a linear model for the relationship between exposure duration, Cu accumulation, and fish weight. Their data suggested that decreased growth in rainbow trout, as a result of chronic Cu exposure, could be predicted if both the duration of exposure and the Cu body burden were known. Rainbow trout exposed to 75 μg Cu/L for up to 100 days accumulated Cu in the liver with a maximum concentration less than four times background values (McGeer et al. 2000a). The response of rainbow trout to Cu suggested an active regulation of tissue burden, and this burden was not a good indicator of physiological impact. McGeer et al. (2000b) reported that the physiological response of rainbow trout to chronic Cu exposure resulted in a metabolic cost. Although no growth effects were detected, a decrease in critical swimming speed and an increase in branchial Na+/K+ATPase activity in rainbow trout exposed to 75 μg Cu/L for 100 days was observed. Other researchers have reported a significant inhibition of Na+/K+ATPase activity and an increase in liver and gill Cu accumulation in freshwater tilapia (Tilapia zillii) exposed to 0.5, 1, 2, and 4 μg Cu/L for 14 days (Ay et al. 1999). Gill Na+/K+ATPase activity decreased by 33% within 24 hours of 55 μg Cu/L exposure to rainbow trout and returned to normal levels by day 14 (Lauren and McDonald, 1987).
Our findings are consistent with those of other researchers, suggesting that plasma ion imbalance and subsequently hematocrit are acutely affected. As the organism acclimates, recovery from these physiological disturbances may occur, which is shown in the results of our 21-day test. Hematocrit was increased in both the 48-hour and 21-day test, possibly indicating that the organisms had not yet fully acclimated.
Results of this research suggest that hybrid striped bass are useful test organisms to examine metal toxicity in freshwater and rank with the most sensitive fish species commonly used in metal-toxicity testing. Impaired ionoregulation in acutely exposed fish and increased gill, intestine, and liver Cu levels in chronically exposed hybrid striped bass are responses typical of other teleosts used in metal-toxicity studies. Growth effects caused by Cu exposure have varied in the existing literature, although in our study, growth was affected in the freshwater Cu-exposed fish and appeared to result from appetite suppression. In salt water, hybrid striped bass seem to be tolerant to Cu exposure through the waterborne-exposure route. However, hybrid striped bass have been shown to be sensitive to dietary Cu exposure in both freshwater and salt water in a previous study (Bielmyer et al. 2005). Conducting toxicity testing with this organism may lead to informative results on mechanisms of metal toxicity at different salinities under different exposure regimes.
References
Harrell RM, Kerby JH, Minton RV (eds) (1990) Culture and propagation of striped bass and its hybrids. Striped Bass Committee Southern Division, American Fisheries Society, Bethesda, MD
American Public Health Association, American Water Works Association, Water Pollution Control Federation (1985) Standard methods for the examination of water and wastewater, 16th ed. American Public Health Association, Washington, DC
Ay O, Kalay M, Tamer L, Canli M (1999) Copper and lead accumulation in tissues of a freshwater fish tilapia zillii and its effects on the branchial Na, K-ATPase activity. Bull Environ Contam Toxicol 62:160–168
Bielmyer GK, Tomasso J, Gatlin D, Isely J, Klaine SJ (2005) Responses of hybrid striped bass to waterborne and dietary copper in freshwater and saltwater. Comp Biochem Physiol C 140:131–137
Benoit D (1975) Chronic effects of copper on survival, growth, and reproduction of the bluegill (Lepomis macrochirus). Trans Am Fish Soc 2:353–358
Birge W, Price D, Shaw J, Spromberg J, Wigginton A (2000) Metal body burden and biological sensors as ecological indicators. Environ Toxicol Chem 19:1199–1212
Blanchard J, Grosell M (2005) Effects of salinity on copper accumulation in the common kilifish (Fundulus heteroclitus) Environ Toxicol Chem 24:1403–1413
Buckley J, Roch M, McCarter J, Rendell C, Matheson A (1982) Chronic exposure of Coho salmon to sublethal concentrations of copper: 1. Effect on growth, on accumulation and distribution of copper, and on copper tolerance. Comp Biochem Physiol C 72:15–19
Cerqueira C, Fernandes M (2002) Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish Prochilodus scrofa. Ecotox Environ Saf 52:83–91
De Boeck G, Vlaeminck A, Balm P, Lock R, De Wachter B, Blust R (2001) Morphological and metabolic changes in common carp, Cyprinus carpio, during short-term copper exposure: Interactions between Cu and plasma cortisol elevation. Environ Toxicol Chem 20:374–381
De Boeck G, Vlaeminck A, Blust R (1997) Effects of sublethal copper exposure on copper accumulation, food consumption, growth, energy stores, and nucleic acid content in common carp. Arch Environ Contam Toxicol 33:415–422
Dethloff G, Schlenk D, Khan S, Bailey H (1999) The effects of copper on blood and biochemical parameters of rainbow trout (Oncorhynchus mykiss). Arch Environ Contam Toxicol 36:415–423
Drummond R, Spoor W, Olson Giron (1973) Some short-term indicators of sublethal effects of copper on brook trout, Salvelinus fontinalis. J Fish Res Board Can 30:698–701
Dunnett CW (1955) Multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50:1096–1121
Grosell M, Hogstrand C, Wood C (1998) Renal Cu and Na excretion and hepatic cu metabolism in both cu acclimated and non acclimated rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 40:275–291
Grosell M, McDonald MD, Wood CM, Walsh PJ (2004a) Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta) I: Hydromineral balance and plasma nitrogenous waste products. Aquat Toxicol 68:249–262
Grosell M, McDonald MD, Walsh PJ, Wood CM (2004b) Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta) II: copper accumulation, drinking rate and Na+/K+ -ATPase activity in osmoregulatory tissues. Aquat Toxicol 68:263–275
Hall Jr LW, Anderson RD (1995) The influence of salinity on the toxicity of various classes of chemicals to aquatic biota. Crit Rev Toxicol 25:281–346
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Hansen J, Lipton J, Welsh P (2002) Relative sensitivity of bull trout (Salvelinus confluentus) and rainbow trout (Oncorhynchus mykiss) to acute copper toxicity. Environ Toxicol Chem 21(3):633–639
Harrell RM (1997) Striped bass and other Morone culture. Develop Aquacult Fish Sci 30:1–300
Lanno R, Slinger S, Hilton J (1985) Effect of ascorbic acid on dietary copper toxicity in rainbow trout (Salmo gairdneri Richardson). Aquaculture 49:269–287
Lauren D, McDonald D (1985) Effects of copper on branchial ionoregultion in the rainbow trout, Salmo gairdneri Richardson. J Comp Physiol B 155:635–644
Lauren D, McDonald D (1986) Influence of water hardness, pH, and alkalinity on the mechanisms of copper toxicity in juvenile rainbow trout, Salmo gairdneri. Can J Fish Aquat Sci 43:1488–1496
Lauren D, McDonald D (1987) Acclimation to copper by rainbow trout, Salmo gairdneri physiology. Can J Fish Aquat Sci 44:99–104
Lett P, Farmer G, Beamish F (1976) Effects of copper on some aspects of the bioenergetics of rainbow trout (Salmo gairdneri). J Fish Res Board Can 33:1335–1342
Lewis S, Lewis W (1971) The effect of zinc and copper on the osmolality of blood serum of the channel catfish, Ictalurus punctatus Rafinesque, and golden shiner, Notemigonus crysoleucas Mitchill. Trans Am Fish Soc 4:639–643
Li J, Quabius E, Bonga Sew F, Lik G, Lock R (1998) Effects of waterborne copper on branchial chloride cells and Na+/K+ ATPase activities in Mozambique tilapia (Oreochrlomis mossambicus). Aquat Toxicol 43:1–11
Lin H, Dunson W (1993) The effect of salinity on the acute toxicity of cadmium to the tropical, estuarine, hermaphroditic fish, Rivulus marmoratus: A comparison of Cd, Cu, and Zn tolerance with Fundulus heteroclitus. Arch Environ Contam Toxicol 25:41–47
Marr J, Lipton J, Cacela D, Hansen J, Bergman H, Meyer J, et al. (1996) Relationship between copper exposure duration, tissue copper concentration, and rainbow trout growth. Aquat Toxicol 36:17–30
McGeer J, Szebedinszky C, McDonald D, Wood C (2000a) Effects of chronic sublethal exposure to waterborne Cu, Cd, or Zn in rainbow trout 2: Tissue specific metal accumulation. Aquat Toxicol 50:245–256
McGeer J, Szebedinszky C, McDondald D, Wood C (2000b) Effects of chronic sublethal exposure to waterborne Cu, Cd, or Zn in rainbow trout. 1: Ionoregulatory disturbances and metabolic costs. Aquat Toxicol 50:231–243
Moore JW, Ramamoorthy S (1984) Heavy metals in natural waters. Springer-Verlag, New York, NY
Playle R, Gensemer R, Dixon D (1992) Copper accumulation on gills of fathead minnows: Influence of water hardness, complexation, and pH of the gill micro-environment. Environ Toxicol Chem 11:381–391
Posthuma L, Suter II GW, Traas TP (2002) Species sensitivity distributions in ecotoxicology. CRC, Boca Raton, FL
Smith TIJ, Jenkins WE, Haggerty RW (1986) Growth and survival of juvenile striped bass (Morone saxatilis) × white bass (M. chrysops) hybrids reared at different salinities. Proc Annu Conf SEAFWA 40:143–151
Stagg RM, Shuttleworth TJ (1982) The accumulation of copper in Platichthys flesus L and its effects on plasma electrolyte concentrations. J Fish Biol 20:491–500
Taylor L, McGeer J, Wood C, McDonald D (2000) Physiological effects of chronic copper exposure to rainbow trout (Oncorhynchus mykiss) in hard and soft water. Evaluation of chronic indicators. Environ Toxicol Chem 19:2298–2308
United States Environmental Protection Agency (2005) USEPA Aquatic Toxicity Information Retrieval (AQUIRE) aquatic toxicology database. Available at: http://www.epa.gov/ecotox/. Accessed: August 2003
United States Environmental Protection Agency (1989) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms, 2nd ed. EPA/600/4-89/001. Final Report. Environmental Monitoring and Support Laboratory, Cincinnati, OH
Weirich CR, Tomasso JR, Smith TIJ (1992) Confinement and transport-induced stress in white bass Morone chrysops × striped bass M. saxatilis hybrids: Effect of calcium and salinity. J World Aqua Soc 23:49–57
Welsh PG, Skidmore JF, J, SD, Dixon DG, Hodson PV, Hutchinson MJ, Hickie BE (1993) Effect of pH and dissolved organic carbon on the toxicity of copper to larval fathead minnows (Pimephales promelas) in natural lake waters of low alkalinity. Can J Fish Aquat Sci 50:1356–1362
Wilson RW, Taylor EW (1992) Differential responses to copper in rainbow trout (Oncorhynchus mykiss) acclimated to sea water and brackish water. J Comp Physiol B 163:239–246
Wood CM, Hogstrand C, Galvez F, Munger RS (1996) The physiology of waterborne silver toxicity in freshwater rainbow trout (Oncorhynchus mykiss). 1. The effects of ionic Ag+. Aquat Toxicol 35:93–109
Wood CM (2001) Toxic responses of the gill. In: Schlenk D, Benson WH (eds) Target organ toxicity in marine and freshwater teleosts. Volume 1. Organs. Taylor & Francis, London, UK, pp 1–89
Acknowledgments
Funding was provided by the Copper Development Association and the South Carolina Agricultural Experimental Station. The authors would also like to thank S. Young, H. Atwood, J. Smink, E. VanGenderen, A. Ryan, S. Knuteson, C. Thomas, L. Frederick, R. Regala, A. Karsten, M. Taylor, and C. Rice for help with this research and L. Bain C. Lee, and K. V. Brix for helpful scientific dialogue.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00244-006-6131-4.
Rights and permissions
About this article
Cite this article
Bielmyer, G.K., Tomasso, J. & Klaine, S.J. Physiological Responses of Hybrid Striped Bass to Aqueous Copperin Freshwater and Saltwater. Arch Environ Contam Toxicol 50, 531–538 (2006). https://doi.org/10.1007/s00244-005-0131-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0131-7